Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
105 results about "Adrenal insufficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adrenal insufficiency is a condition in which the adrenal glands do not produce adequate amounts of steroid hormones, primarily cortisol; but may also include impaired production of aldosterone (a mineralocorticoid), which regulates sodium conservation, potassium secretion, and water retention. Craving for salt or salty foods due to the urinary losses of sodium is common.
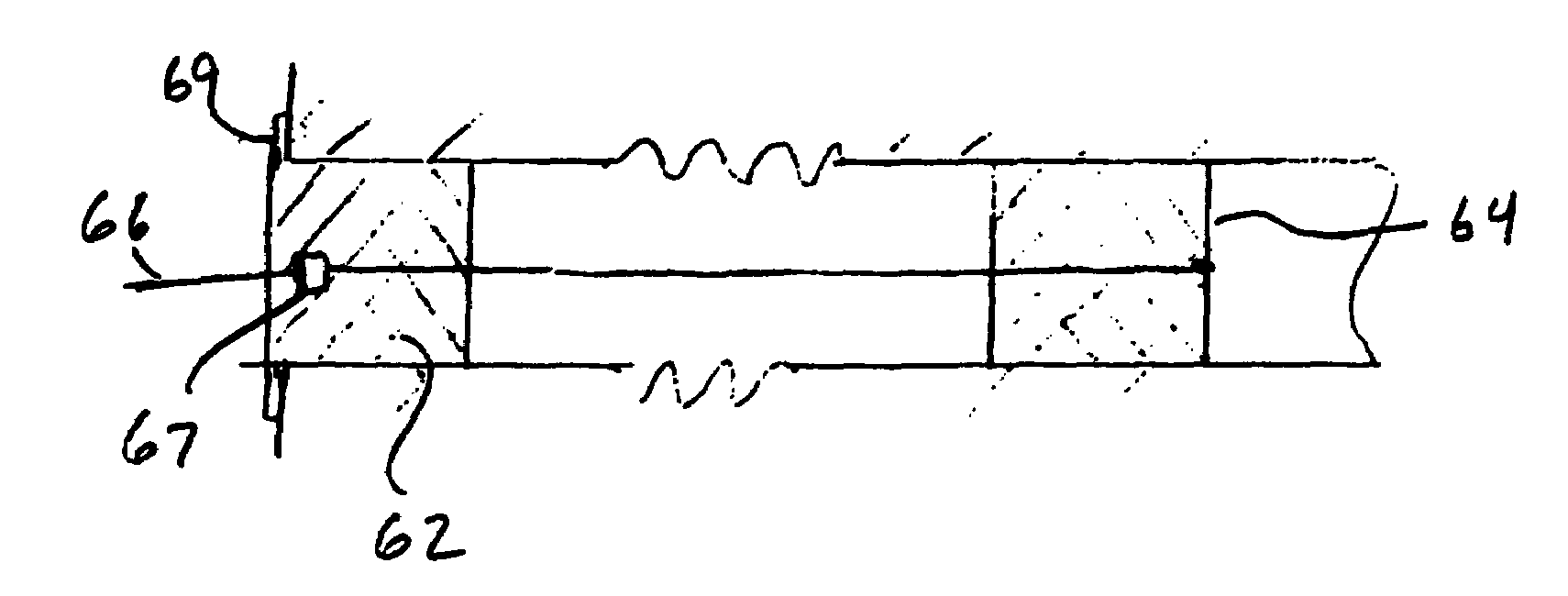
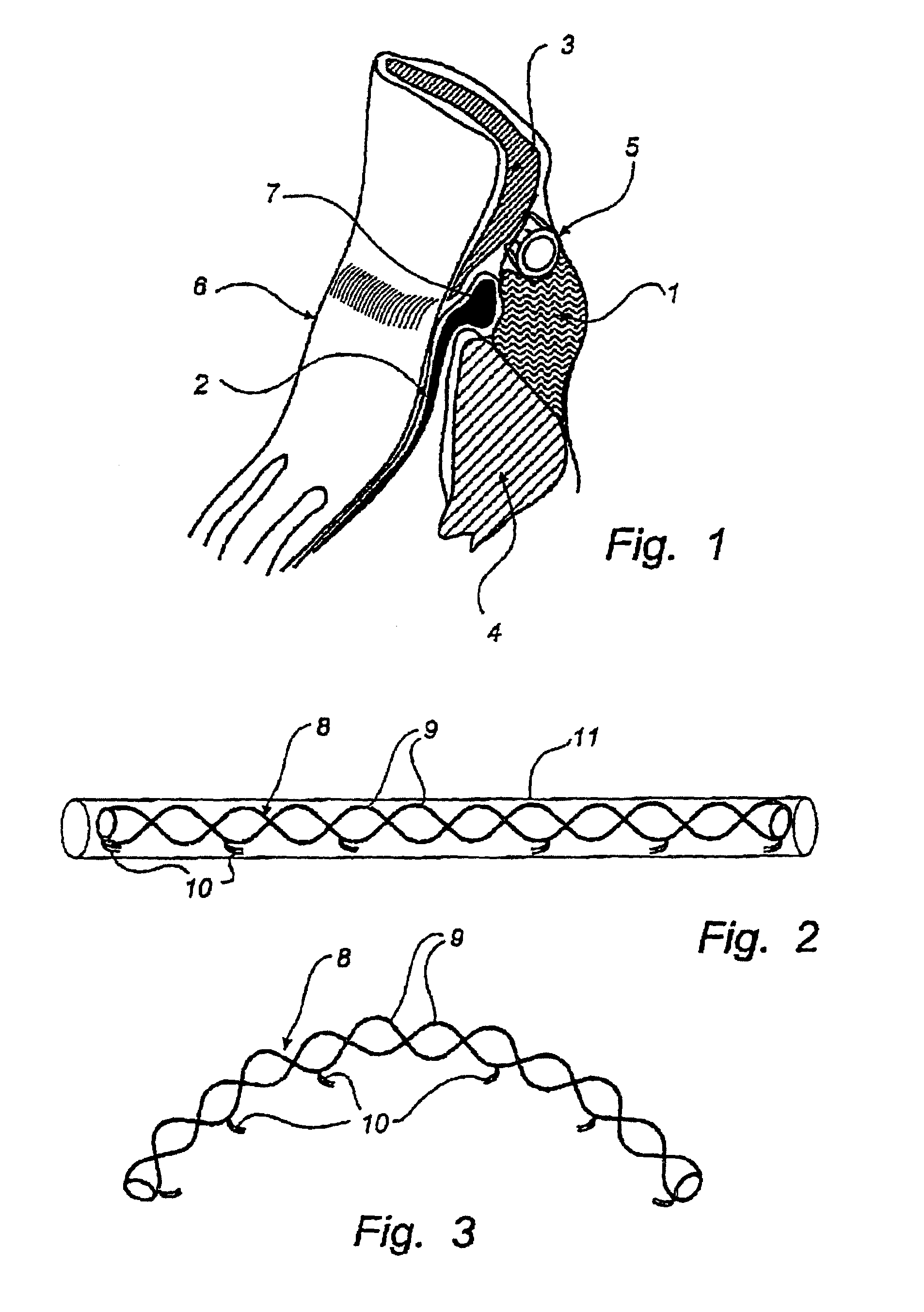
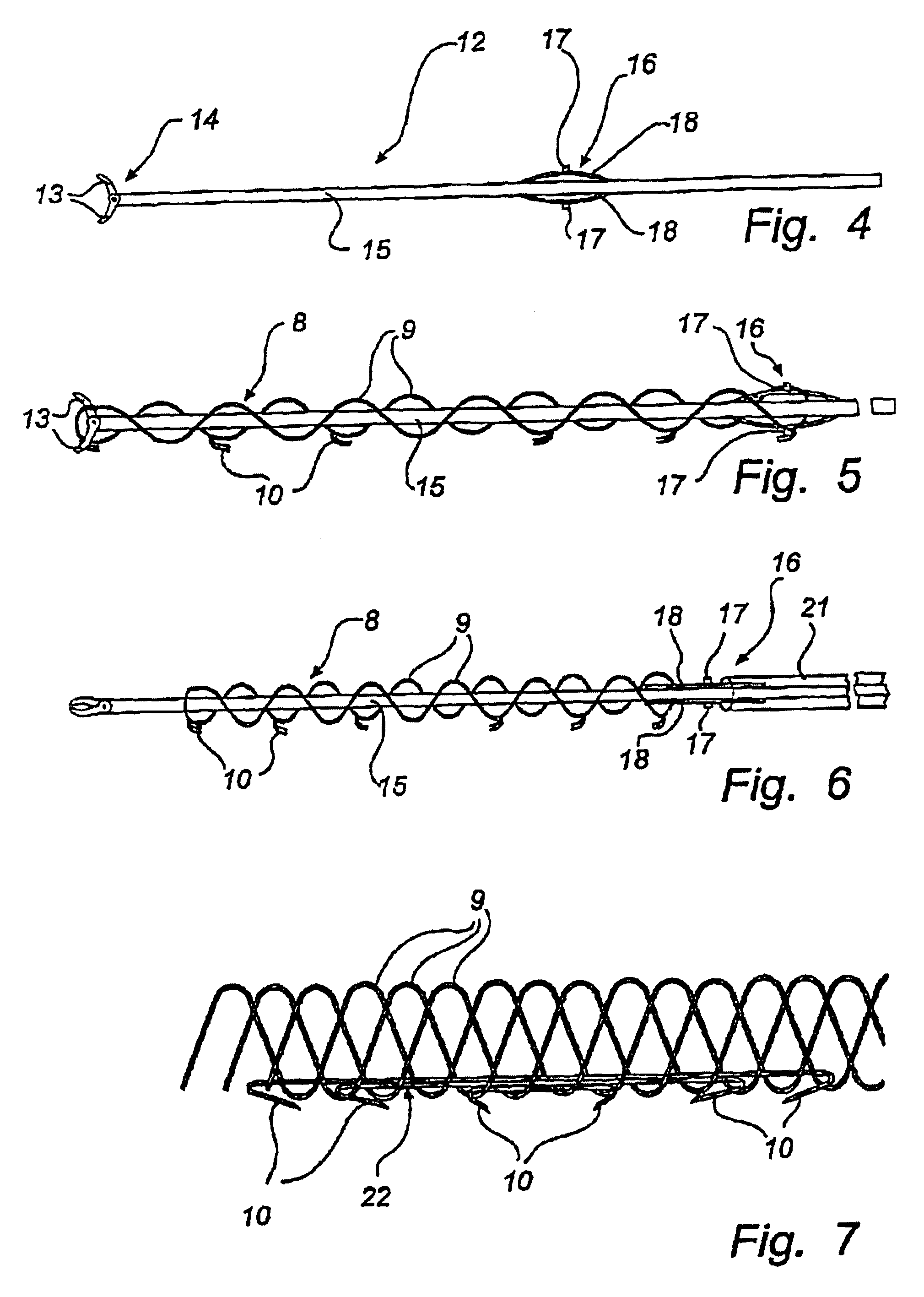
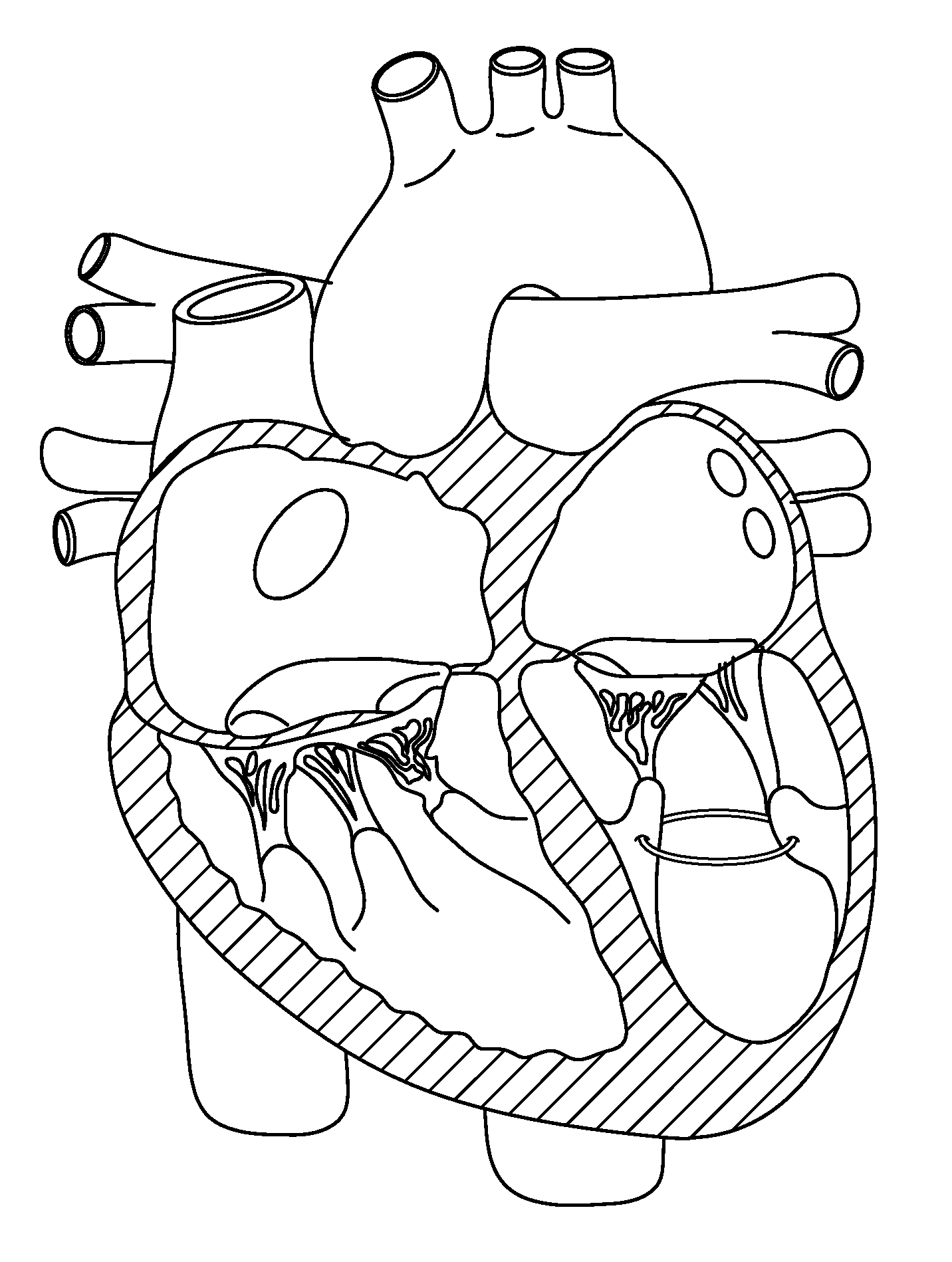
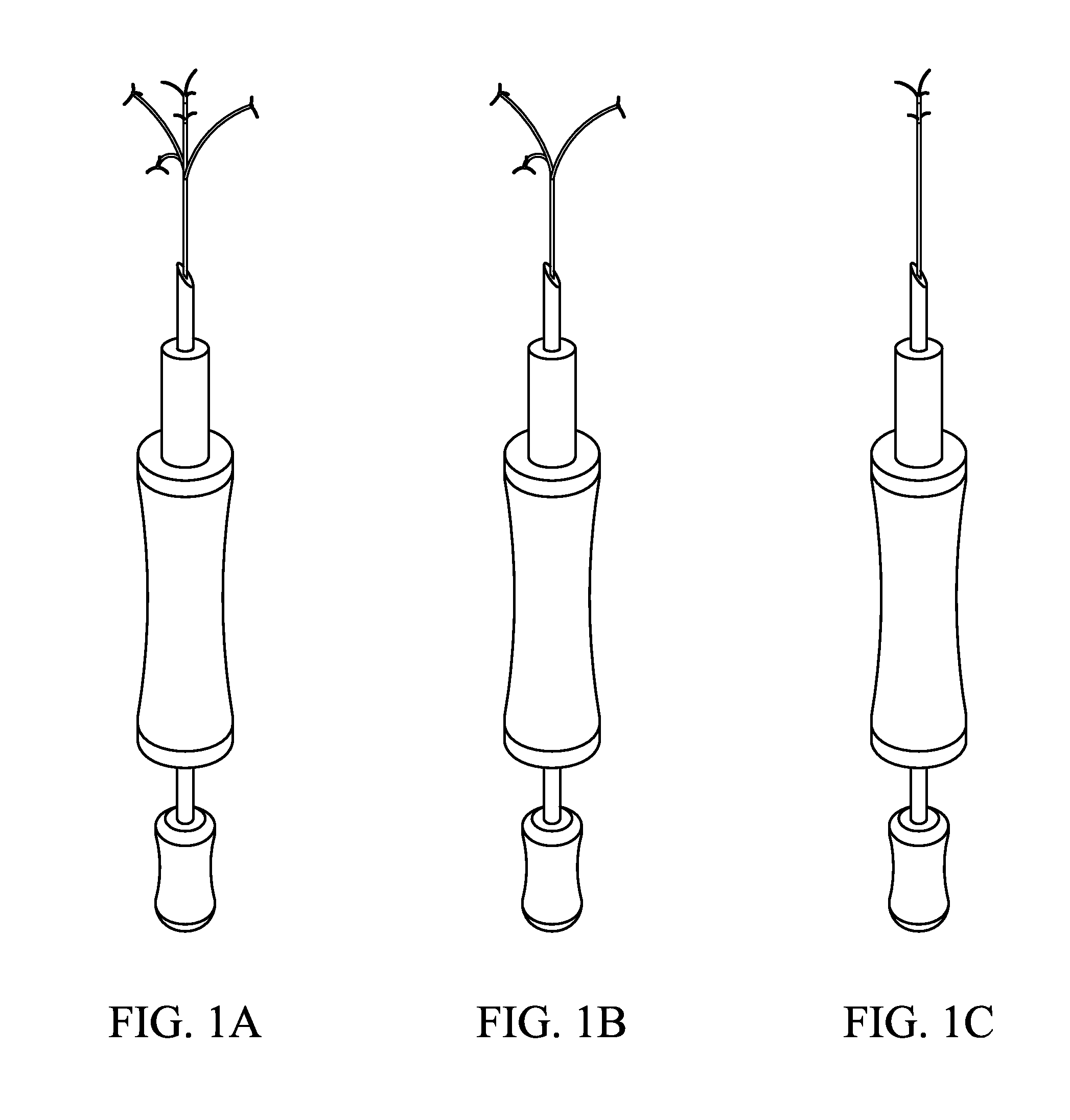
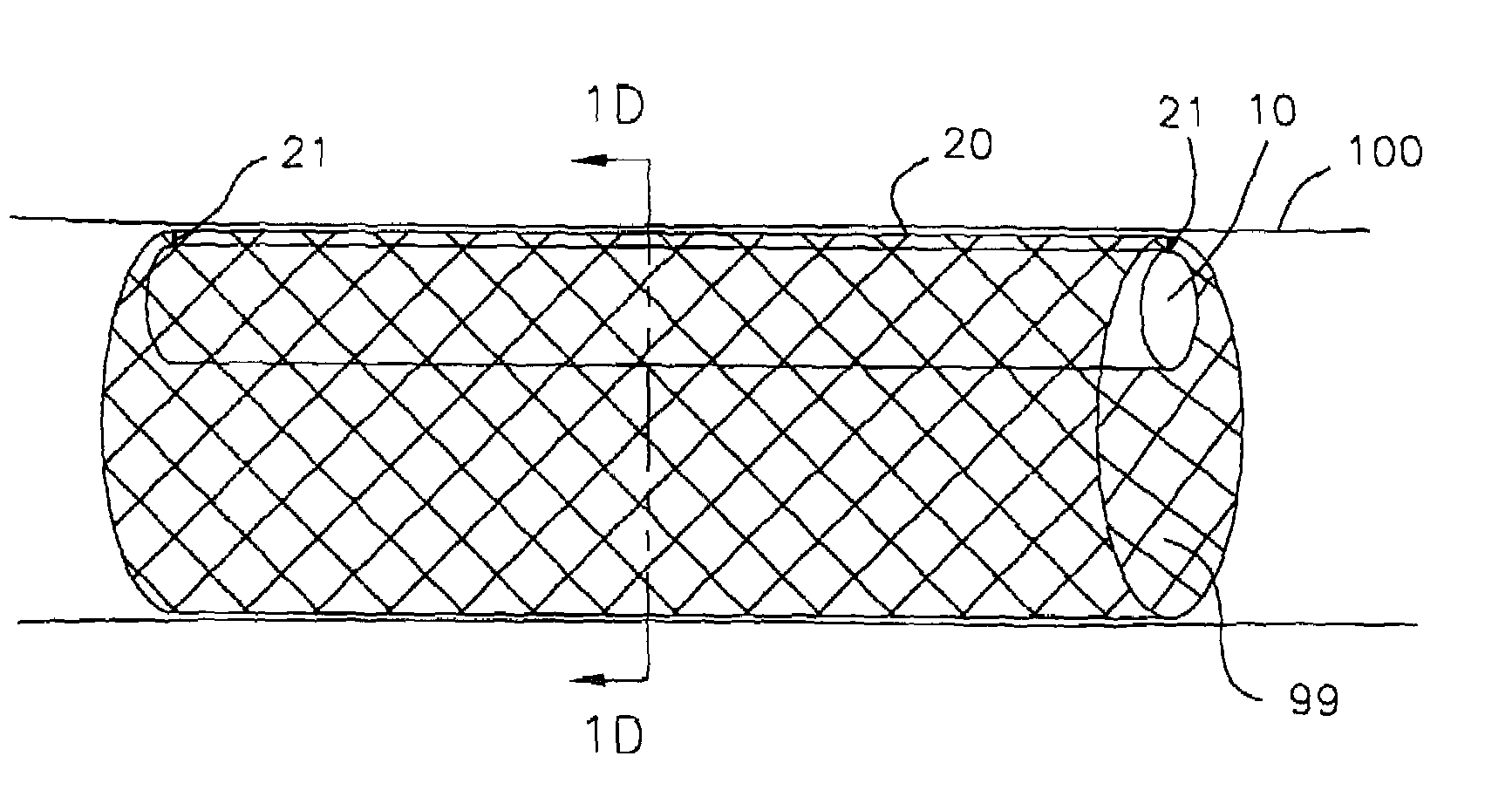
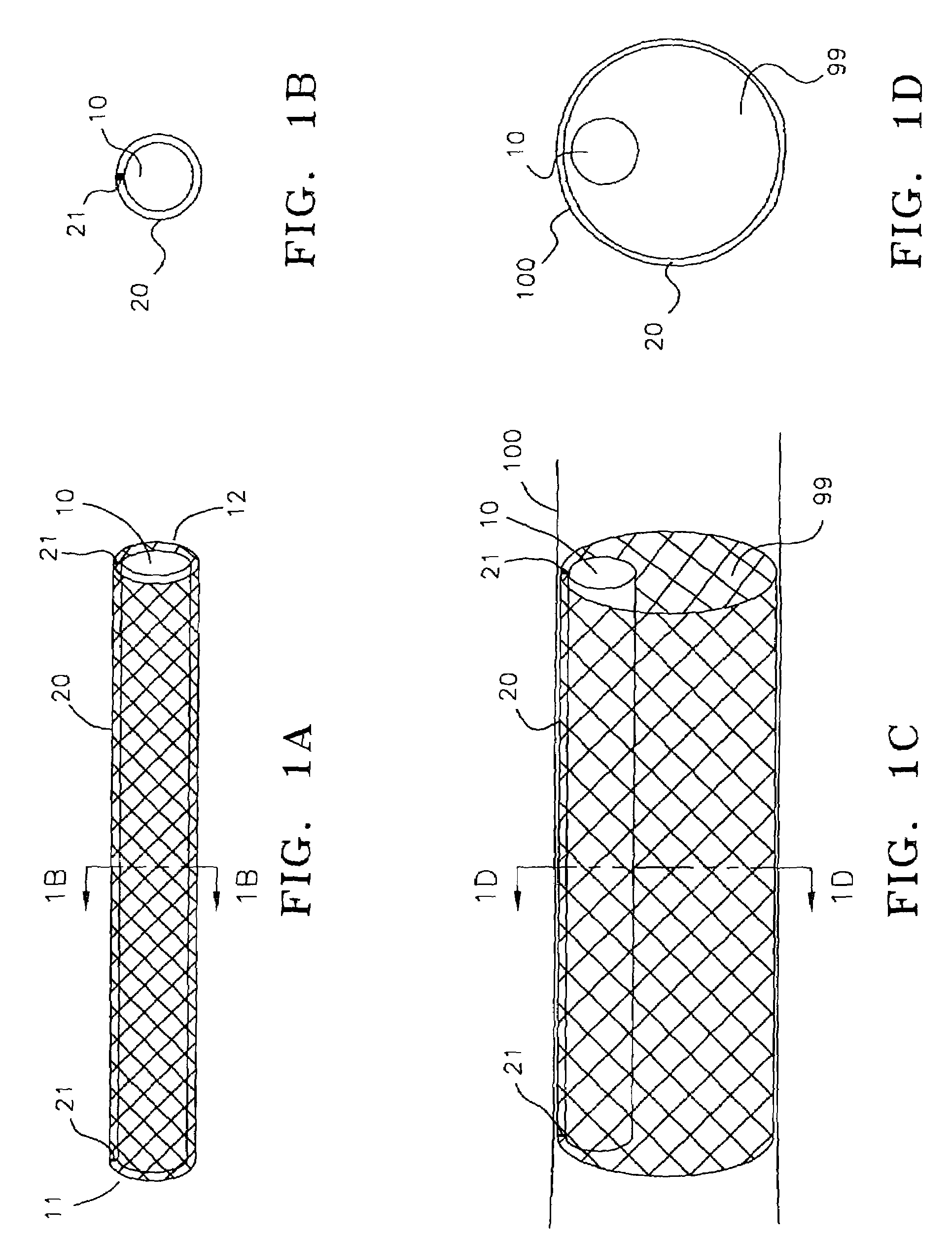
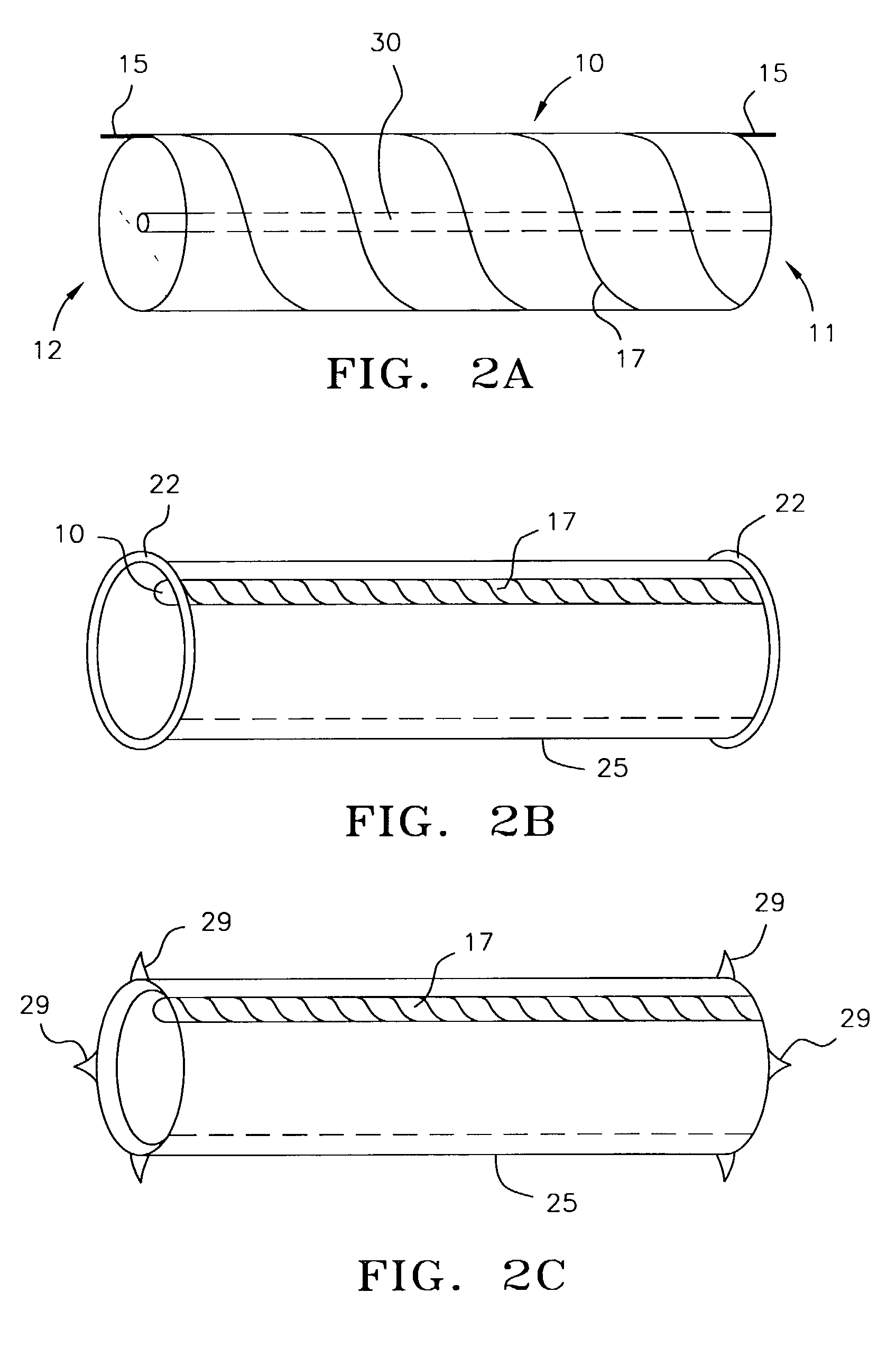
Method and device for treatment of mitral insufficiency
InactiveUS6997951B2Length of device can be decreasedShorten the lengthStentsBone implantCoronary sinusMitral annulus
A device for treatment of mitral annulus dilation is disclosed, wherein the device comprises two states. In a first of these states the device is insertable into the coronary sinus and has a shape of the coronary sinus. When positioned in the coronary sinus, the device is transferable to the second state assuming a reduced radius of curvature, whereby the radius of curvature of the coronary sinus and the radius of curvature as well as the circumference of the mitral annulus is reduced.
Owner:EDWARDS LIFESCIENCES AG +1
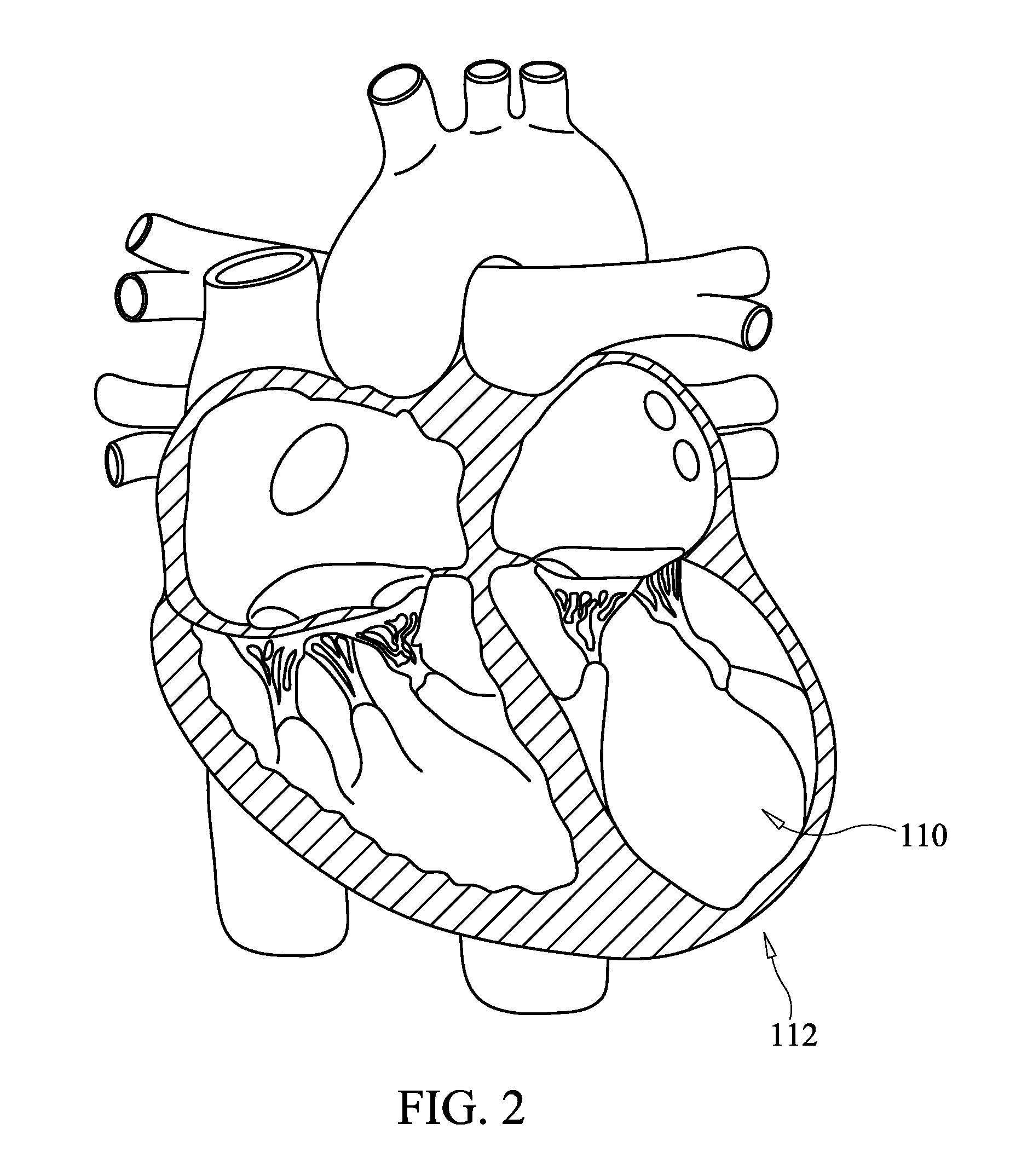
Method for percutaneous lateral access to the left ventricle for treatment of mitral insufficiency by papillary muscle alignment
InactiveUS20100210899A1Improve heart functionLower the volumeSuture equipmentsHeart valvesLeft ventricular sizePapillary muscle
This invention relates to devices and methods for the therapeutic changing of the geometry of the left ventricle of the human heart. Specifically, the invention relates to the left-ventricular lateral wall introduction of an anchoring device to align the papillary muscles.
Owner:TENDYNE MEDICAL
Endoscopic arterial pumps for treatment of cardiac insufficiency and venous pumps for right-sided cardiac support
Methods for using blood pumps to treat heart failure are disclosed. The pump is mounted on an interior of a stent, and the stent is releasably mounted on a distal end of a catheter. The distal end of the catheter is inserted into a peripheral artery and advanced to position at a region of interest within the descending aorta, the ascending aorta, or the left ventricle. The stent and the pump are released from the catheter, and the pump is activated to increase blood flow downstream of the pump. The pump can also be positioned in the vena cava or used to treat right-sided heart failure following the insertion of an LVAD, or to improve venous return in patients with varicose veins. Non-stent pumps are described for insertion between the pulmonary vein and aorta, and between the vena cava and pulmonary artery designed for use during cardiac surgery.
Owner:BARBUT DENISE R +2
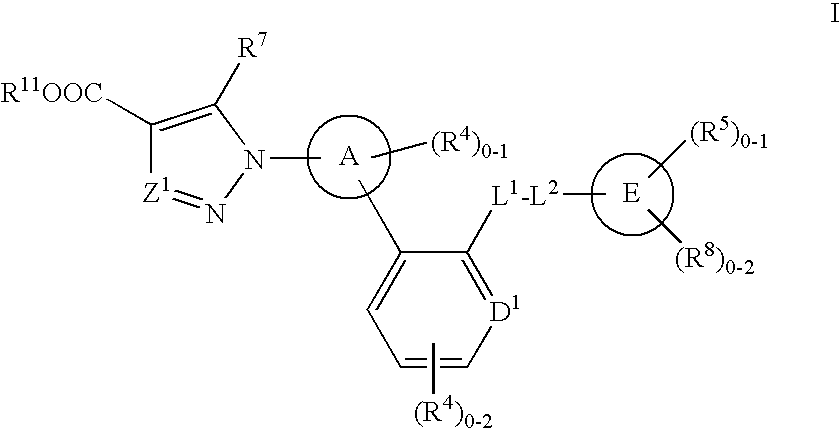
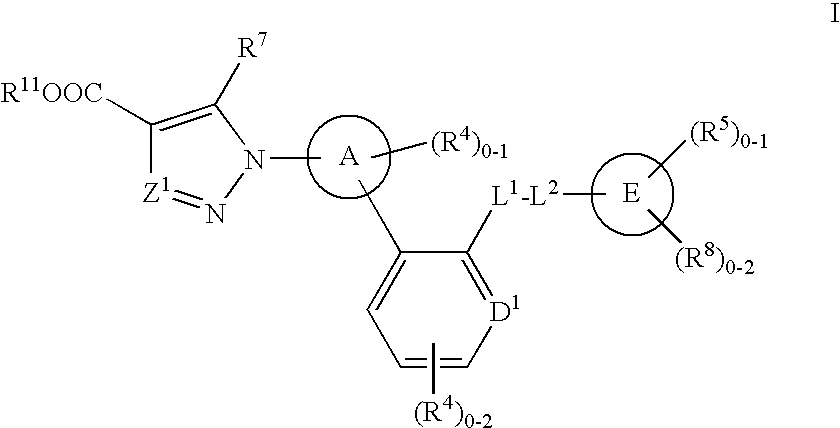
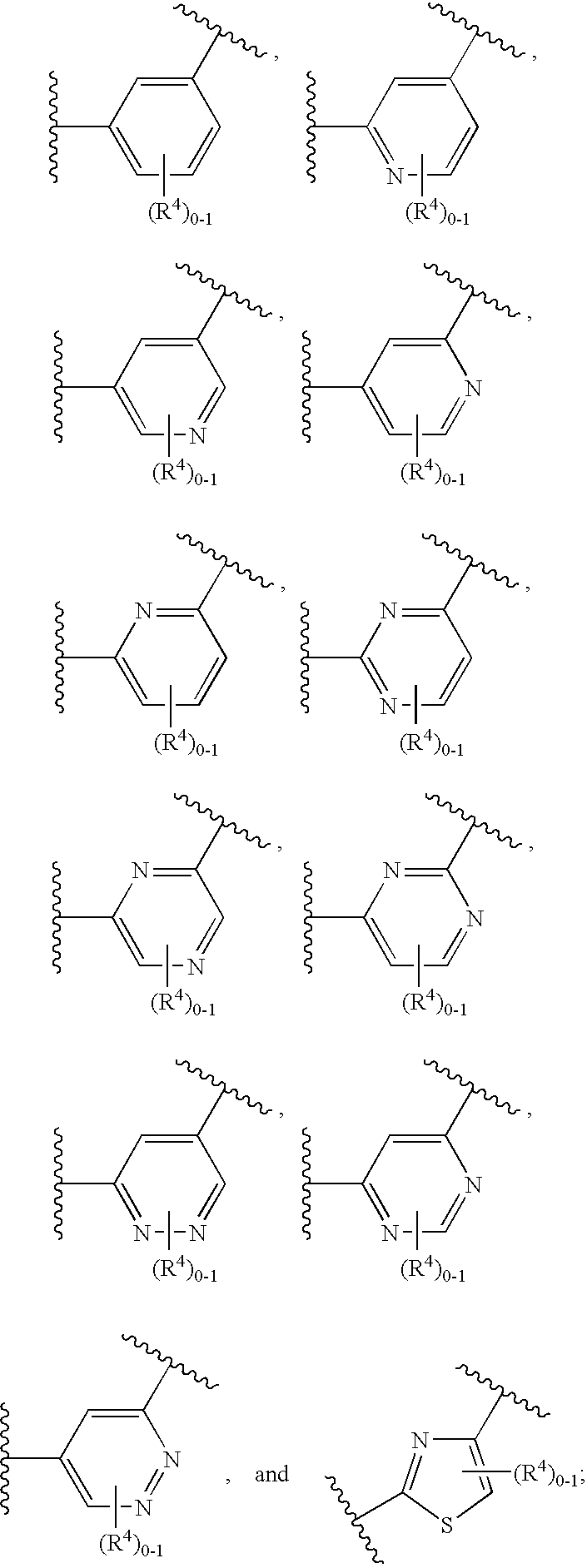
Soluble guanylate cyclase activators
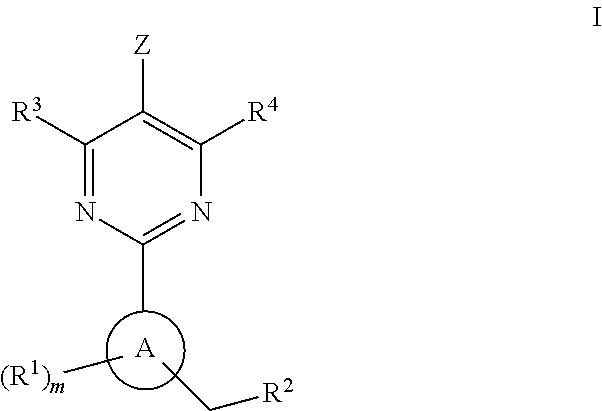
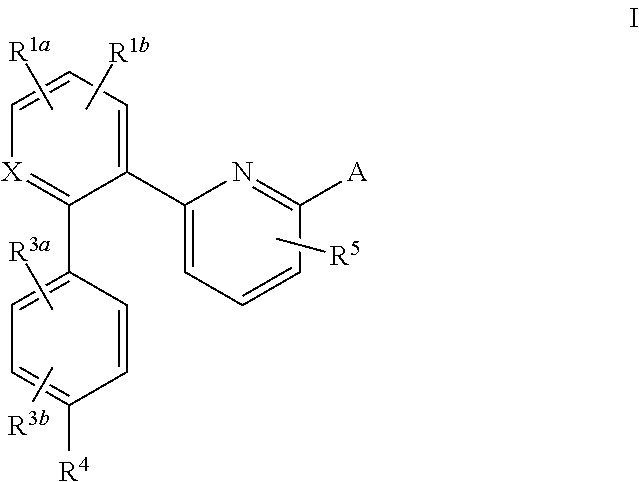
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME CORP
Combination enzyme for cystic fibrosis
InactiveUS20080166334A1Well formedFormulation stabilityPeptide/protein ingredientsDigestive systemExocrine pancreatic insufficiencyCystic fibrosis lungs
A stable preparation of digestive / pancreatic enzymes which can be readily formed into a dosage formulation is provided as a treatment of pancreatic insufficiency in persons having cystic fibrosis. The dosage formulation can be administered either by an oral preparation including, but not limited to, a microcapsule, mini-capsule, time released capsule, sprinkle or other methodology. A further object of this invention is to provide a stabilized preparation of a combination medicant which resists degradation by light, heat, humidity or association with commonly used excipients.
Owner:CUREMARK
Method and device for treatment of mitral insufficiency
A device for treatment of mitral annulus dilatation comprises an elongate body having two states. In a first of these states the elongate body is insertable into the coronary sinus and has a shape adapting to the shape of the coronary sinus. When positioned in the coronary sinus, the elongate body is transferable to the second state assuming a reduced radius of curvature, whereby the radius of curvature of the coronary sinus and the radius of curvature as well as the circumference of the mitral annulus is reduced.
Owner:EDWARDS LIFESCIENCES AG
Compositions and methods for treating pancreatic insufficiency
InactiveUS7718169B2Stable enzyme componentEffective low dose treatment regimensPeptide/protein ingredientsDigestive systemProteinase activityExocrine pancreatic insufficiency
The present invention relates to compositions for the treatment of conditions, including pancreatic insufficiency. The compositions of the present invention comprise lipase, protease and amylase in a particular ratio that provides beneficial results in patients, such as those afflicted with pancreatic insufficiency. This invention also relates to methods using such compositions for the treatment of pancreatic insufficiency. The compositions specifically comprise crosslinked Burkholderia cepacia lipase crystals, Aspergillus melleus protease crystals and amorphous Aspergillus oryzae amylase in a ratio of about 1:1:0.15 USP units.
Owner:ELI LILLY & CO
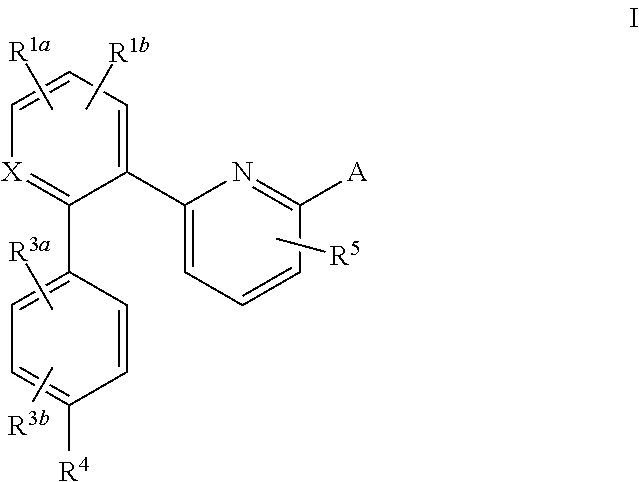
Soluble guanylate cyclase activators
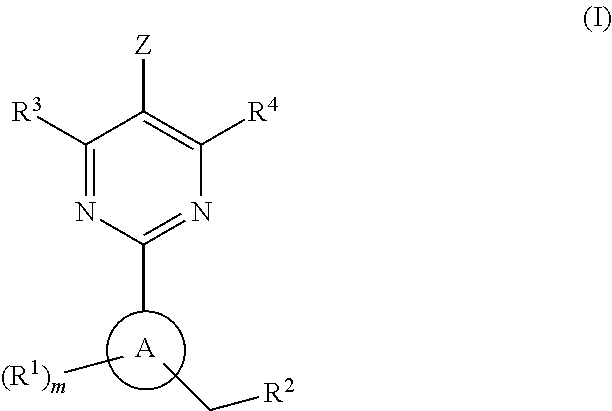
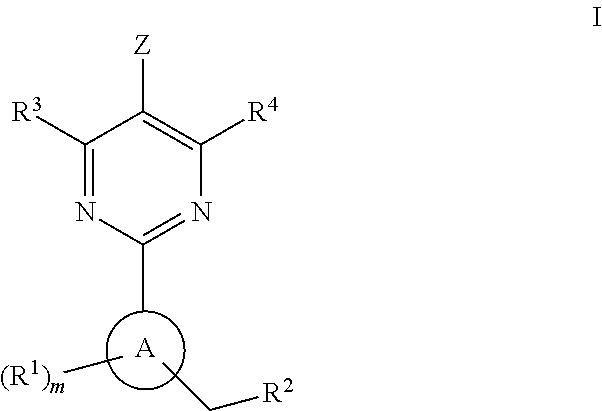
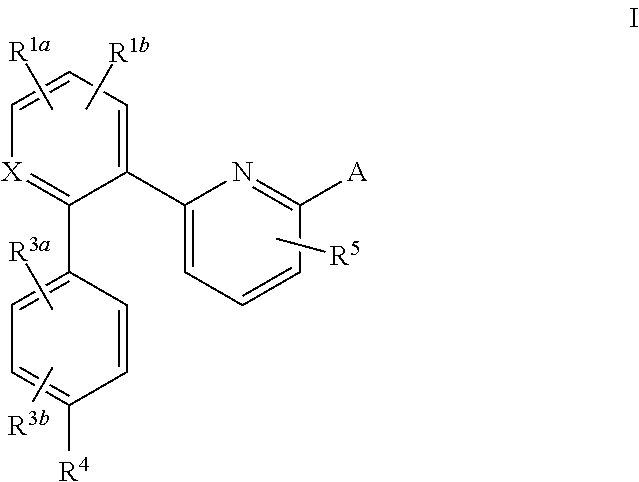
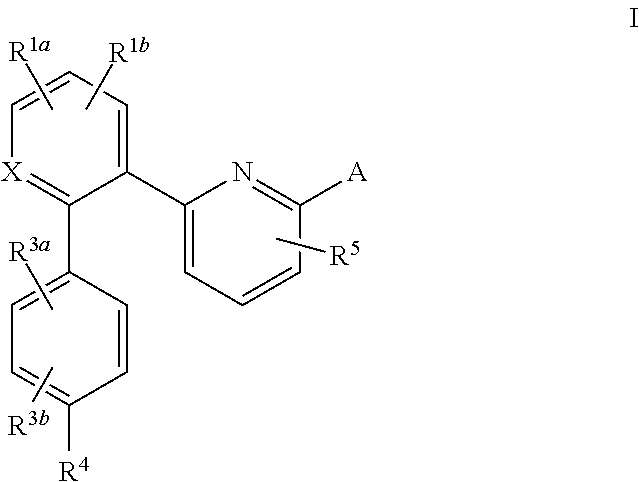
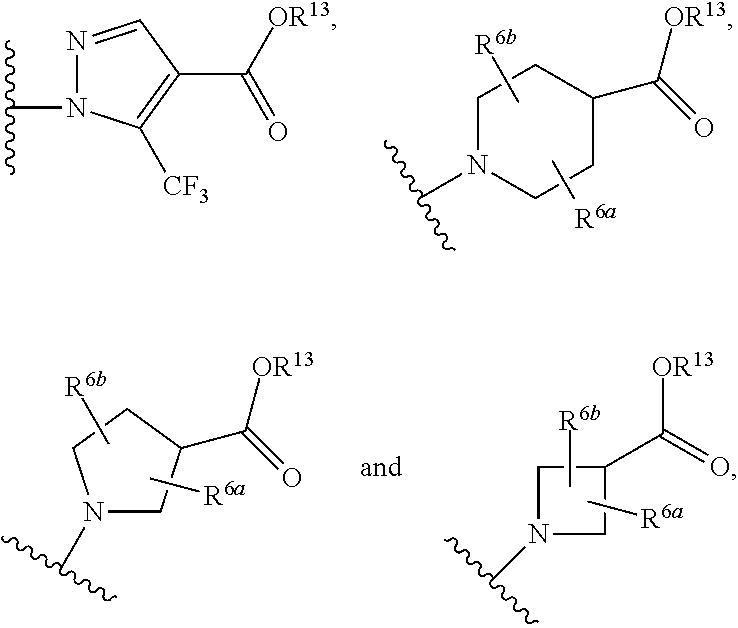
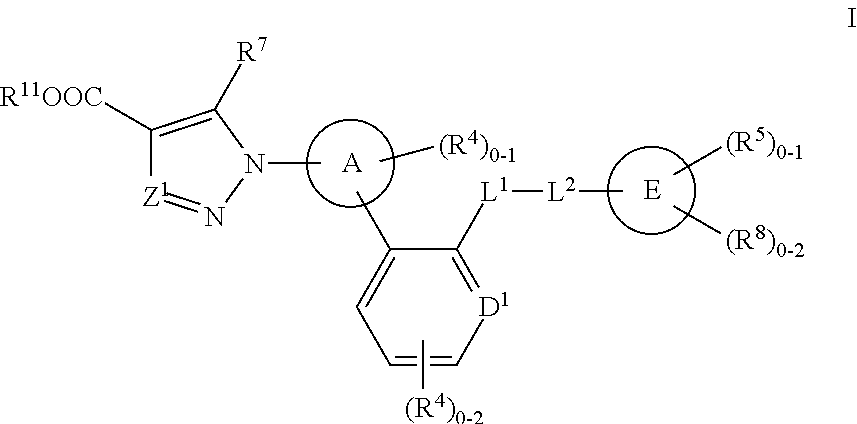
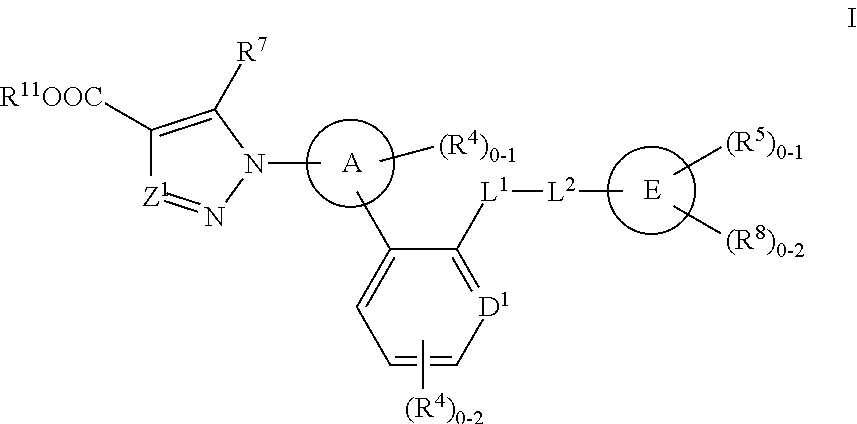
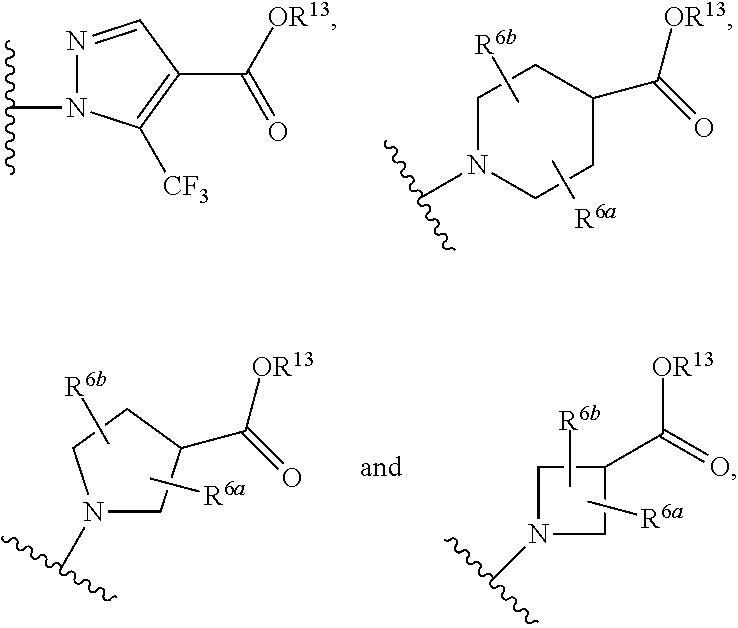
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
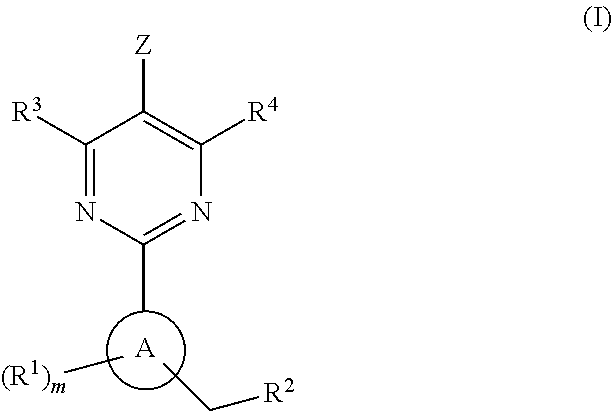
This inventions relates to compounds having the structure Formula I and pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Soluble Guanylate Cyclase Activators
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME LLC
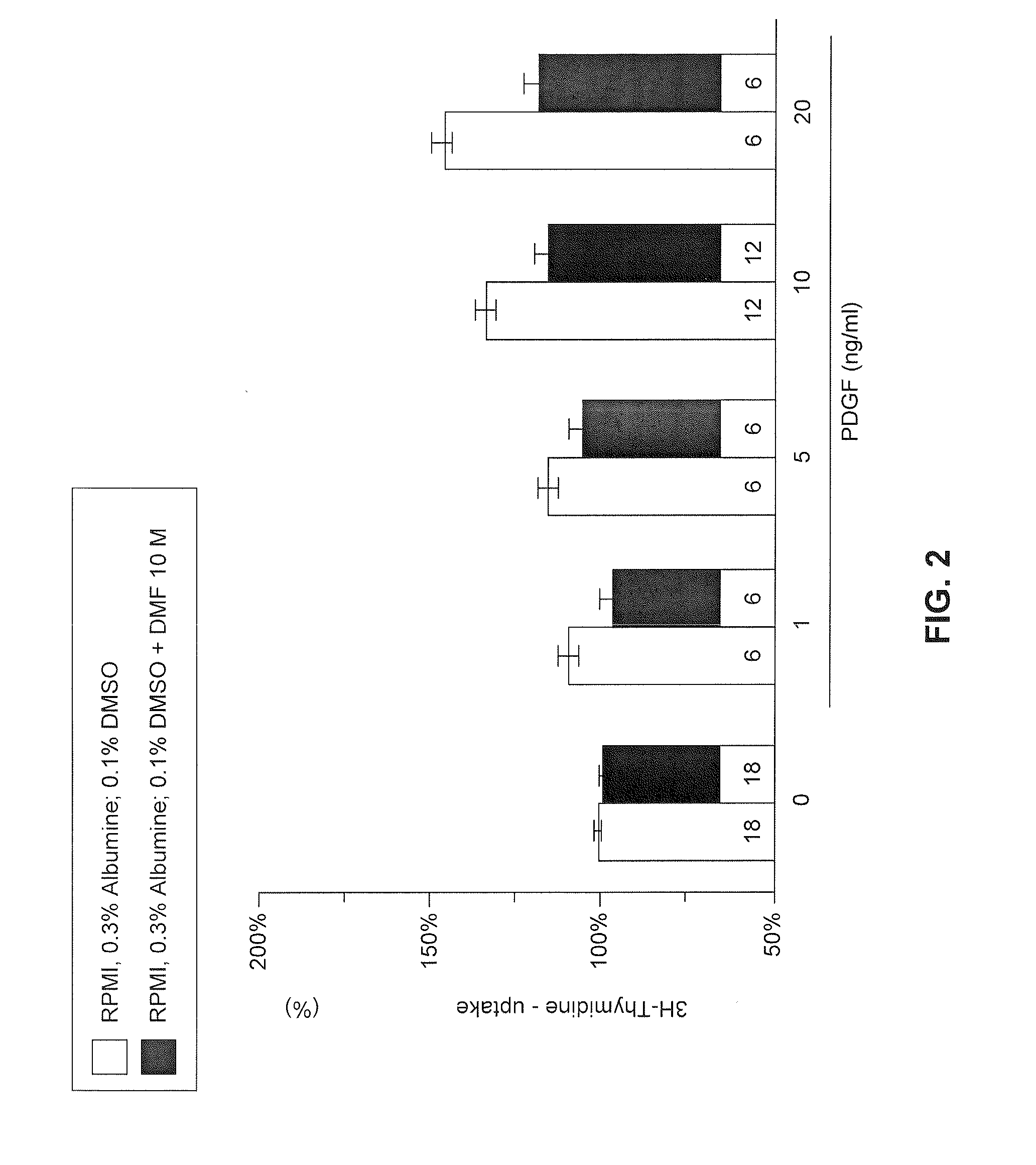
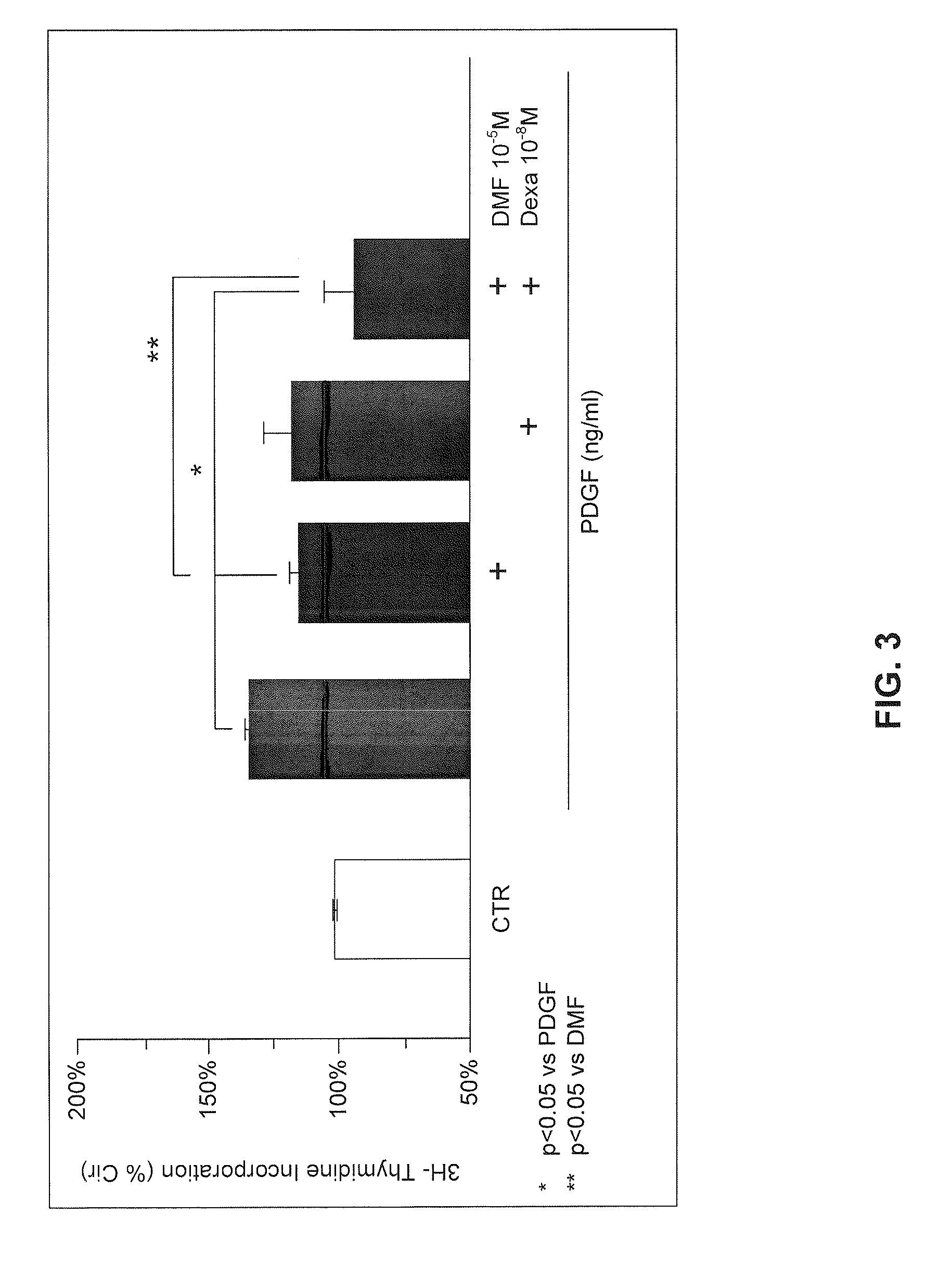
Use of fumaric acid derivatives for treating cardiac insufficiency, and asthma
According to a first aspect the invention relates to the use of fumaric acid derivatives selected from the group consisting of dialkyl fumarates, monoalkyl hydrogen fumarates, fumaric acid monoalkyl ester salts, fumaric acid monoamides, monoamido fumaric acid salts, fumaric acid diamides, monoalkyl monoamido fumarates, carbocyclic and oxacarbocyclic oligomers of these compounds and mixtures thereof for preparing a drug for the treatment or prevention of cardiac insufficiency, in particular left ventricular insufficiency, myocardial infarction and angina pectoris.According to a second aspect the invention relates to the use of fumaric acid derivatives, selected from the group consisting of dialkyl fumarates, monoalkyl hydrogen fumarates, fumaric acid monoalkyl ester salts, fumaric acid monoamides, monoamido fumaric acid salts, fumaric acid diamides, monoalkyl monoamido fumarates, carbocyclic and oxacarbocyclic oligomers of these compounds and mixtures thereof for preparing a drug for the treatment of asthma and chronic obstructive pulmonary diseases, especially asthma caused by allergies, infections, analgesics, job conditions or physical effort, mixed forms of asthma, or asthma cardiale.
Owner:BIOGEN INT
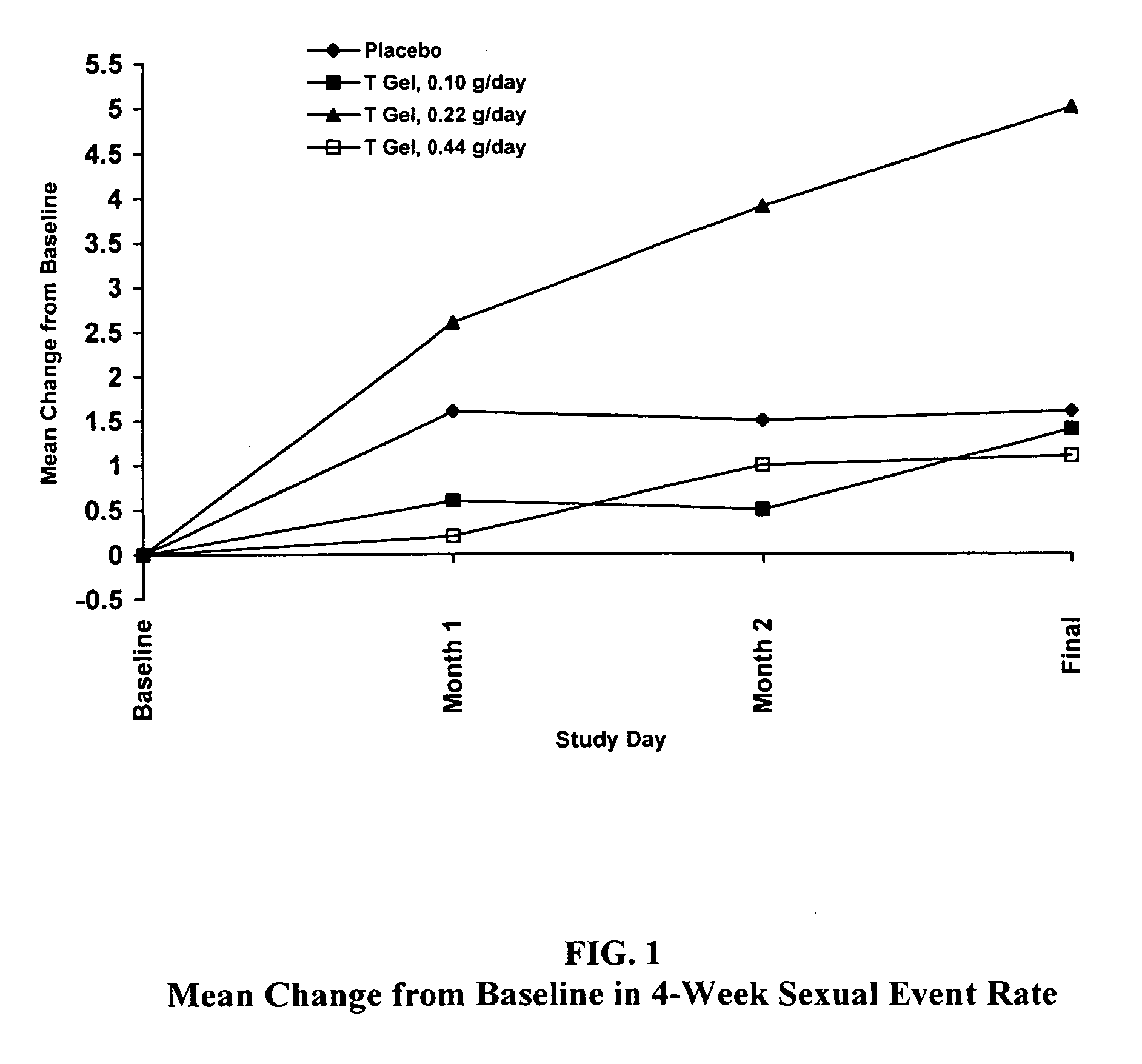
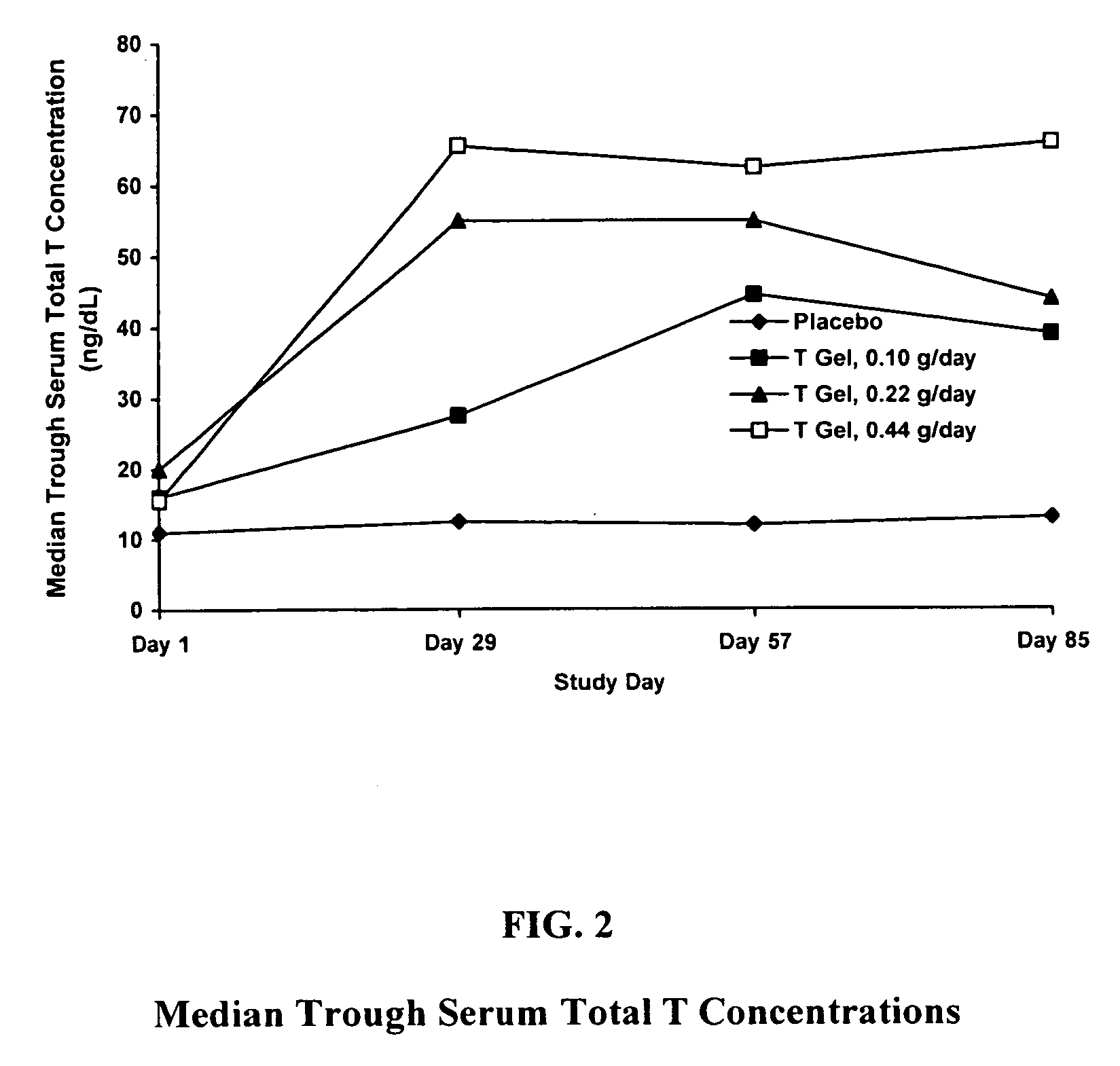
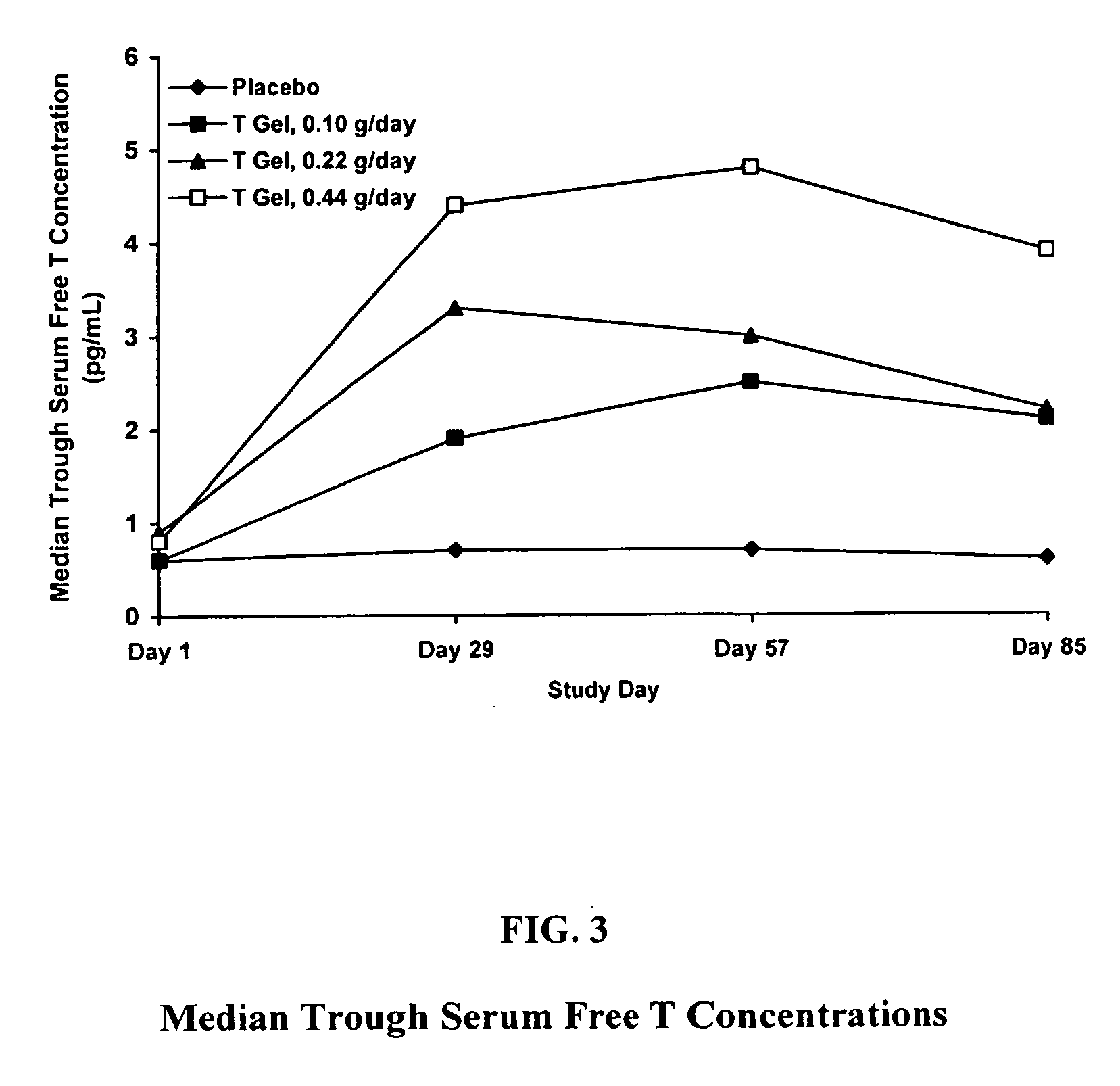
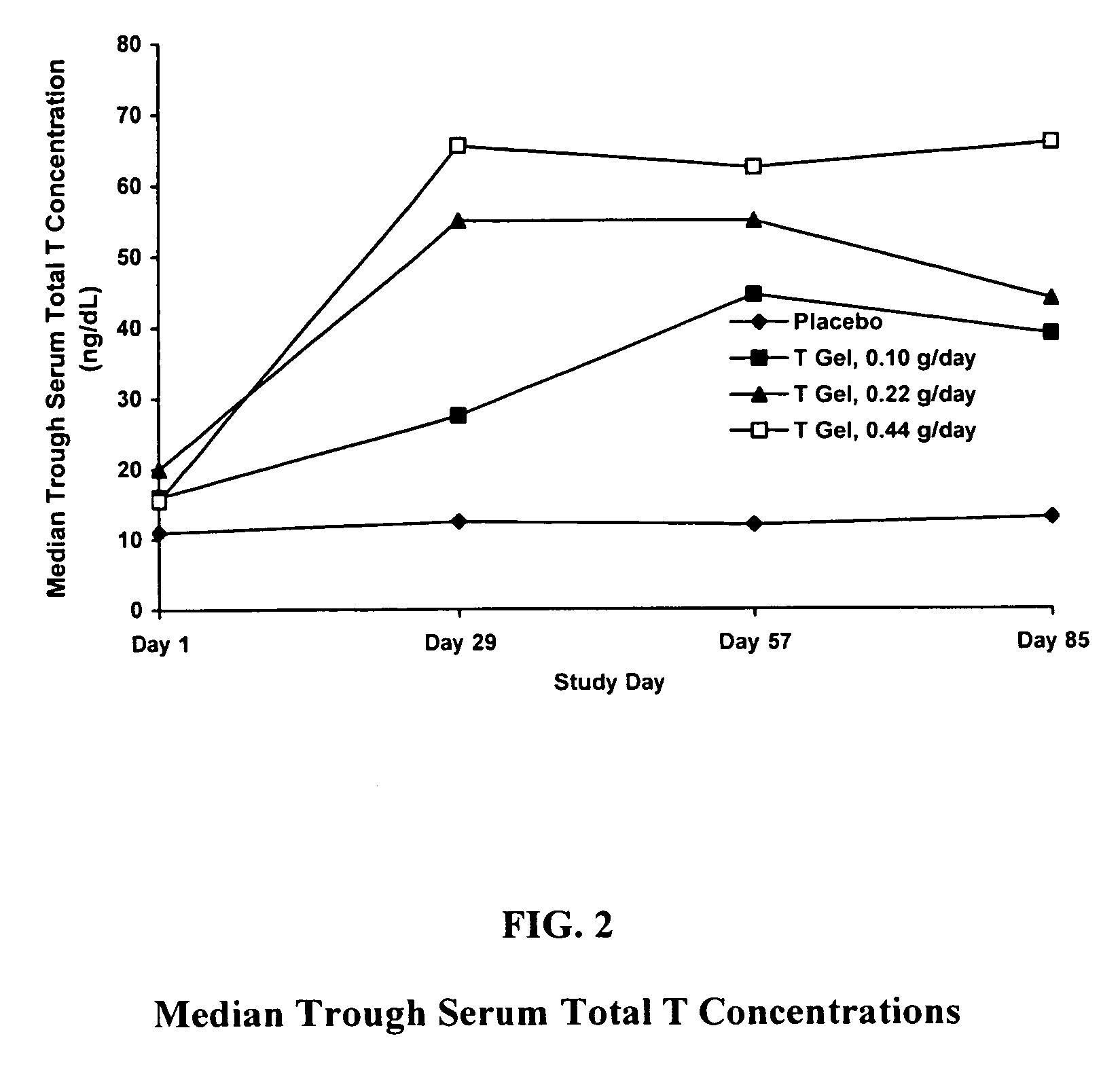
Method and apparatus for transdermal or transmucosal application of testosterone
InactiveUS20060270642A1Effective treatmentDecrease and alleviate clinical symptomBiocideOrganic active ingredientsActive agentMenopausal disorders
Methods, formulations, and devices for providing transdermal or transmucosal delivery of active agents to subjects in need thereof. The formulations and methods treat symptoms of hormonal disorders including hypogonadism, female sexual desire disorder, female menopausal disorder, and adrenal insufficiency.
Owner:ANTARES PHARMA IPL
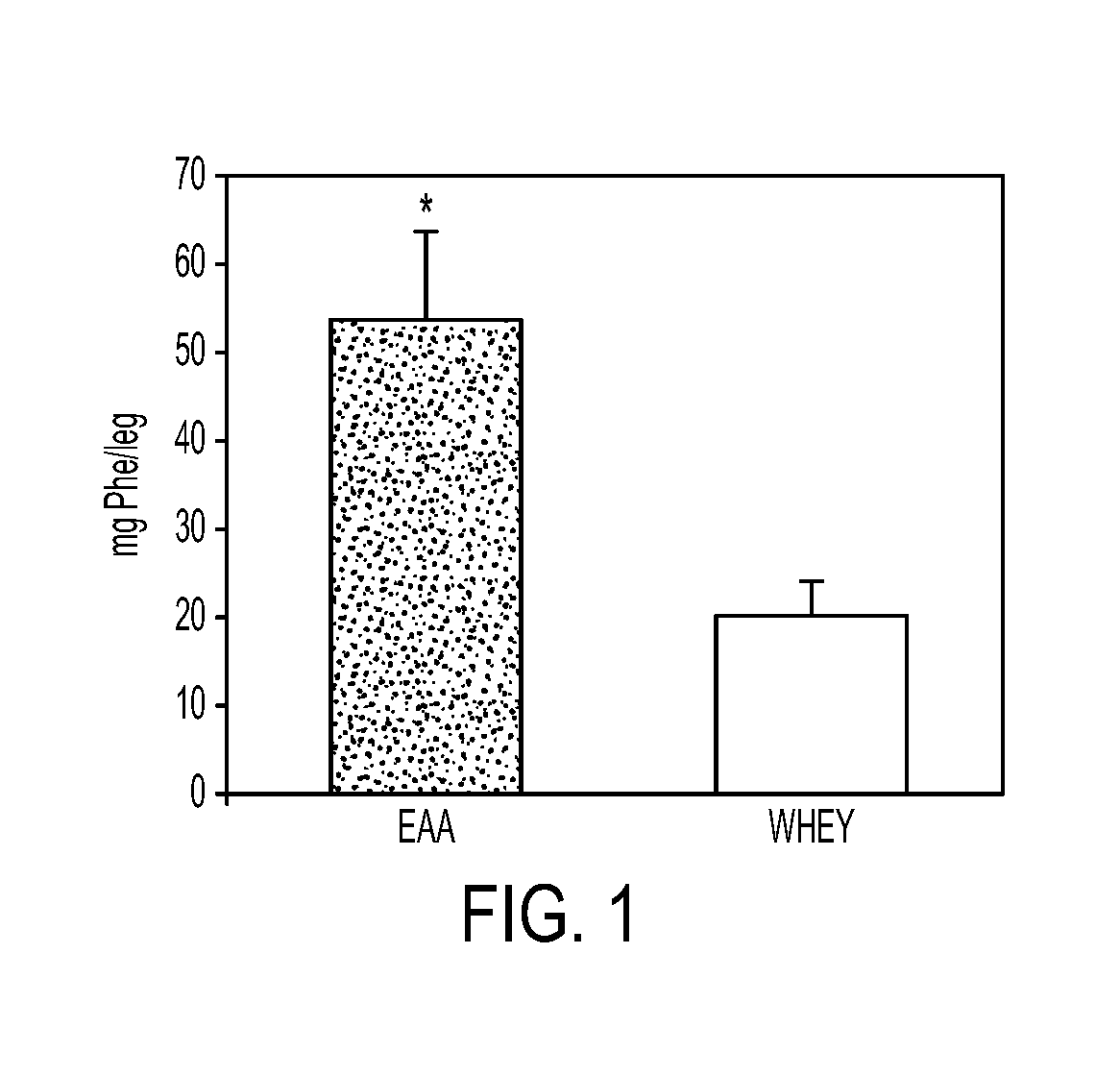
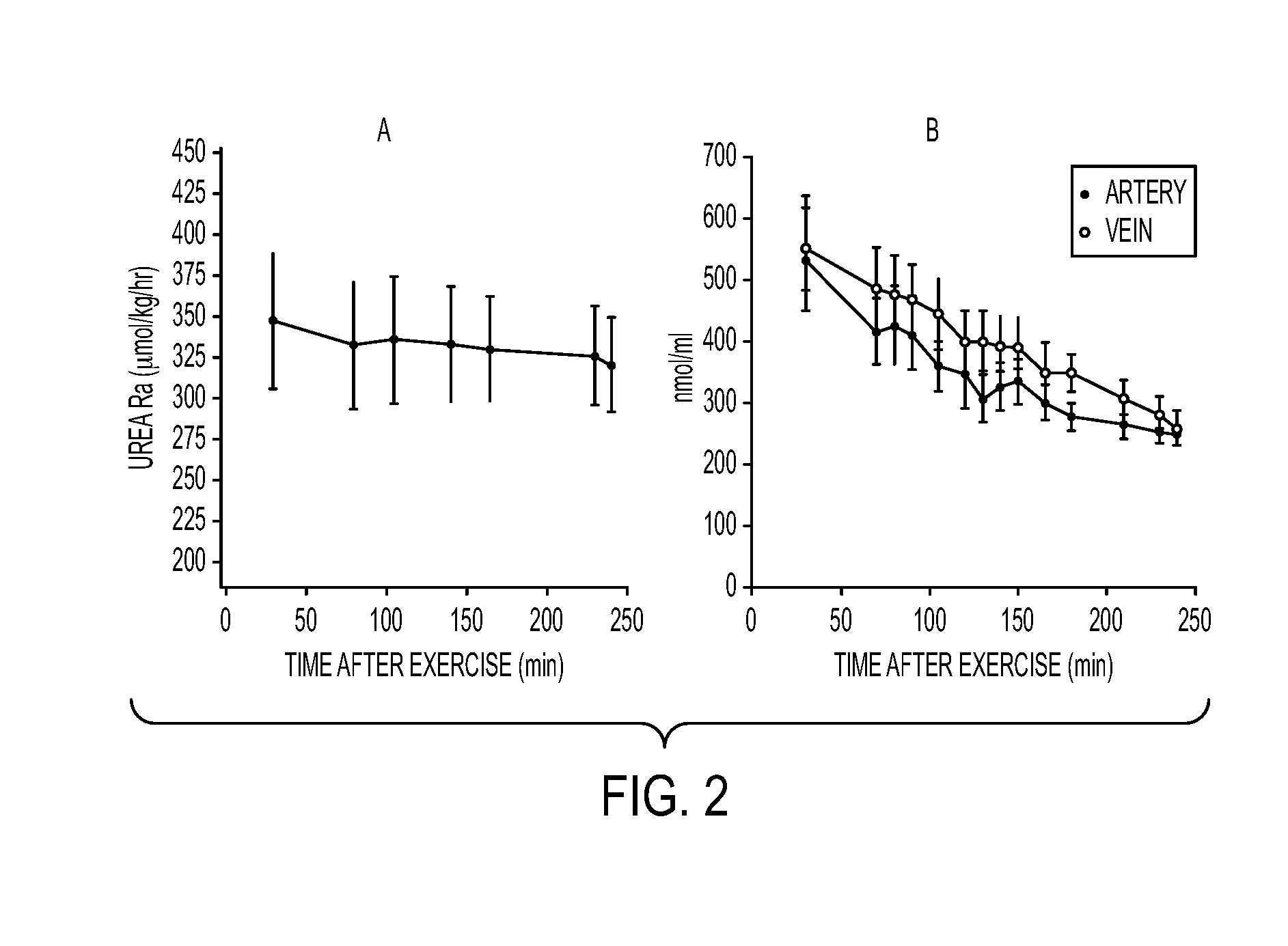
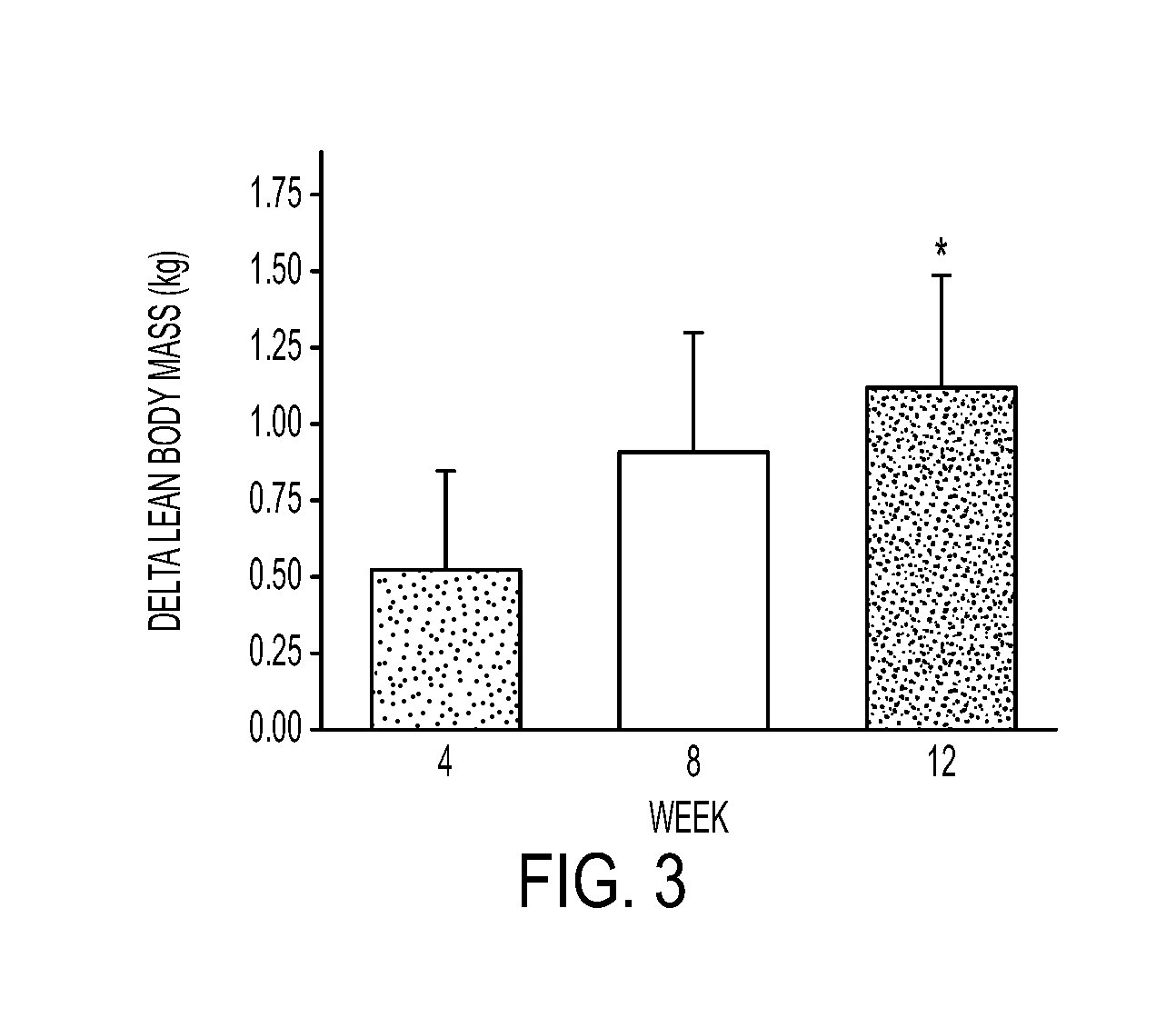
Compositions and Methods for Sparing Muscle in Renal Insufficiency and During Hemodialysis
InactiveUS20120178672A1Improves net balanceImproved protein intakeBiocidePeptide/protein ingredientsHemodialysisProtein intake
A nutritional composition and method of use that improves the net balance in skeletal muscle by targeting both the synthetic and breakdown processes. The disclosed composition provides for improved protein intake to increase skeletal muscle protein accretion in stressed patients who are at risk for the development of renal insufficiency by stimulating protein synthesis.
Owner:ENERGY LIGHT
Method and apparatus for transdermal or transmucosal application of testosterone
InactiveUS8067399B2Convenient, pain-free, and non-invasiveEasy to manageBiocideOrganic active ingredientsMedicineActive agent
Methods, formulations, and devices for providing transdermal or transmucosal delivery of active agents to subjects in need thereof. The formulations and methods treat symptoms of hormonal disorders including hypogonadism, female sexual desire disorder, female menopausal disorder, and adrenal insufficiency.
Owner:ANTARES PHARMA IPL
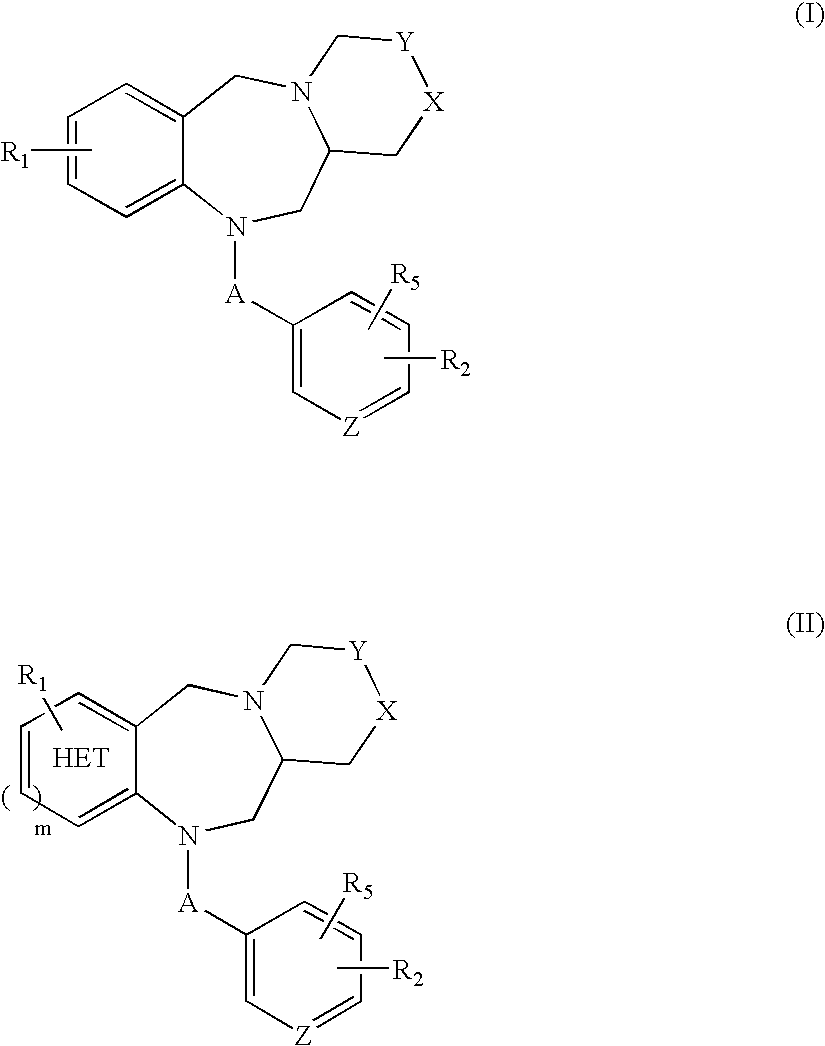
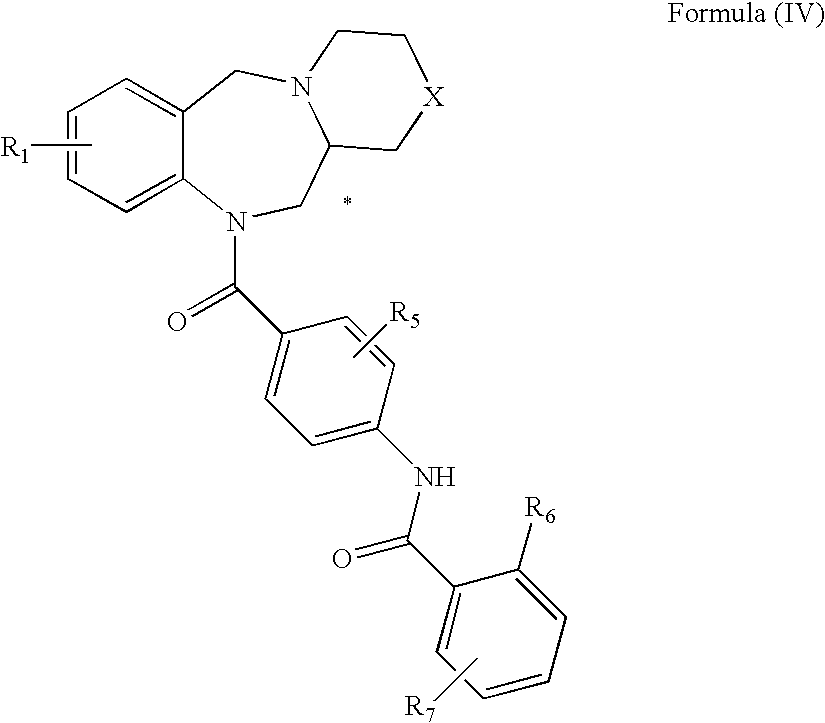
Tricyclic benzodiazepines as vasopressin receptor antagonists
The invention is directed to tricyclic benzodiazepines useful as vasopressin receptor antagonists for treating conditions involving increased vascular resistance and cardiac insufficiency. Pharmaceutical compositions comprising tricyclic benzodiazepines of the present invention and methods of treating conditions such as hypertension, congestive heart failure, cardiac insufficiency, coronary vasospasm, cardiac ischemia, liver cirrhosis, renal vasospasm, renal failure, cerebral edema and ischemia, stroke, thrombosis, or water retention are also disclosed.
Owner:ORTHO MCNEIL PHARM INC
Soluble guanylate cyclase activators
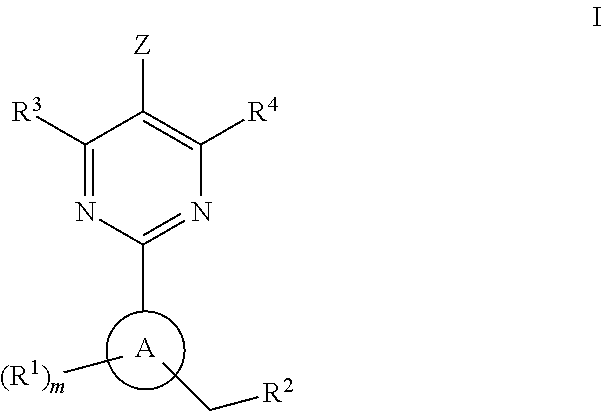
This inventions relates to compounds having the structure Formula I and pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Pancreatic enzyme compositions and methods for treating pancreatitis and pancreatic insufficiency
Compositions of the present invention, comprising the combination of enterically coated and uncoated pancreatic enzyme-containing beads are useful for treating or preventing pancreatitis pain, and optionally disorders associated with digestive enzyme deficiencies.
Owner:SOC DES PROD NESTLE SA
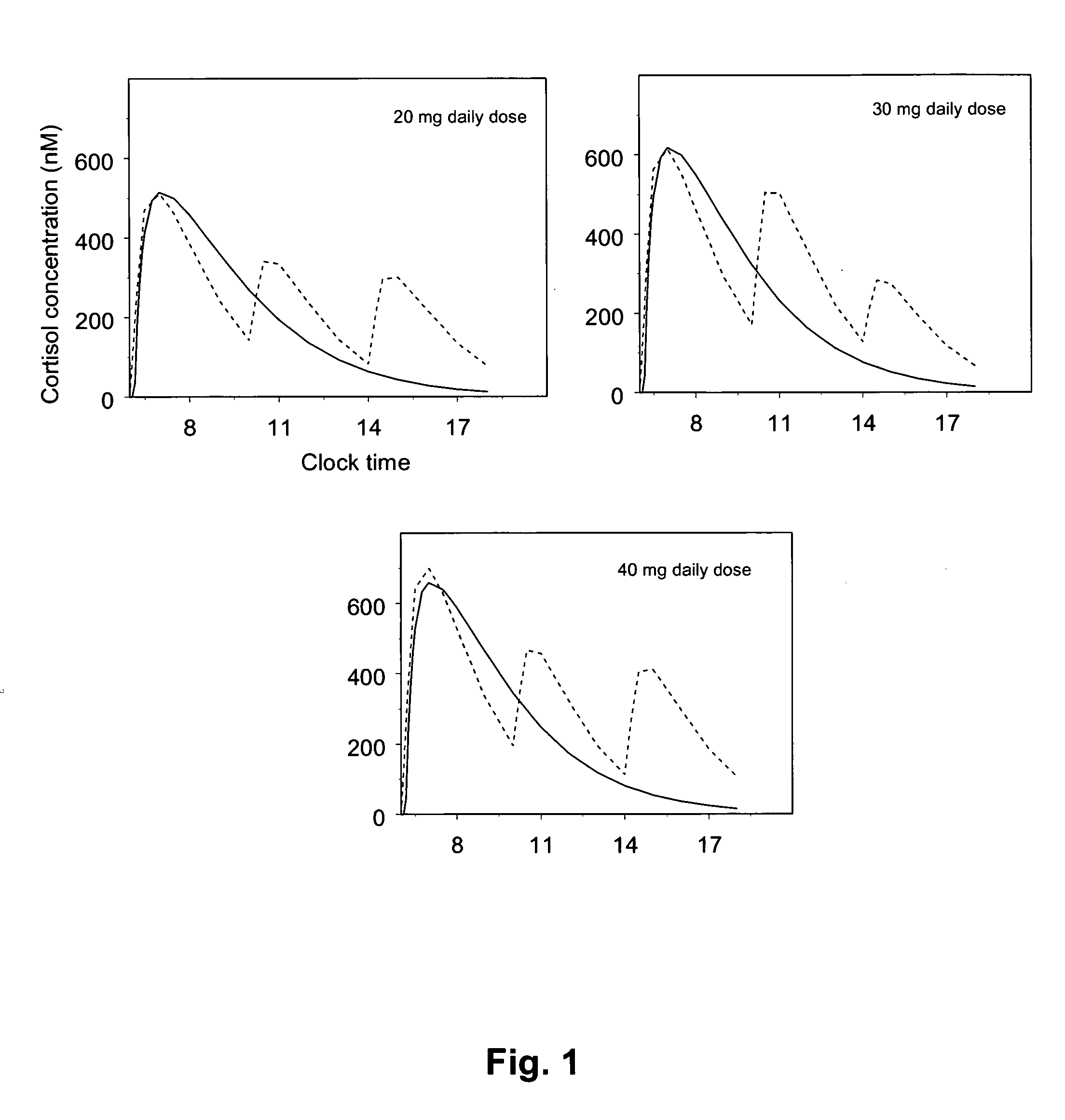
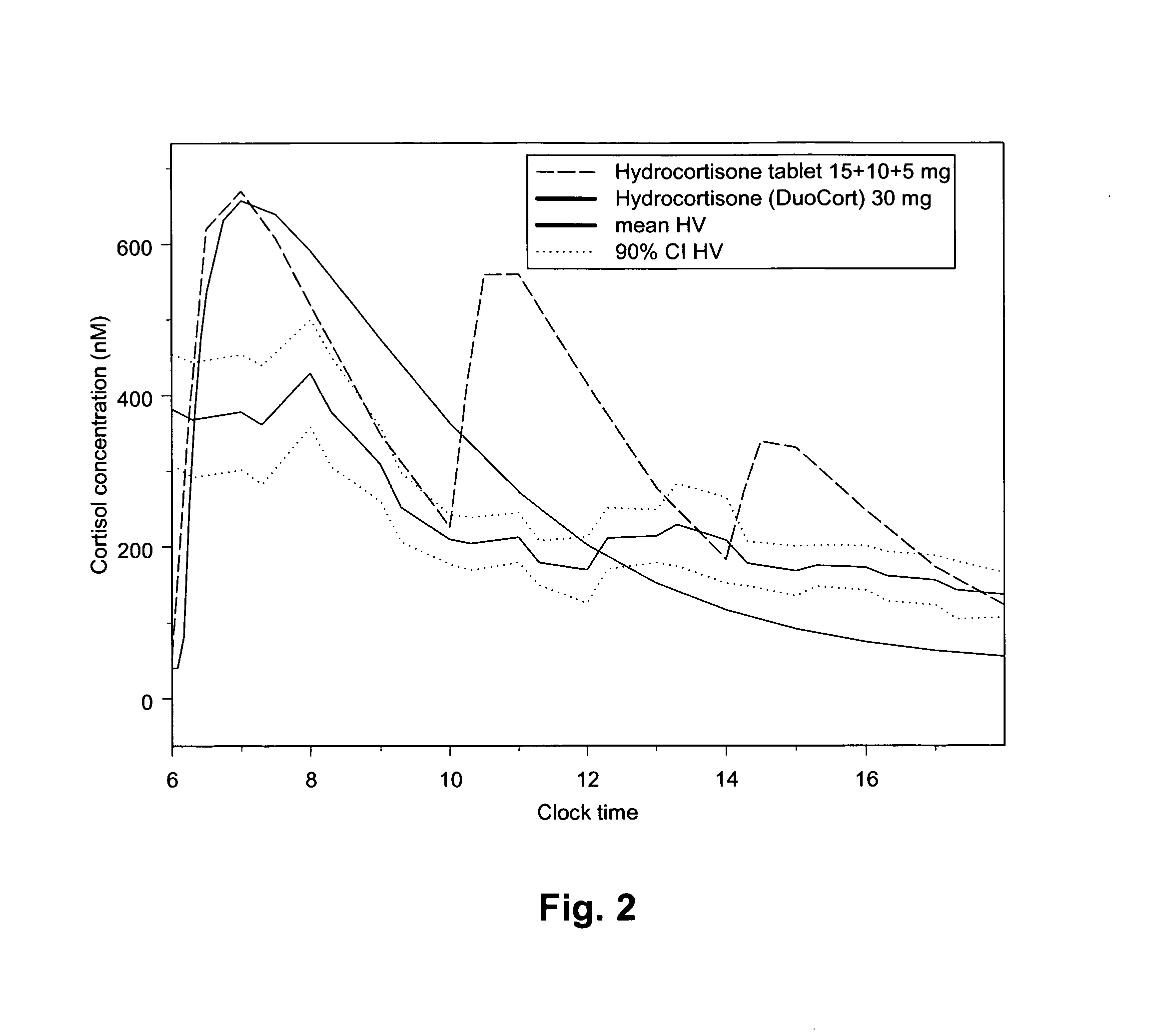
Posology and administration of glucocorticoid based compositions
InactiveUS20130209565A1More exposureReliable, informed and objectiveOrganic active ingredientsSteroidsRegimenGlucocorticoid
The present invention relates to an improved method of administration of glucocorticoid based compositions in glucocorticoid replacement therapies enabling an objectively based regimen for administration enabling correct individual dosing of glucocorticoids resulting in an optimised individual replacement therapy and thus an improved long-term outcome for patients with temporary or chronic adrenal insufficiency.
Owner:DUOCORT PHARMA
Use of hypericum japonicum in preparation of medicine against nephritis and renal insufficiency
ActiveCN101028317AGood preventive effectGood prevention effectUrinary disorderPlant ingredientsMedicineRenal function
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
Traditional Chinese medicine composition for treating yang insufficiency of both spleen and kidney
InactiveCN104083743ASimple recipeEasy to prepareDigestive systemPlant ingredientsSide effectRadix Aconiti
The invention discloses a traditional Chinese medicine composition for treating the yang insufficiency of both the spleen and the kidney. The traditional Chinese medicine composition is mainly prepared through the following raw materials in parts by weight: 10-20 parts of poria cocos, 10-20 parts of paeonia lactiflora, 5-15 parts of rhizoma atractylodis macrocephalae, 10-20 parts of ginger and 5-15 parts of radix aconiti carmichaeli. The medicine disclosed by the invention is scientifically blended by selecting 5 natural traditional Chinese medicines, is simple in formula, achieves the effects of promoting blood circulation to remove blood stasis, warmly invigorating the liver and the kidney and strengthening muscles and bones through the synergistic effect of crude medicines, is quick in effect without toxic or side effect, simple in preparation method, convenient to take and low in cost and achieves the effective rate more than 95%.
Owner:谢桂斌
Autologous stem cells for treating renal insufficiency and preparation method thereof
InactiveCN102146357AEasy accessRelieve painArtificial cell constructsVertebrate cellsProgenitorTreatment effect
The invention provides autologous stem cells for treating renal insufficiency and a preparation method thereof. In the preparation method, autologous progenitor cells for treating renal insufficiency are generated by induced differentiation of adipose-derived stem cells which are obtained by separation from the adipose tissue of a patient, purification and amplification culture. The resource of the autologous progenitor cells is rich, the autologous progenitor cells can be obtained conveniently, and the preparation method is simple, convenient, easy, economic and practical. The prepared stem cells are subjected to autotransfusion to treat renal insufficiency with obvious treatment effect, high safety, no immunological rejection, no obvious toxic or side effects, no adverse reaction and less pain in patients. The invention provides a brand-new treatment means for patients with renal insufficiency and has a profound active significance.
Owner:SHANGHAI BIOMED UNION BIOTECHNOLOGY CO LTD
Pharmaceutical preparation
InactiveUS20110293590A1Reduce steatorroheaPeptide/protein ingredientsDigestive systemPharmaceutical formulationPancreatic Insufficiencies
Owner:NORDMARK-WERKE GMBH 2000 HAMBURG
Treatment of cardiac insufficiency and cardiac failure by using 'Shen Gui' capsule
The invention relates to the use of a medicinal capsule for treating cardiac functional insufficiency and cardiac failure, wherein the medicinal preparation comprises the formulations of red ginseng 300g, Ligusticum wallichii 500g and cassia twig 200g.
Owner:SHANGHAI YUDAN PHARMA
Composition comprising hydrocortisone
ActiveUS20160081942A1Prevent attritional effectInhibitionOrganic active ingredientsPharmaceutical non-active ingredientsAdrenal tissueAdrenal insufficiency
The disclosure relates to pharmaceutical compositions useful in the treatment of adrenal insufficiency in paediatric or elderly subjects.
Owner:DIURNAL
Yanghe decoction oral formulations and preparation method thereof
The invention relates to a Yang-Activating Decoction medicine oral preparation prepared from seven Chinese traditional medicines including prepared rhizome of rehmannia, and a preparation method thereof. The oral preparation is prepared from mixed extracts (extract or dried powder and volatile oil) of prepared rhizome of rehmannia, white mustard seed, carrageenin, cinnamon, dry ginger charcoal, ephedra, and licorice root, and pharmaceutically acceptable carrier or excipient as ground substance in the conventional preparation method. The oral preparation can be mainly used for treating dorsal furuncle due to yang insufficiency, blood deficiency, accumulation of pathogenic cold and phlegm stagnation. The long term clinical application of the prescription indicates that the oral preparation has good curative effect on dorsal furuncle due to yang insufficiency, accumulation of pathogenic cold, menoxenia due to blood deficiency, and phlegm dampness and stagnation in joint and meridian.
Owner:苏州世林医药技术发展有限公司
Use of hypericum japonicum in preparation of medicine against nephritis and renal insufficiency
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
Traditional Chinese medicine composition for treating chronic nephritis and renal insufficiency
InactiveCN105998936AQuick resultsShort course of treatmentOrganic active ingredientsAnthropod material medical ingredientsSalvia miltiorrhizaSide effect
The invention relates to traditional Chinese medicine composition for treating chronic nephritis and renal insufficiency. The traditional Chinese medicine composition is prepared from the following substances in parts by weight: 18-22 parts of rhubarb, 12-30 parts of mole crickets, 10-15 parts of centipedes, 40-50 parts of radix pseudostellariae, 25-35 parts of coastal glehnia roots, 23-30 parts of ophiopogon japonicas, 6-8 parts of Chinese magnoliavine fruits, 30-40 parts of milkvetch roots, 20-25 parts of Chinese angelica, 20-25 parts of stir-fried largehead atractylodes rhizomes, 20-25 parts of poria cocos, 20-25 parts of dried tangerine peels, 20-25 parts of uncooked coix seeds, 20-25 parts of common macrocarpium fruits, 30-35 parts of Chinese taxillus herbs, 20-25 parts of stir-fried spina date seeds, 30-35 parts of spreading hedyotis herbs, 30-35 parts of honeysuckle flowers, 30-35 parts of charred triplet, 20-25 parts of rice beans, 20-25 parts of dandelions, 25-30 parts of glossy privet fruits. 25-30 parts of dodder seeds, 30-40 parts of fructus lycii, 20-30 parts of semen plantaginis, 20-30 parts of cherokee rose fruits and 30-40 parts of salvia miltiorrhiza. The traditional Chinese medicine composition takes effect quickly, the treatment course is short, the efficiency is high, the cost is low, and toxic and side effects are avoided.
Owner:孙福强
Enzyme composition and application thereof in the treatment of pancreatic insufficiency
InactiveUS20110171294A1Easy to transportPeptide/protein ingredientsHydrolasesMicroorganismExocrine pancreatic insufficiency
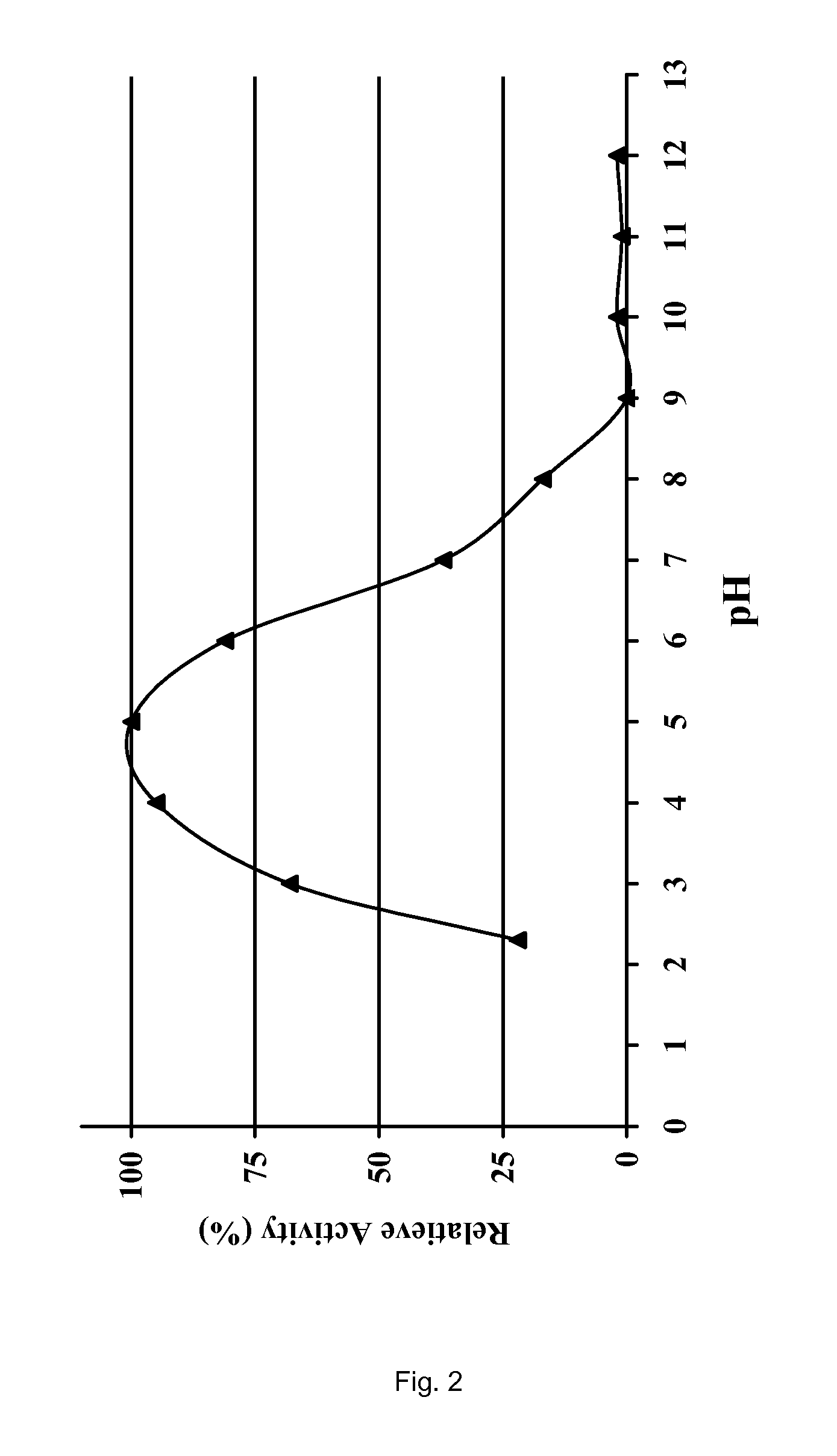
The present invention relates to a composition of at least one protease and a mode of application for treating patients suffering from pancreatic enzyme insufficiency, pancreatitis or cystic fibrosis. The composition of enzymes comprises at least one protease which has a pH optimum below 5.0 and wherein said protease is further active in the presence of pepsin. In a preferred embodiment, said protease is of microbial origin.
Owner:DSM IP ASSETS BV
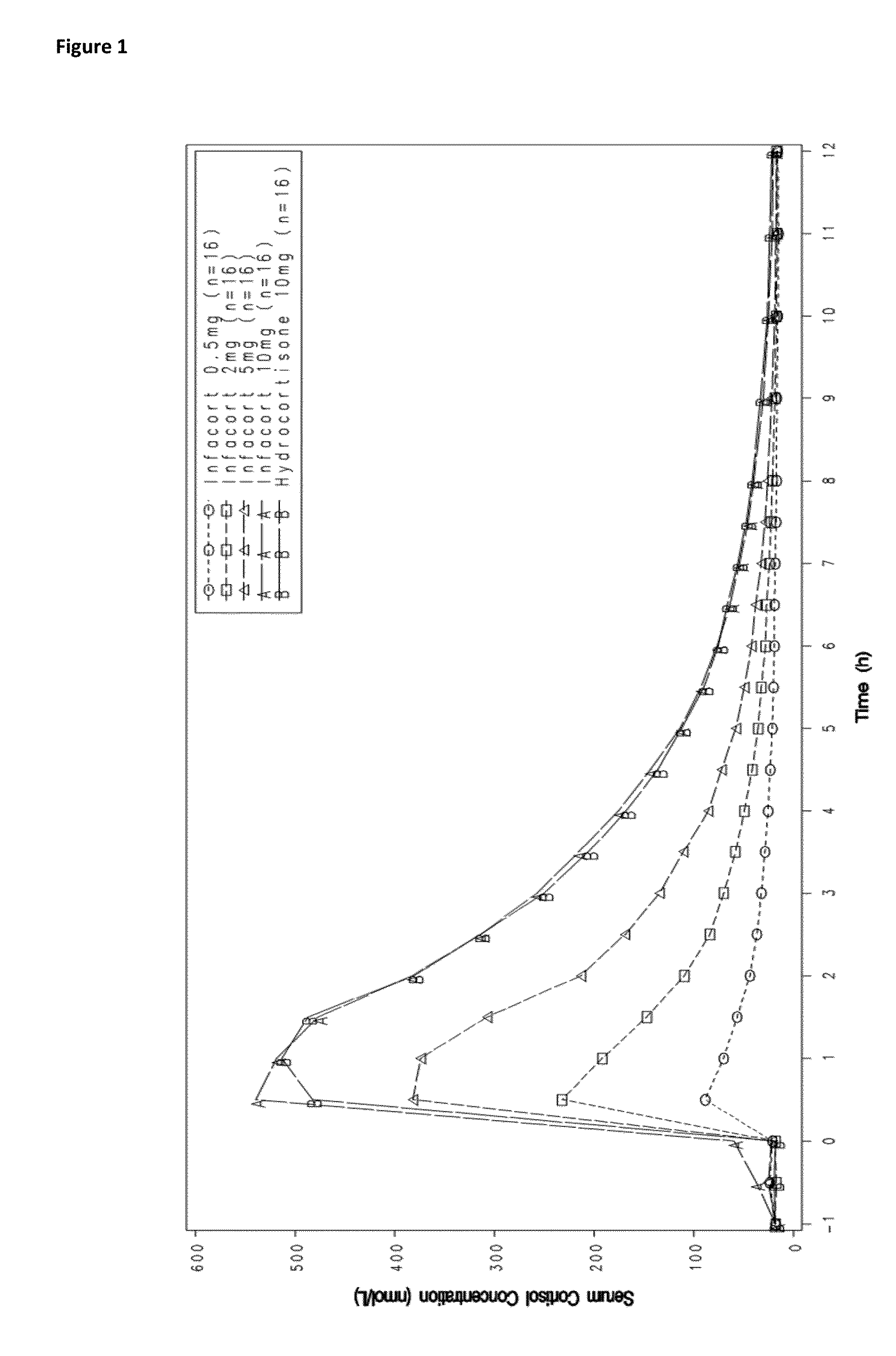
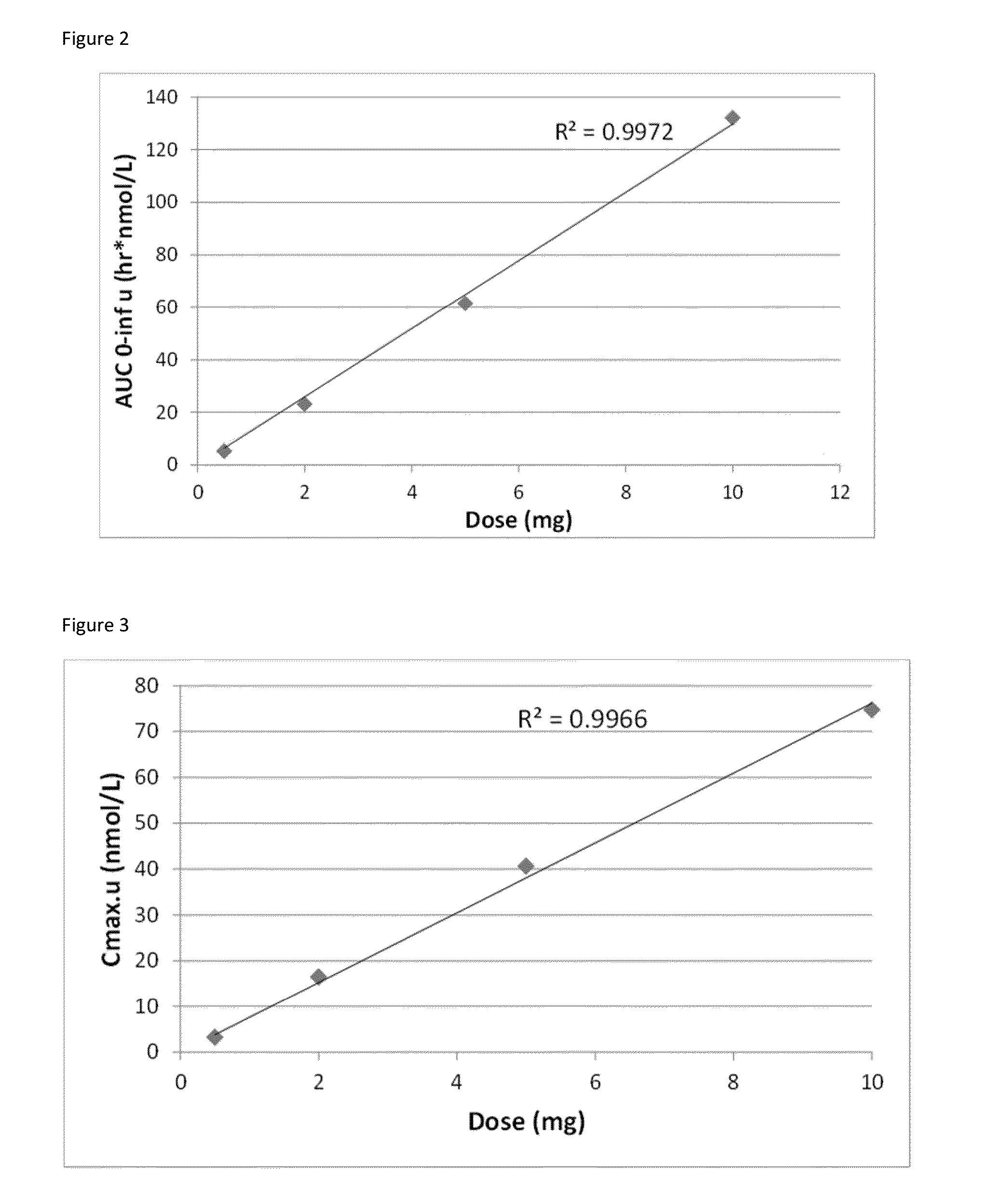
Hydrocortisone controlled release formulation
ActiveUS20140370113A1Organic active ingredientsNervous disorderHydrocortisonePharmaceutical formulation
The disclosure relates to a pharmaceutical formulation comprising hydrocortisone and its use in the treatment of conditions that would benefit from a delayed release of hydrocortisone, in particular conditions such as adrenal insufficiency, inflammatory conditions and depression.
Owner:DIURNAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com