Synthesis of hydrocortisone butyrate
A technology of hydrocortisone acetate and hydrocortisone, applied in steroids, organic chemistry and other directions, can solve the problems of impure products, direct esterification steric hindrance, and increasing the number of refining times.
Active Publication Date: 2010-08-25
TIANJIN JINYAO GRP
View PDF3 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
But because acid is to 17,21 cyclic orthoesters ring-opening process, although most of them generate corresponding 17-esters, some corresponding 21-esters will also occur, resulting in impure products, especially 17-esters and 21-esters. The polarity of esters is similar, and it is not easy to separate by recrystallization. In order to obtain the desired product, the number of refining times will be increased accordingly, and the yield will be reduced.
In fact, there are many methods for synthesizing esters. For example, the corresponding esters can be directly obtained by reacting alcohols with acids, acid anhydrides or acid chlorides. Since the above-mentioned methods have obvious problems, why are the above-mentioned methods with obvious problems still used? The reason is that there is a β-acetone side chain and an α-hydroxyl group at the 17th position of the pregnant compound. Due to the existence of the β-acetone side chain, the direct esterification of the α-hydroxyl group has a large steric hindrance, especially when the β-acetone side chain This is especially true when there is an ester group (ie 21-ester) at the other end of the 17α-hydroxyl group, which causes the 17α-hydroxyl group to be unable to be esterified by a direct method. It will also be esterified at the same time, causing more impurities and not obtaining high-purity products
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
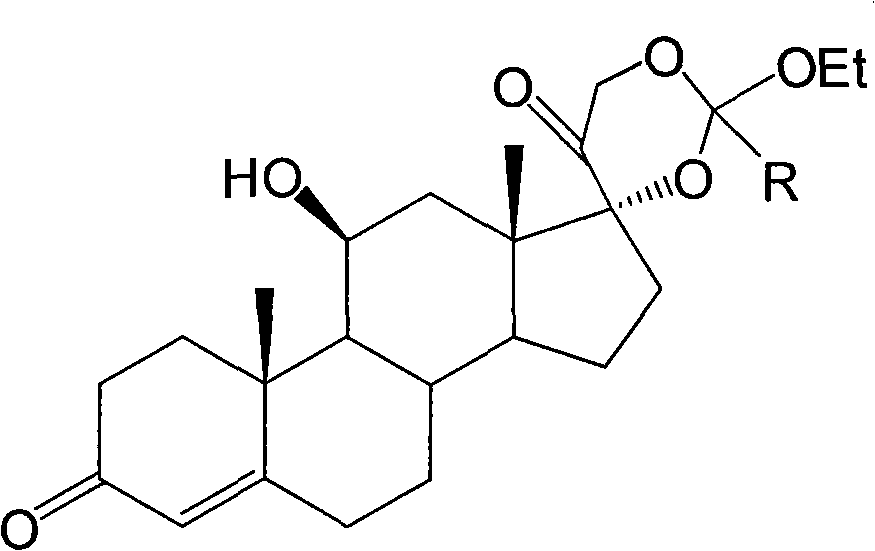
The invention relates to synthesis of hydrocortisone butyrate, which comprises the following steps of: A. carrying out acylation reaction on hydrocortisone acetate adopted as a raw material and butyl chloride in an organic solvent I under the condition of existence of a catalyst; after the reaction is finished, adding acid to adjust a pH value being smaller than 5; diluting or recrystallizing to obtain hydrocortisone 17alpha-butyrate-21-acetate; and B. adopting the hydrocortisone 17alpha-butyrate-21-acetate as a raw material, adding inorganic alkali in an organic solvent II and carrying out selective hydrolysis reaction to obtain hydrocortisone 17alpha-butyrate.
Description
field of invention The invention relates to a preparation method of hydrocortisone 17-butyrate. Background technique: Hydrocortisone 17α-butyrate (Hydrocortisone-17-butyrate, CAS: 13609-67-1) is a common skin drug and should be widely used in China (the application of hydrocortisone butyrate in dermatology , "Continuing Medical Education", 2006, Vol. 20, No. 33, No. 59), preparations with hydrocortisone 17-butyrate as the active ingredient are relatively common in the market, for example, the product produced by Tianjin Pharmaceutical Group Co., Ltd. is named "Udrole" cream. The synthesis technology of hydrocortisone 17-butyrate has been studied by scientists in the seventies of last century, such as JP52136157, JP 56040700, Synthesis, (8), 700-1 1984, JP 60048998 and other documents all show that the compound The synthesis of hydrocortisone is reacted with triethyl orthobutyrate to form 17,21 cyclic orthoester, which is formed by acid-selective ring opening at position 2...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07J21/00
Inventor 孙亮陈松赵琳
Owner TIANJIN JINYAO GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com