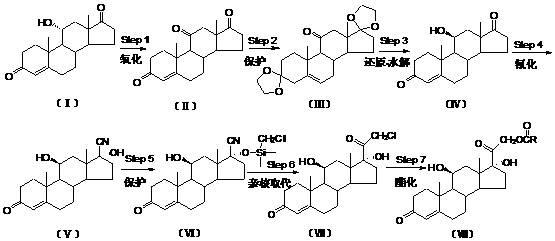
Preparation method of hydrocortisone acetate or analogue thereof
A technology for hydrocortisone acetate and its analogues, which is applied in the field of preparation of pine or its analogues, can solve the problems of unfavorable environmental protection, high price, etc., and achieve the effect of stable and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Oxidation reaction: 4-ene-pregna-3,11,17-trione;
[0022] At room temperature, under the protection of nitrogen, add 180ml of glacial acetic acid and 50ml of manganese acetate aqueous solution with a mass concentration of 50% to a clean and dry 500ml four-necked round-bottomed flask equipped with a thermometer, a reflux condenser, and mechanical stirring, and add 100.0g of it under stirring. For compound I, the temperature of the system was lowered to 0 to 10°C, and 50ml of chromium trioxide aqueous solution with a mass concentration of 50% was added dropwise at a temperature of 0 to 10°C. After the dropwise addition, the temperature was raised to 30°C-35°C to react for 2 hours. TLC followed the reaction until no raw material remained. The system was cooled to room temperature. The reaction system was added dropwise to 2L of purified water, a large amount of solids were precipitated, and the stirring was continued for 2 hours. The system was suction-filtered, the fil...
Embodiment 2
[0036] Oxidation reaction: 4-ene-pregna-3,11,17-trione;
[0037] At room temperature, under the protection of nitrogen, add 200 ml of acetone and 50 ml of periodic acid solution with a mass concentration of 40% to a clean and dry 500 ml four-necked round-bottomed flask equipped with a thermometer, a reflux condenser, and mechanical stirring, and stir. 100.0 g of compound I was added, the temperature of the system was lowered to 0 to 10°C, and 50ml of chromium trioxide aqueous solution with a mass concentration of 60% was added dropwise at a temperature of 0 to 10°C. After the dropwise addition, the temperature was raised to 35°C-37°C to react for 3 hours. TLC followed the reaction until no raw material remained. The system was cooled to room temperature. The reaction system was added dropwise to 3 L of purified water, a large amount of solids were precipitated, and the stirring was continued for 2.5 hours. The system was suction-filtered, the filter cake was washed with wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com