Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Hydrocortisone acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrocortisone acetate is a synthetic glucocorticoid corticosteroid and a corticosteroid ester.
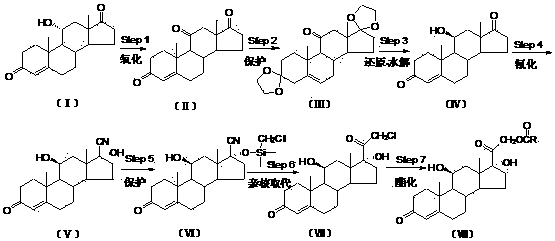
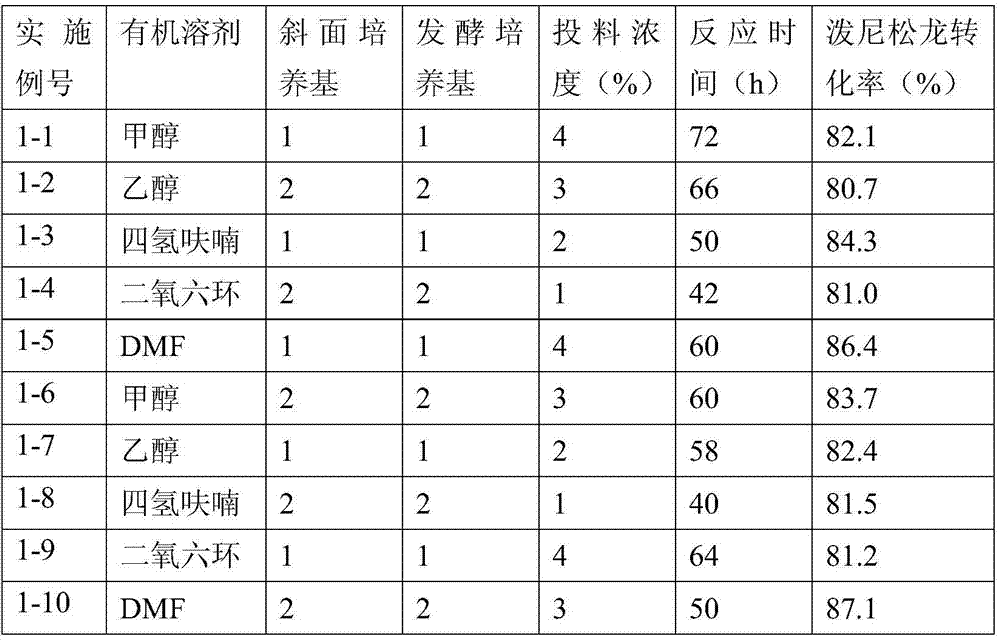
Preparation method of hydrocortisone acetate or analogue thereof
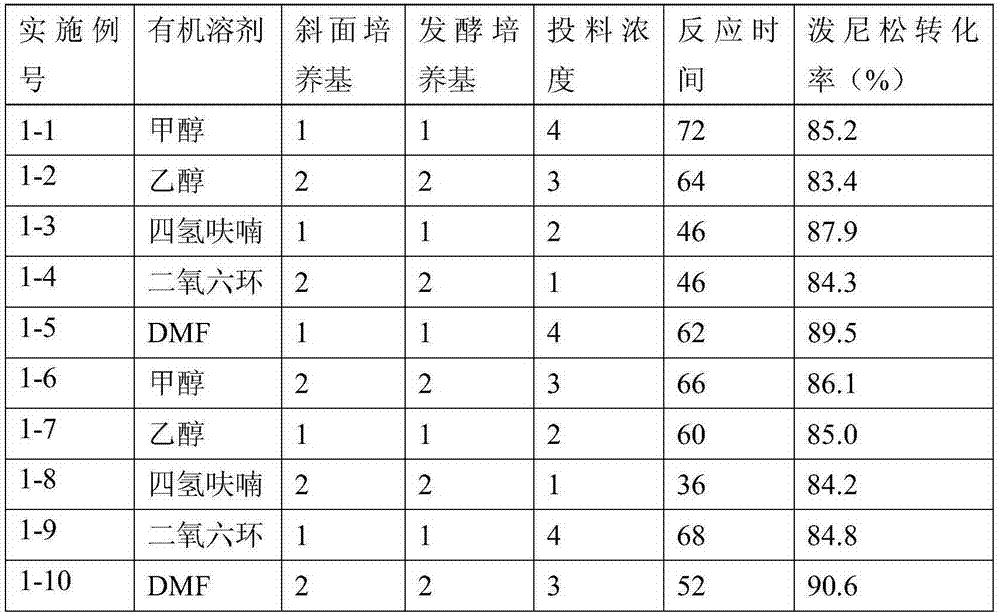
ActiveCN102603842AStarting materials are readily availableHigh and stable yieldSteroidsSNiStructural formula
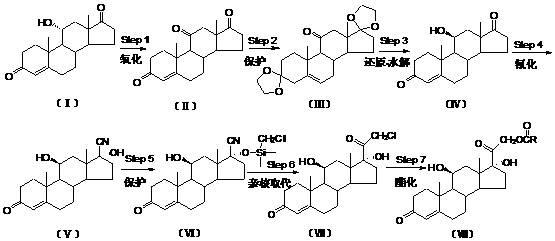
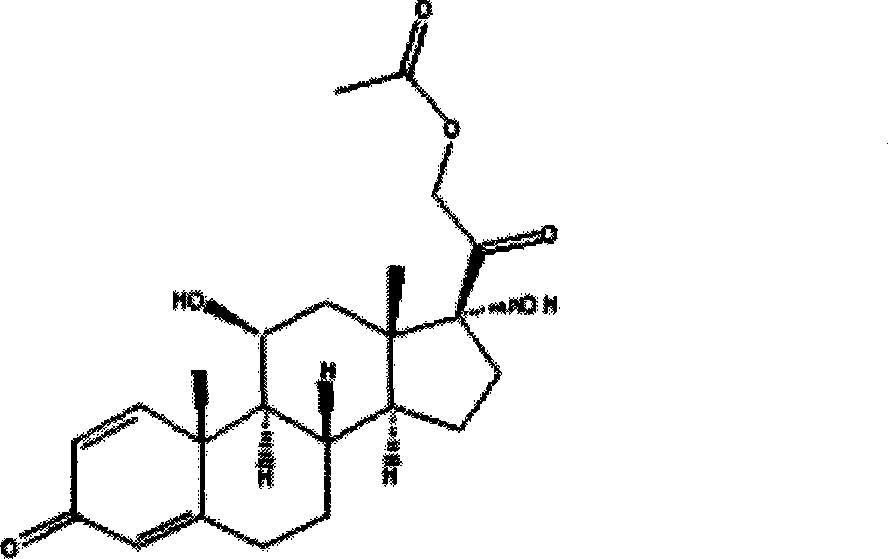
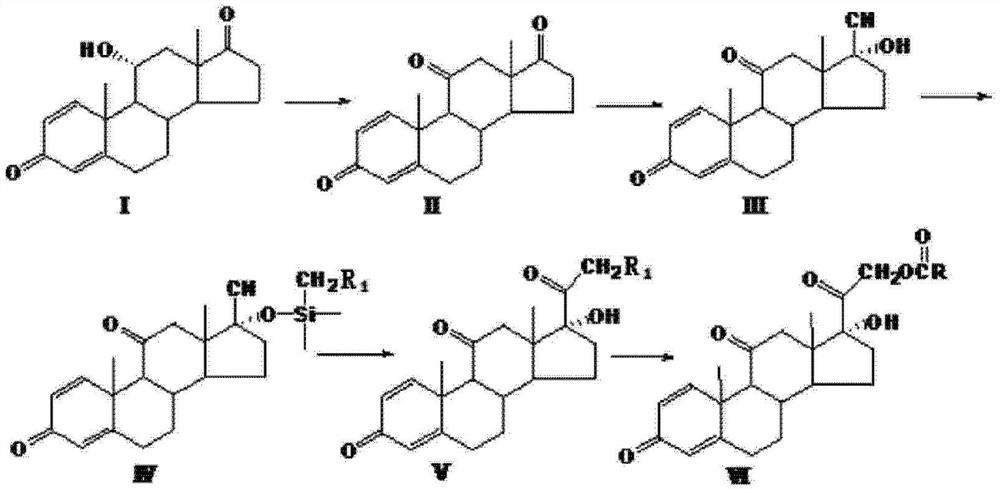
The invention relates to a preparation method of hydrocortisone acetate or an analogue thereof. Hydrocortisone acetate or the analogue thereof has a structural formula represented as a formula VIII. According to the invention, a compound I is subject to an oxidation reaction, a carbonyl protection reaction, a reduction and hydrolysis reaction, a cyano substitution reaction, a silicon alkyloxy protection reaction, an intramolecular nucleophilic substitution reaction, and a substitution reaction, such that hydrocortisone acetate or the analogue thereof is prepared. The initial raw materials areeasy to obtain, and the yield is high and stable. The compound I is represented by a formula I, wherein R is H or alkyl.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Preparation of hydrocortisone and derivatives thereof
The invention relates to a preparation method of steroids compounds, in particular to the preparation of hydrocortisone and derivatives thereof. The invention adopts 17-hydroxy-4, 9-diene-pregna-3, 20-diketone as the original material which is modified by 21 bits and 9, 11 bits so as to obtain anecortave acetate retaane, the hydrocortisone and hydrocortisone acetate, and the like. The technical process of the invention adopts the existing intermediates of manufacturers as the original material; the route is simple; the feasibility is high; the operability is strong; the materials are available; the use of expensive accessories is avoided so as to largely reduce the cost of industrial manufacturing; a plurality of products can be obtained on the same manufacturing line; the yield and the cost are dramatically better than that of the prior methods used for synthesizing the hydrocortisone and the derivatives thereof; moreover, the adoption of the existing intermediates realizes the combined-line production of triamcinolone acetonide series products, hydrocortisone series products and anecortave acetate series products so as to greatly reduce the manufacturing cost and the conditions for industrial manufacturing; wherein, R is equal to minus OCOR1 and R1 is equal to the alkyl with the carbon less than 11.
Owner:TIANJIN PHARMA GROUP CORP
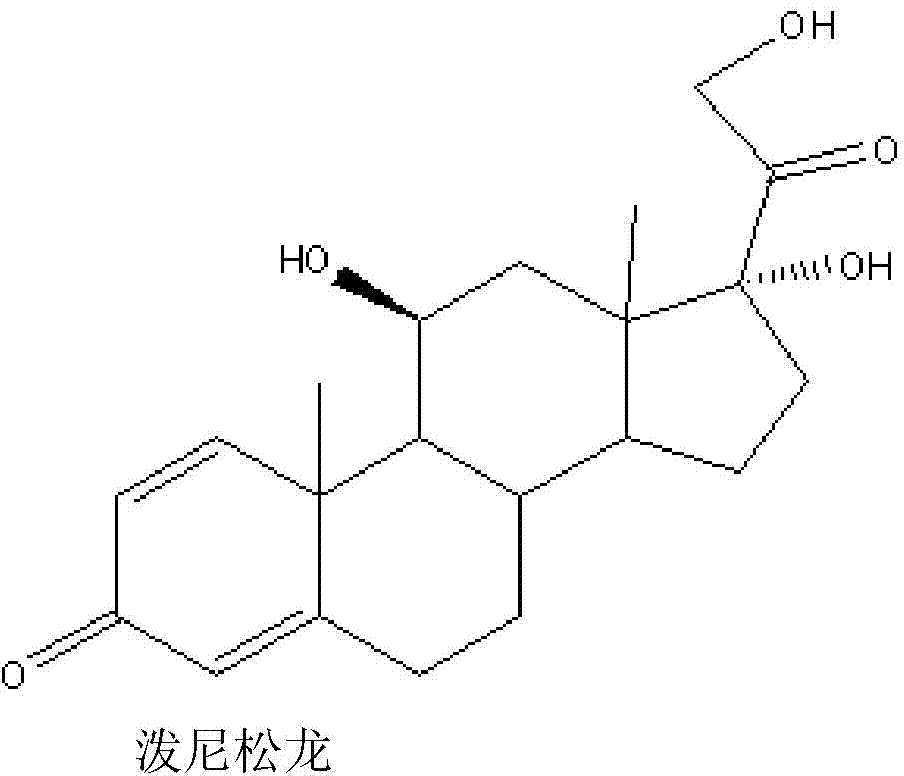
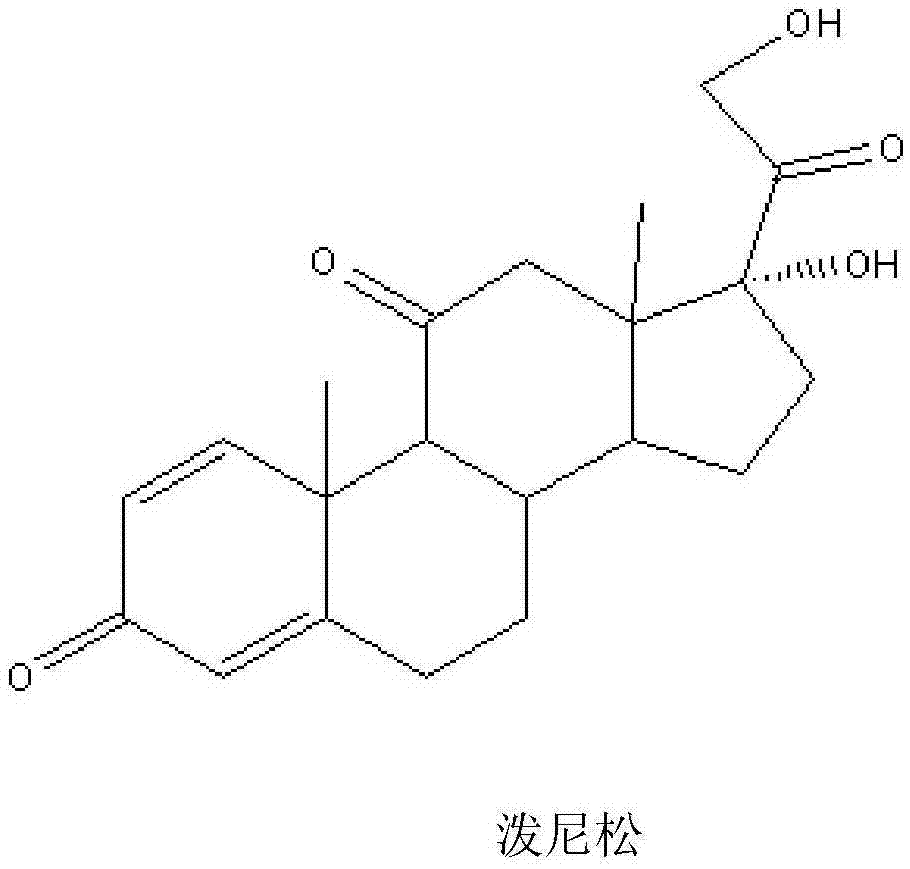
Method for producing prednisolone acetate
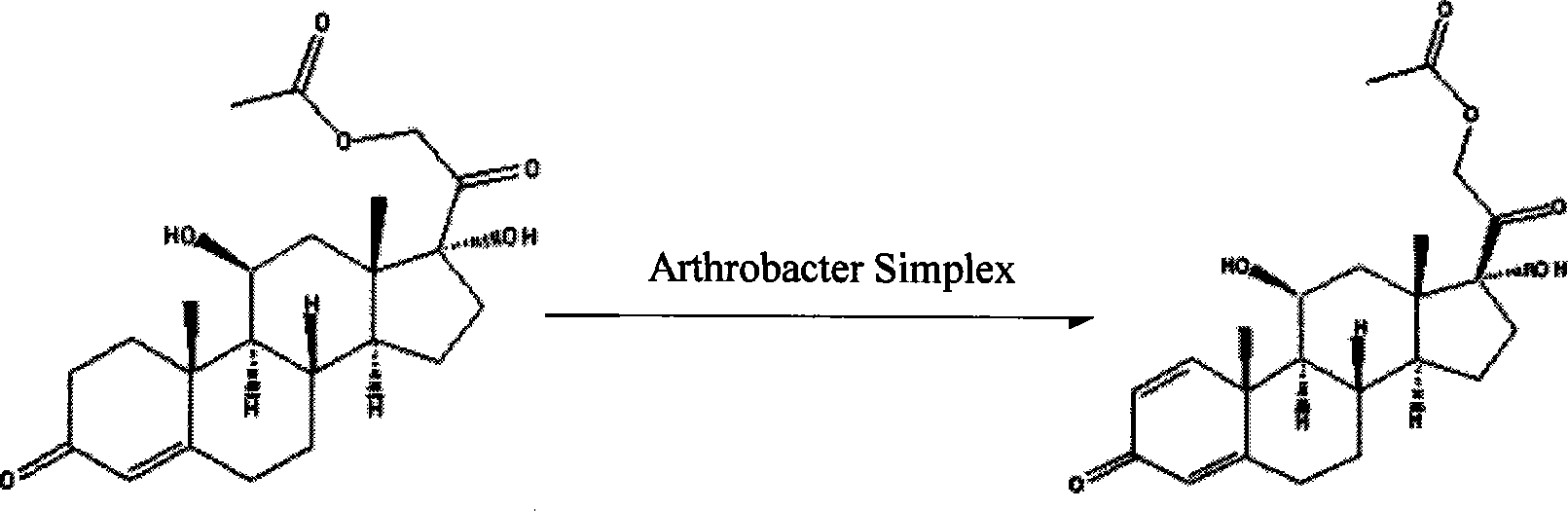
InactiveCN101210259AStrong response specificityEasy to operateMicroorganism based processesFermentationMicrobial transformationArthrobacter simplex
The invention belongs to the field of microbial pharmaceutics and pharmaceutical engineering, specifically relates to a production method of prednisone acetate by microbial transformation with Arthrobacter simplex as bacteria strain and hydrocortisone acetate as substrate. The method uses Arthrobacter simplex as bacteria strain and comprises the following steps of: performing primary seed culture, performing second fermentation culture, adding hydrocortisone acetate into the fermentation liquid of Arthrobacter simplex to transform hydrocortisone acetate into prednisone acetate, filtering, and collecting cake to obtain prednisone acetate. The bacteria can be prepared into double liquid phase, broken cells or protoplast, each of which has high transformation ratio. The inventive production method replaces cortisone acetate with hydrocortisone acetate as raw material, and has the advantages of high yield, simple process, good economical and practical performance, and less use of harmful reagents; and is important for steroids production with biotransformation method.
Owner:TIANJIN UNIV OF SCI & TECH
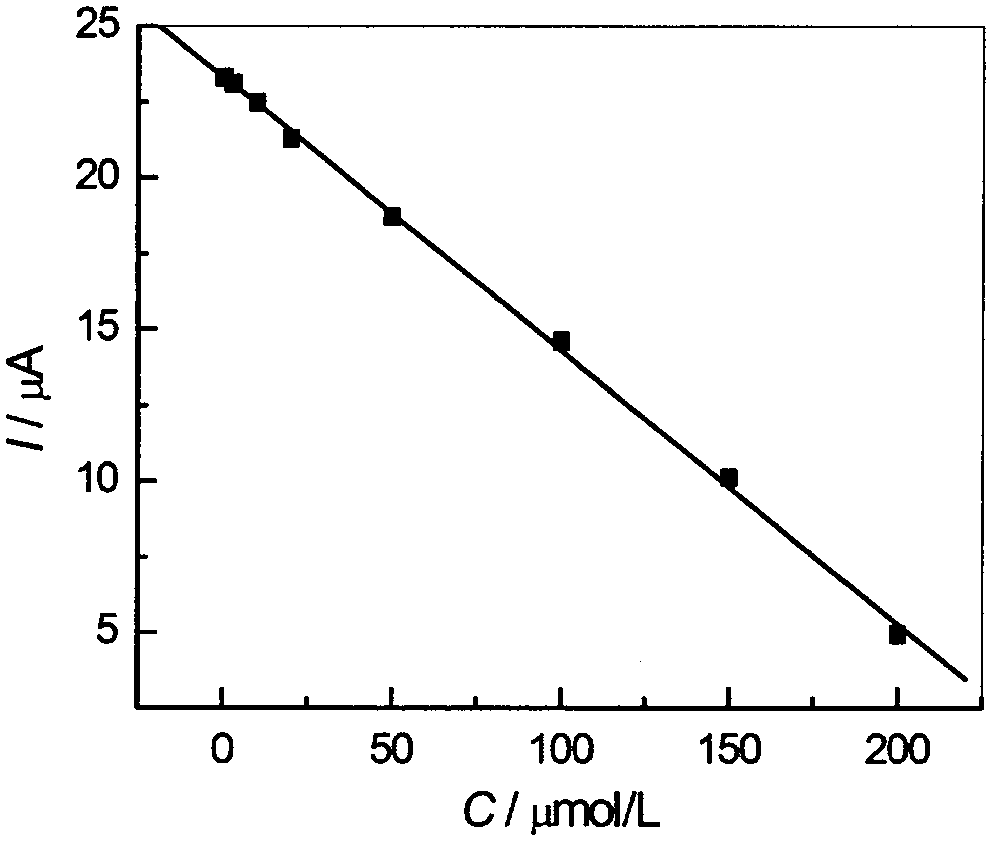
Preparation method for high-sensitivity functionalized gold nanoparticle-doped meprobamate molecularly-imprinted electrochemical sensor
The invention discloses a preparation method for a high-sensitivity functionalized gold nanoparticle-doped meprobamate molecularly-imprinted electrochemical sensor. According to the method, meprobamate serves as a template molecule, aminotadalafil serves as a functional monomer, azodiisobutyronitrile serves as an initiating agent, dodecanethiol functionalized gold nanoparticles serve as a doping agent, hydrocortisone acetate serves as a cross-linking agent, and then the high-sensitivity dodecanethiol functionalized gold nanoparticle-doped meprobamate molecularly-imprinted electrochemical sensor is prepared. The analytical method is simple and practical, and the defects that an original analytical method is complex, expensive in equipment, and low in sensitivity are overcome.
Owner:GUANGXI UNIV FOR NATITIES
Preparation method of hydrocortisone acetate or analogue thereof
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
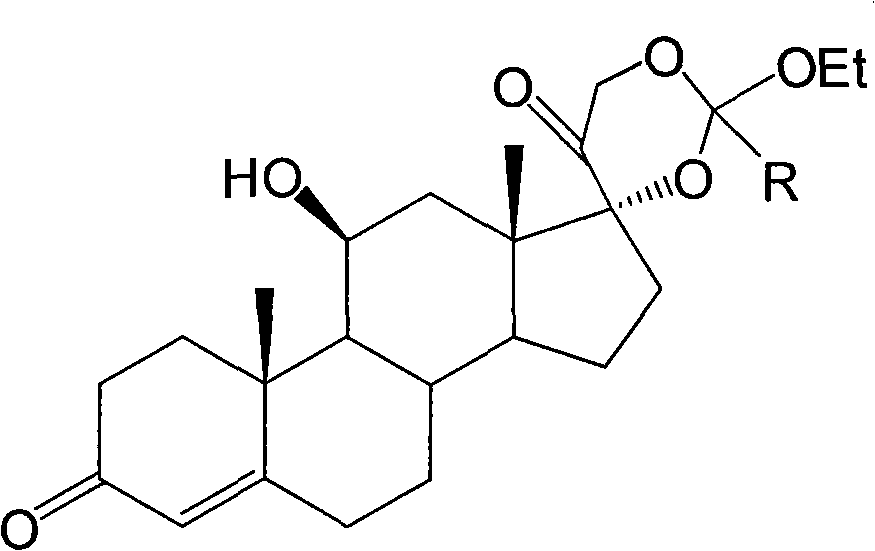
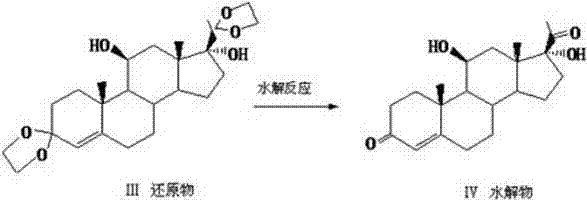
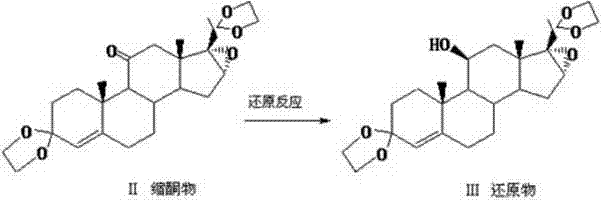
Preparation method of hydrocortisone
ActiveCN102367262AAvoid it happening againRaw materials are easy to getSteroidsDisplacement reactionsHydrocortisone acetate
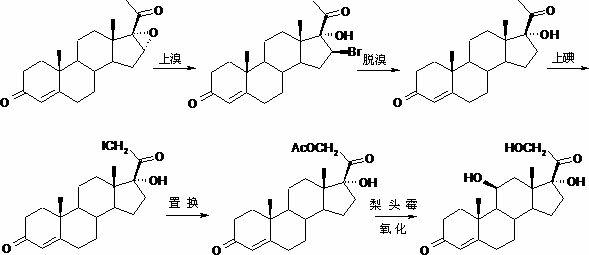
The invention, relating to the field of pharmaceutical synthesis, particularly relates to a preparation method of hydrocortisone, comprising the following steps: using an intermediate 17 alfa-hydroxy-4-pregnene-3,11,20- triketone (II) in a Rhizopus nigricans method as raw material, successively carrying out ketal reaction, reduction reaction, hydrolysis reaction, iodine adding reaction, and displacement reaction to obtain hydrocortisone acetate (VII), and finally carrying out sodium hydroxide hydrolysis reaction to obtain the hydrocortisone (I). The invention has the advantages of easy obtainment of raw material, common auxiliary materials, and no need of using toxic, highly toxic, and carcinogenic reagents. The method is more efficient than the process in the prior art, and the products has good quality and yield.
Owner:ZHEJIANG XIANJU PHARMA
Process for preparing hydrocortisone acetate
This invention refers to a chemical synthesis preparation technique of acetic acid hydrocortisone. C3~C5 fat ketone is used as solvent and certain acetate is added. It is got by synthesizing hydrocortisone, acetic acid and acetic anhydride. Pyridine is replaced by low toxicity solvent, then pollution is reduced and yield is raised, the yield of acetic acid hydrocortisone can reach above 95%. The product quality is stabilized and cost is reduced, it is more acceptable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Method for preparing prednisolone through bio-fermentation in one step
The invention relates to a method for directly generating prednisolone through bio-fermentation in one step by using cortisone acetate as a raw material and Arthrobacter Simple ATCC 21032.
Owner:TIANJIN JINYAO GRP
Preparation method of hydrocortisone acetate
ActiveCN107400153AResolve incomplete responsesImprove product qualitySteroidsHydrocortisone acetateAlkali metal halide
The invention belongs to the field of preparation of steroid drugs and particularly relates to a preparation method of hydrocortisone acetate. The preparation method comprises steps as follows: a bromohydroxy compound is synthesized from a compound C5 as a raw material through a bromohydroxy reaction, a crude hydrocortisone acetate product is produced through a debromination reaction, and finally, a refined hydrocortisone acetate product is obtained through refining, wherein alkali halide is added as a catalyst of the bromohydroxy reaction and a feeding mass ratio of the compound C5 to alkali halide is 1:(0.08-0.1). The preparation method of hydrocortisone acetate has the advantages as follows: alkali halide is adopted as the catalyst of the bromohydroxy reaction, so that the key of the problem that the reaction is incomplete in the bromohydroxy reaction process is solved, and one novel preparation method of hydrocortisone acetate with stable product quality and higher yield is provided.
Owner:ZHEJIANG XIANJU PHARMA
Method for preparing hydrocortisone acetate
ActiveCN101781349AGood quality and stableMeet the requirementsSteroidsHydrocortisone acetatePyridine
The invention relates to a method for preparing hydrocortisone acetate. Three to twenty parts of dipolar aprotic solvent (including but not limited to dimethyl formamide or dimethyl sulfoxide and the like) is taken as the solvent, 0.1 to 1.5 parts of cheap organic amine (including but not limited to triethylamine, ethanediamine or piperidine and the like) is taken as the catalyst, one part of hydrocortisone and 0.4 to 1.0 part of acid anhydride react for 2 to 8 hours at 15 to 80 DEG C, and the hydrocortisone acetate can be prepared through adding water. The method replaces pyridine with low-toxic solvents, thereby reducing the environmental pollution and increasing the yield. The yield of the hydrocortisone acetate can reach more than 95 percent, the single maximum impurity is 0.82 percent, and the total impurity is 1.68 percent. The method stabilizes the product quality, reduces the cost, and is more suitable for large-scale industrialized production.
Owner:TIANJIN JINJIN PHARMA
Synthesis of hydrocortisone butyrate
The invention relates to synthesis of hydrocortisone butyrate, which comprises the following steps of: A. carrying out acylation reaction on hydrocortisone acetate adopted as a raw material and butyl chloride in an organic solvent I under the condition of existence of a catalyst; after the reaction is finished, adding acid to adjust a pH value being smaller than 5; diluting or recrystallizing to obtain hydrocortisone 17alpha-butyrate-21-acetate; and B. adopting the hydrocortisone 17alpha-butyrate-21-acetate as a raw material, adding inorganic alkali in an organic solvent II and carrying out selective hydrolysis reaction to obtain hydrocortisone 17alpha-butyrate.
Owner:TIANJIN JINYAO GRP
Production technology of hydrocortisone acetate
The invention discloses a production technology of hydrocortisone acetate, which belongs to the technical field of chemical pharmacy. The technology employs reaction of hydrocortisone and acetic anhydride to prepare the product, a reaction of hydrocortisone and acetic anhydride is carried out in a dipolar aprotic solvent, an alkali salt acetate is taken as a catalyst, the reaction is carried out under nitrogen or inert gas protection, and the dipolar aprotic solvent is dimethyl formamide or dimethyl sulfoxide. The technology has the advantages of high yield and good product quality.
Owner:HENAN LIHUA PHARMA
Moisturizing skin ointment - composition consisting of polymyxin B Sulfate, bacitracin zinc, neomycin (the combination of which totals 1 ounce), hydrocortisone acetate (1 ounce) and white petrolatum (13 ounces)
This invention relates to a moisturizing skin ointment composition consisting of polymyxin B Sulfate, bacitracin zinc, neomycin (the combination of which totals 1 ounce), hydrocortisone acetate (1 ounce) and white petrolatum (13 ounces). When combined and water evaporated, an external ointment that penetrates the skin and alleviates dry skin conditions, itching and minor scrapes and scratches is created. The properties of the antibiotic products help to promote healing of minor scrapes and scratches as well as preventing the spread of dry skin conditions. The properties of the hydrocortisone aids in the alleviation of itching associated with dry skin. Because this ointment penetrates the dermis almost immediately, the moisturizing properties of petrolatum allows the full benefit of the antibiotic products and hydrocortison to remain on / in the skin through several washings thereby alleviating the need to reapply several times a day.
Owner:MEEKS JOYCE ANN
Preparation method of hydrocortisone acetate
InactiveCN102827230AAvoid it happening againRaw materials are easy to getSteroidsEthylic acidAcetylation
The invention discloses a preparation method of hydrocortisone acetate, belonging to the field of chemical pharmacy. The preparation method comprises the following steps: by using an intermediate Pu's oxide in the traditional cortisone acetate synthesis technique as a raw material, sequentially carrying out ketalation reaction, reduction reaction, hydrolysis reaction, iodine reaction and acetylation reaction under certain conditions to obtain the product hydrocortisone acetate. The preparation method of hydrocortisone acetate has the advantages of accessible raw materials and common auxiliary materials, and does not need some toxic, virulent and cancerigenic reagents in the original technique. The invention is environment-friendly and effective; the product has both high quality and yield; and the HPLC (high performance liquid chromatography) content is up to higher than 98.5%, and the yield is up to higher than 80%.
Owner:HENAN LIHUA PHARMA
Preparation method of halcinonide and derivative thereof
The invention provides a preparation method of halcinonide and a derivative thereof. The method comprises the following steps: taking hydrocortisone acetate as a raw material, performing dehydration and then epoxidation on the raw material, performing ring opening, performing hydrolysis and chlorination, and then performing oxidation and ketalization, so as to obtain halcinonide; or performing dehydration and then epoxidation on the raw material, performing ring opening, performing hydrolysis, and then performing oxidation and ketalization, so as to obtain 9-fluoro-16a,17-(isopropylidenedioxy)corticosterone. In ring opening fluorination unit reaction, safe and mild reaction environment is selected, a fluorizating agent with low concentration is used as a reaction reagent, the reaction rate is effectively controlled, the production of side reaction products is restrained, and the product quality and yield are greatly improved. Moreover, in 16, 17-ketalization unit reaction, an acid catalyst which is low in toxicity and easy to control is adopted to replace boron trifluoride with high toxicity to perform catalysis, and the catalytic effect is effectively improved.
Owner:SHANDONG TAIHUA BIO & TECH
Refining method for hydrocortisone acetate
ActiveCN104610407AImprove responseSimple and fast operationSteroidsChloride sodiumHydrocortisone acetate
The invention discloses a refining method for hydrocortisone acetate. The refining method comprises the step of purifying a hydrocortisone acetate crude product with chlorine gas or with hypochorite, glacial acetic acid and sodium chloride, so as to obtain a hydrocortisone acetate refined product. The refining method is simple, convenient, low in manufacturing cost, high in yield and product purity, stable in process, and suitable for producing a large quantity of the hydrocortisone acetate refined product.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Medicinal Cream Made Using Hydrocortisone Acetate and A Process To Make The Same
The present invention relates to a composition for treating skin inflammation, along with skin rejuvenation. More particularly, the present invention relates to a pharmaceutical cream comprising a biopolymer, and a corticosteroid. It discloses a composition for treating skin inflammation, along with skin rejuvenation containing a) a biopolymer in the form of chitosan, b) an active pharmaceutical ingredient (API) composition in the form of hydrocortisone acetate, used in treating skin inflammation c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The active ingredients, namely chitosan, and a corticosteroid in the form of hydrocortisone acetate, are incorporated in cream base for use in treating skin inflammation due to allergy & itching & wounds on human skin involving contacting human skin with the above identified composition.
Owner:APEX LAB PRIVATE LTD
Method for preparing prednisolone through bio-fermentation in one step
The invention relates to a method for directly generating prednisolone through bio-fermentation in one step by using hydrocortisone acetate as a raw material and Arthrobacter Simple ATCC 21032.
Owner:TIANJIN JINYAO GRP
Preparation method of hydrocortisone acetate
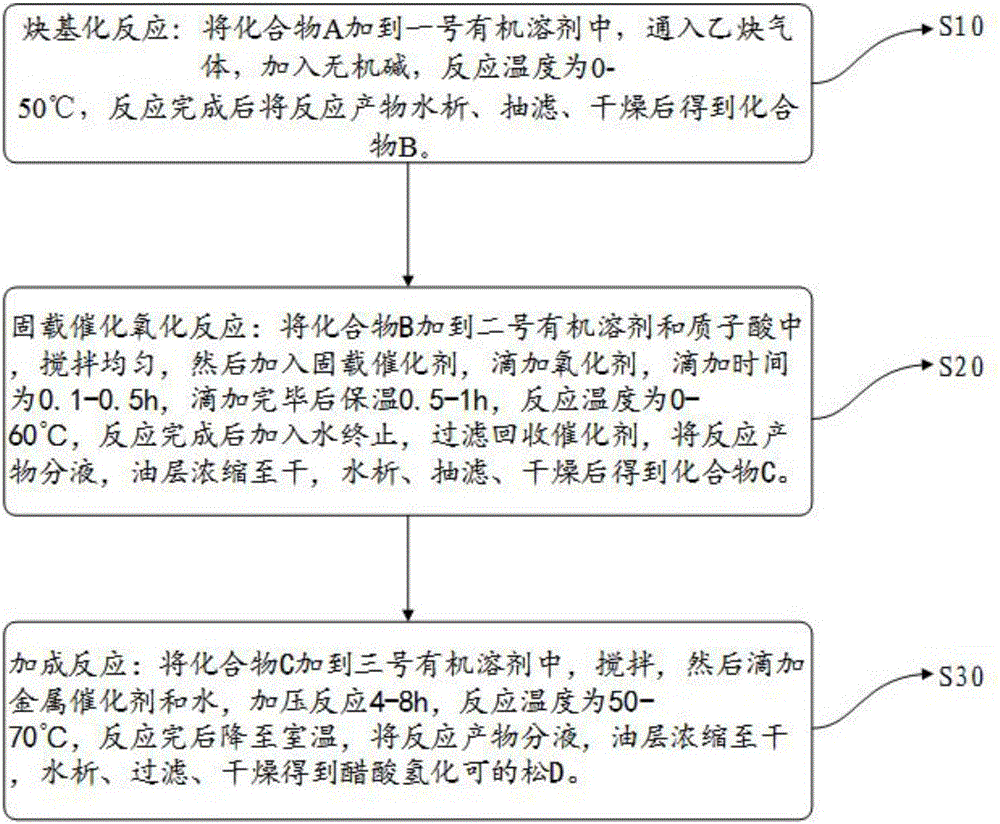
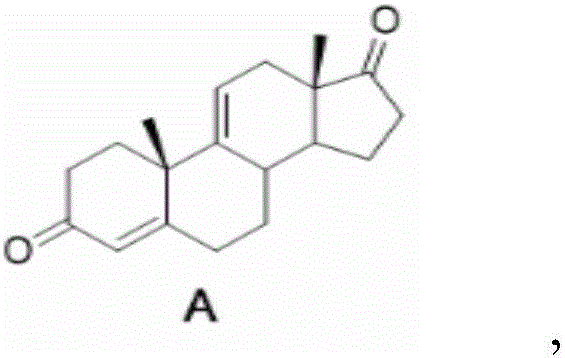
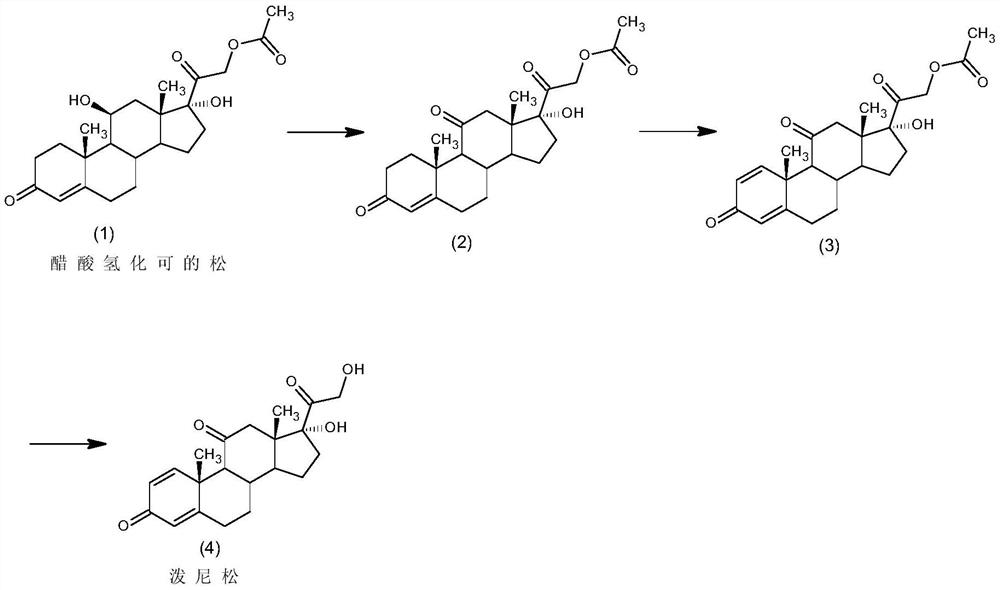
The invention relates to a preparation method of hydrocortisone acetate. A steroid raw material compound A is subjected to alkynylation reaction, solid-supported catalytic oxidation reaction and addition reaction to prepare hydrocortisone acetate. According to the method, the starting material is a basic steroid raw material, the source is easy to obtain, the cost is low, the synthesis procedure is short, all steps of reaction are relatively easy to implement, the yield is high, the catalyst and the carrier can be recycled, dangerous and toxic materials such as bromine are avoided, the dependence on high-end equipment is greatly reduced, the environment pollution is reduced, the production is more economic and environmental-friendly and safer, and the preparation method of the hydrocortisone acetate is more suitable for industrial production.
Owner:湖南成大生物科技有限公司
Refining method of hydrocortisone acetate
The invention discloses a refining method for hydrocortisone acetate. The refining method comprises the step of purifying a hydrocortisone acetate crude product with chlorine gas or with hypochorite, glacial acetic acid and sodium chloride, so as to obtain a hydrocortisone acetate refined product. The refining method is simple, convenient, low in manufacturing cost, high in yield and product purity, stable in process, and suitable for producing a large quantity of the hydrocortisone acetate refined product.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Preparation method of high-sensitivity functionalized silver nanoparticle-doped aniracetam MIECS (Molecular Imprinting Electrochemical Sensor)
InactiveCN105628762AHigh sensitivityEasy to manufactureMaterial electrochemical variablesDopantFunctional monomer
The invention discloses a preparation method of a high-sensitivity functionalized silver nanoparticle-doped aniracetam MIECS (Molecular Imprinting Electrochemical Sensor). The preparation method is characterized by taking aniracetam as a template molecule, dihydromyrcenol as a functional monomer, hydrocortisone acetate as a coupling agent and decyl mercaptan functionalized silver nanoparticles as a doping agent, thus preparing the high-sensitivity decyl mercaptan functionalized silver nanoparticle-doped aniracetam MIECS. An analysis method is simple and practical, and the disadvantages of complexity, expensive price of equipment and low sensitivity of a previous analysis method are overcome.
Owner:GUANGXI UNIV FOR NATITIES
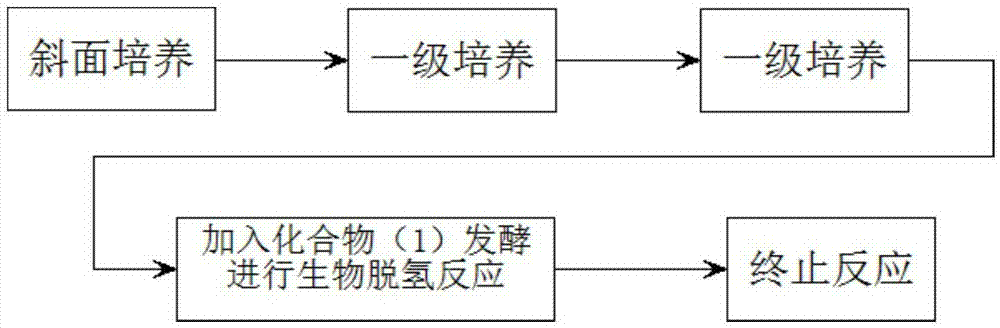
Preparation method of prednisone
ActiveCN111777654ALower requirementLow running costMicroorganism based processesSteroidsBiotechnologyDehydrogenation
The invention discloses a preparation method of prednisone, and belongs to the technical field of preparation and processing of medicines. According to the method, hydrocortisone acetate is used as aninitial raw material, and the prednisone is prepared through three steps of oxidation, biological fermentation dehydrogenation and hydrolysis. According to the preparation method of prednisone, the defects of a traditional process are overcome, the target product is high in purity, good in quality stability, high in yield, low in production cost and mild in reaction condition, a highly toxic cyanide reagent is prevented from being used, and the method is easy and convenient to operate, suitable for industrial production and wide in market prospect.
Owner:ZHEJIANG SHENZHOU PHARMA
Medicinal fusidic acid cream made using sodium fusidate and incorporating a biopolymer, a corticosteroid, and an antifungal agent, and a process to make it.
InactiveUS20120035144A1Improve shelf life stabilityFine granularityAntibacterial agentsBiocideBiopolymerOxygen
The present invention is directed to a medicinal composition for treating skin inflammations, fungal / bacterial skin infections and related wounds, and also other skin wounds including those caused by burns. The cream also causes skin rejuvenation through an epithelisation process. The cream comprises:a) a biopolymer in the form of Chitosan, b) active Pharmaceutical Ingredients (APIs), in the form of fusidic acid that has been generated in situ from sodium fusidate Hydrocortisone acetate & clotrimazole, c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The invention also discloses a process to make medicinal cream containing Fusidic acid formed in situ from Sodium Fusidate by converting it into Fusidic acid under oxygen-free environment. The cream has greater shelf-life and the finer particle size of the API than the conventional creams containing Fusidic acid.
Owner:APEX LAB PRIVATE LTD
Method for preparing hydrocortisone acetate
The invention relates to a method for preparing hydrocortisone acetate. The method comprises the following steps: 9beta, 11beta-epoxy-17alpha-dihydroxypregn-4-ene-3,20-dione-21-acetate is subjected toa ring-opening reaction with a fluorinating reagent, wherein the fluorinating reagent is an organic amine salt or solution of hydrogen fluoride. The reagent and conditions for ring-opening fluorination of epoxy compounds in key process steps are mainly improved, organic amine salt of hydrogen fluoride substitutes for high-toxicity and high-corrosiveness hydrogen fluoride gas, a small quantity ofthe fluorinating reagent is used, and the method is safe and convenient to operate and suitable for industrial production.
Owner:江苏福锌雨医药科技有限公司
Medicinal cream containing miconazole nitrate, hydrocortisone acetate, and a biopolymer, and a process to make it
InactiveUS20120115828A1Effective treatment against fungal infectionActively healOrganic active ingredientsBiocideBiopolymerAdditive ingredient
The present invention is directed to a composition for treating bacterial skin infections & skin inflammation, along with skin rejuvenation. More particularly, the present invention relates to a pharmaceutical cream comprising a biopolymer, a corticosteroid and an antibacterial active ingredient. It discloses a composition for treating fungal skin infections & skin inflammation, along with skin rejuvenation containing a) a biopolymer in the form of chitosan, b) a combination of active pharmaceutical ingredients (APIs), miconazole and hydrocortisone acetate used in treating bacterial skin infections & skin inflammations, c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The active ingredients, namely chitosan, corticosteroid in the form of hydrocortisone acetate, and an antibacterial agent in the form of miconazole, are incorporated in cream base for use in treating bacterial skin infections and skin inflammation due to allergy & itching, & wounds on human skin involving contacting human skin with the above identified composition.
Owner:APEX LAB PRIVATE LTD
Hydrocortisone acetate suppository formulation for treatment of disease
ActiveUS10653623B2High retention rateLow variabilityOrganic active ingredientsDigestive systemSuppository drugCortisone
The present invention relates, in various embodiments, to formulations comprising hydrocortisone and silicon dioxide. In additional embodiments, the invention relates to suppositories comprising hydrocortisone and silicon dioxide. The formulations of the present invention are useful for administration to patients who have gastrointestinal diseases and disorders such as, for example, inflammatory bowel disease.
Owner:CRISTCOT LLC
Fluorohydrocortisone acetate tablet and preparation method thereof
ActiveCN114569569AAvoid negative effectsGood storage stabilityOrganic active ingredientsPharmaceutical non-active ingredientsFludrocortisoneLactose
The fluhydrocortisone acetate tablet is mainly prepared from fluhydrocortisone acetate, lactose, a disintegrating agent, a flow aid and a lubricant, the disintegrating agent is directly pressed starch, and the fluhydrocortisone acetate tablet does not contain ionic auxiliary materials. The preparation method comprises the following steps: (1) sieving the fluorohydrocortisone acetate and the lactose, adding the sieved fluorohydrocortisone acetate and lactose into a mixer, and then adding the directly pressed starch for mixing; (2) adding a flow aid into the mixed material obtained in the step (1), and continuously mixing; (3) adding a lubricant into the mixed material obtained in the step (2), and carrying out final mixing; and (4) tabletting and molding the mixed material obtained in the step (3) to obtain the hydrocortisone acetate tablet. The direct-pressed starch is innovatively adopted to replace a traditional ionic auxiliary material to serve as a disintegrating agent, so that negative effects caused by the use of the ionic auxiliary material can be avoided, and the storage stability of the hydrocortisone acetate tablet can be improved under the condition of ensuring that the dissolution rate of the product is consistent with that of an original developed agent; and the use safety of the product is further ensured.
Owner:湖南醇健制药科技有限公司
Preparation method of a highly sensitive functionalized silver nanoparticle-doped aniracetam molecularly imprinted electrochemical sensor
InactiveCN105628762BHigh sensitivityEasy to manufactureMaterial electrochemical variablesDopantFunctional monomer
The invention discloses a preparation method of a high-sensitivity functionalized silver nanoparticle-doped aniracetam MIECS (Molecular Imprinting Electrochemical Sensor). The preparation method is characterized by taking aniracetam as a template molecule, dihydromyrcenol as a functional monomer, hydrocortisone acetate as a coupling agent and decyl mercaptan functionalized silver nanoparticles as a doping agent, thus preparing the high-sensitivity decyl mercaptan functionalized silver nanoparticle-doped aniracetam MIECS. An analysis method is simple and practical, and the disadvantages of complexity, expensive price of equipment and low sensitivity of a previous analysis method are overcome.
Owner:GUANGXI UNIV FOR NATITIES
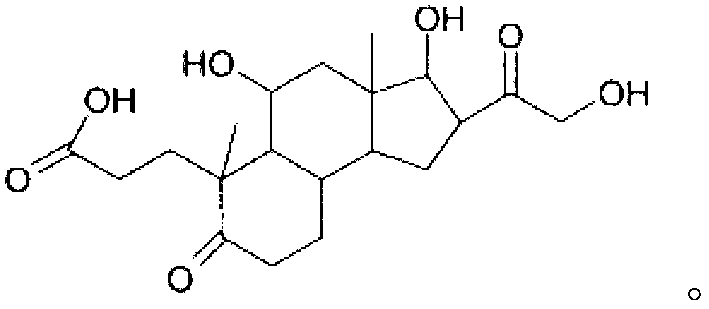
Pharmaceutical compositions and therapeutic applications of a hydrocortisone derivative designated as deina
InactiveCN102858331ANormal physiological tissue functionOrganic active ingredientsCosmetic preparationsPropanoic acidHydrocortisone acetate
The invention relates to the hydrocortisone derivative of formula (I), whose IUPAC name is 3-[3, 5-Dihydroxy-3-(2-hydroxy-acetyl)-3a, 6-dimethyl- 7-oxo-dodecahydro-cyclo- penta[alpha]naphthalen-6-yl] -propionic acid, designated as Deina TM , for use in the treatment of atrophic tissues, particularly skin, cartilage, connective, and mucosal tissues and scalp skin.
Owner:乔瓦尼・巴尔科
A kind of preparation method of hydrocortisone acetate
ActiveCN107400153BResolve incomplete responsesImprove product qualitySteroidsHydrocortisone acetateAlkali metal halide
The invention belongs to the field of preparation of steroid drugs and particularly relates to a preparation method of hydrocortisone acetate. The preparation method comprises steps as follows: a bromohydroxy compound is synthesized from a compound C5 as a raw material through a bromohydroxy reaction, a crude hydrocortisone acetate product is produced through a debromination reaction, and finally, a refined hydrocortisone acetate product is obtained through refining, wherein alkali halide is added as a catalyst of the bromohydroxy reaction and a feeding mass ratio of the compound C5 to alkali halide is 1:(0.08-0.1). The preparation method of hydrocortisone acetate has the advantages as follows: alkali halide is adopted as the catalyst of the bromohydroxy reaction, so that the key of the problem that the reaction is incomplete in the bromohydroxy reaction process is solved, and one novel preparation method of hydrocortisone acetate with stable product quality and higher yield is provided.
Owner:ZHEJIANG XIANJU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com