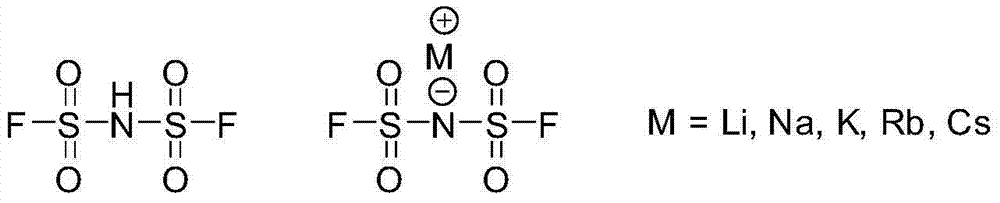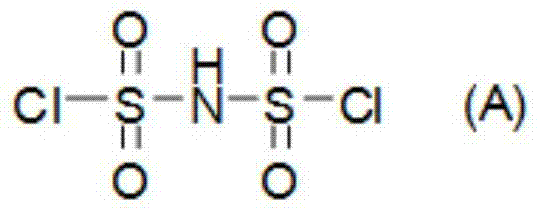Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
423 results about "Alkali metal halide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alkali metal halides (also known as alkali halides) are the family of inorganic compounds with the chemical formula MX, where M is an alkali metal and X is a halogen. These compounds are the often commercially significant sources of these metals and halides. The best known of these compounds is sodium chloride, table salt.
Method for removing mercury vapor in gas
When sulfur oxides are present in mercury vapor-containing gas, the adsorption of mercury vapor by activated carbon is inhibited. Therefore, there has been demand for development of a method for effective adsorption removal of mercury vapor even in the coexistence of sulfur oxides.Efficient and long-term removal of mercury vapor was made successful by contacting an activated carbon adsorbent consisting of 100 parts by weight of activated carbon impregnated with 5 to 70 parts by weight of only an alkali metal halide, with mercury vapor in sulfur oxide-containing gas.
Owner:JAPAN ENVIROCHEM
Ammonothermal process for bulk synthesis and growth of cubic GaN
InactiveUS20030209191A1Easy to transportQuality improvementPolycrystalline material growthFrom chemically reactive gasesLanthanideSingle crystal
A method of growing single-crystals of a cubic (zinc blende) form of gallium nitride, the method comprising the steps of: placing into a reaction tube or acid resistant vessel a gallium source, anhydrous ammonia, an acid mineralizer and a metal halide salt selected from the group consisting of alkali metal halides, copper halides, tin halides, lanthanide halides and combinations thereof; closing said reaction tube or vessel; heating said reaction tube; cooling said reaction tube or vessel; and collecting single-crystals of cubic (zinc blende) form of GaN; wherein said reaction tube or vessel has a temperature gradient with a hot zone of at least 250° C., wherein said reaction tube or vessel has a temperature gradient with a cool zone of at least 150° C., and wherein said acid mineralizer has a sufficient concentration to permit chemical transport of GaN in said reaction tube or vessel from said hot zone to said cool zone due to said temperature gradient within said reaction tube or vessel.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
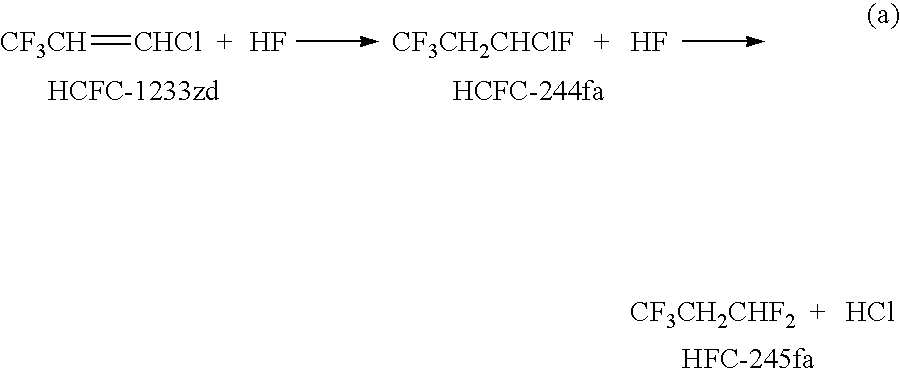
Process for the manufacture of 1,3,3,3-tetrafluoropropene
ActiveUS7829748B1Physical/chemical process catalystsPreparation by hydrogen halide split-offAlkaline earth metalOxidation state
The invention provides an economic process for the manufacture of 1,3,3,3-tetrafluoropropene (HFO-1234ze) by a two stage process. A vapor phase hydrofluorination of 1-chloro-3,3,3-trifluoropropene (HCFC-1233zd) into 1-chloro-1,3,3,3-tetrafluoropropane (HCFC-244fa) and / or 1,1,1,3,3-pentafluoropropane (HFC-245fa) is conducted, followed by the thermal dehydrochlorination of HCFC-244fa and dehydro fluorination of HFC-245fa into HFO-1234ze in the presence of a catalyst which comprises one or more of alkali metal halides, alkaline earth metal halides, halogenated metal oxides, zero oxidation state metals, zinc halides, palladium halides, and activated carbon.
Owner:HONEYWELL INT INC
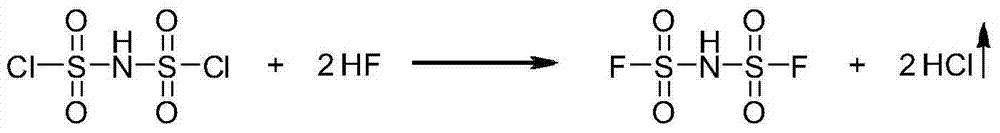
Preparation methods of bis(fluorosulfonyl)imide and alkali metal salts thereof
InactiveCN103935970AEasy to getLow costHybrid capacitor electrolytesAmidosulfonic acidImideHydrogen fluoride
The invention relates to preparation methods of bis(fluorosulfonyl)imide and alkali metal salts thereof. The bis(fluorosulfonyl)imide is prepared by subjecting bis(chlorosulfonyl)imide to react with a fluorinating agent namely hydrogen fluoride. The alkaline metal salts of bis(fluorosulfonyl)imide is prepared by subjecting bis(chlorosulfonyl)imide and alkali metal halides to react with hydrogen fluoride taken as the fluorinating agent and the solvent. The preparation methods can reduce the raw material costs, reduce the material consumption and generation of wastes, improve the raw material utilization rate, product yield, and product purity; moreover, the product purification is easier, the preparation technology process is more concise and more efficient, and the preparation methods provides a very good technical foundation for massive industrial production of bis(fluorosulfonyl)imide and alkali metal salts thereof.
Owner:SHENZHEN CAPCHEM TECH CO LTD
Cathode for an electrochemical cell
ActiveUS20100068610A1Good hygroscopicityA large amountConductive materialSecondary cellsMetal halidesElectrochemical cell
The invention provides a cathode for an electrochemical cell comprising: a first particulate material having particles comprising a mixture of at least one alkali metal halide and at least one metal; and a second particulate material comprising at least one alkali metal halide, wherein the second particulate material has a particle size smaller than that of the first particulate material.
Owner:GENERAL ELECTRIC CO
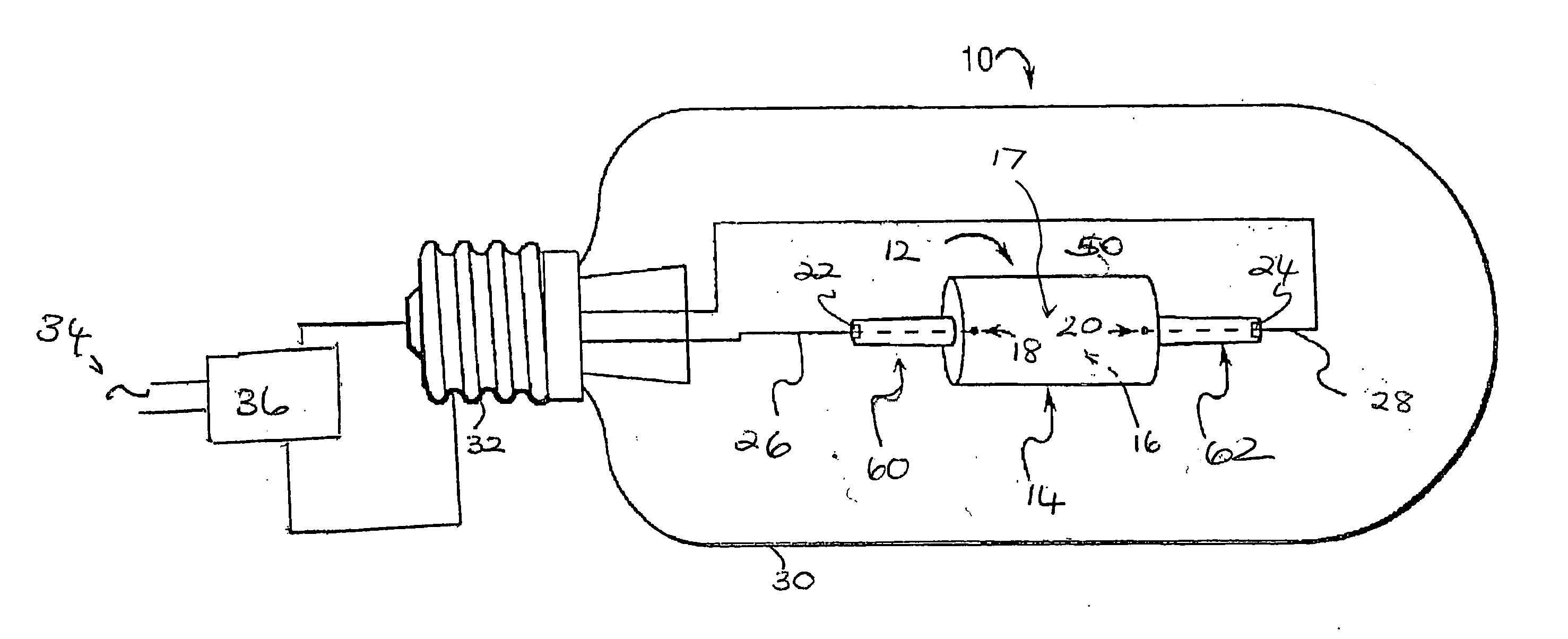
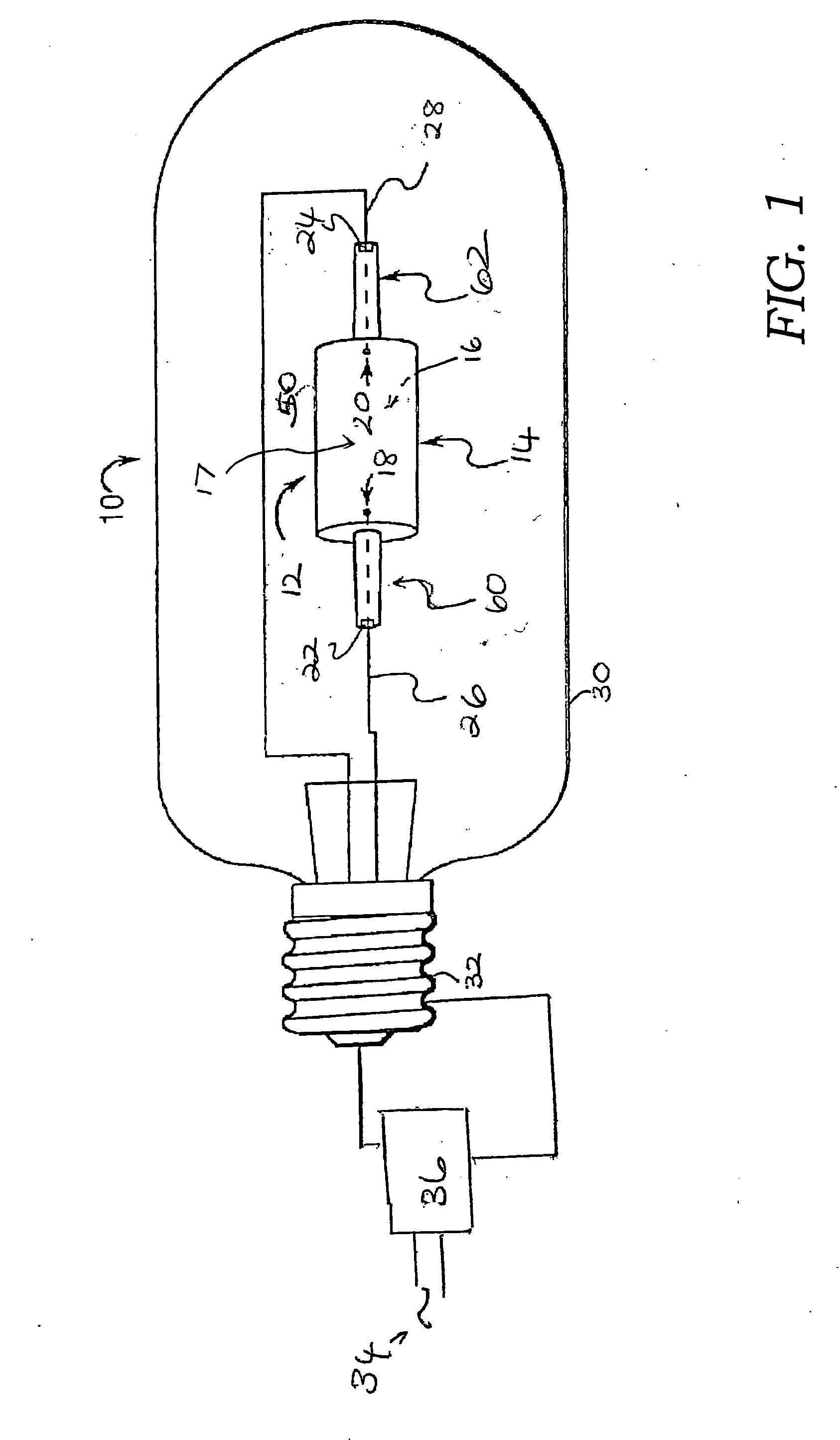
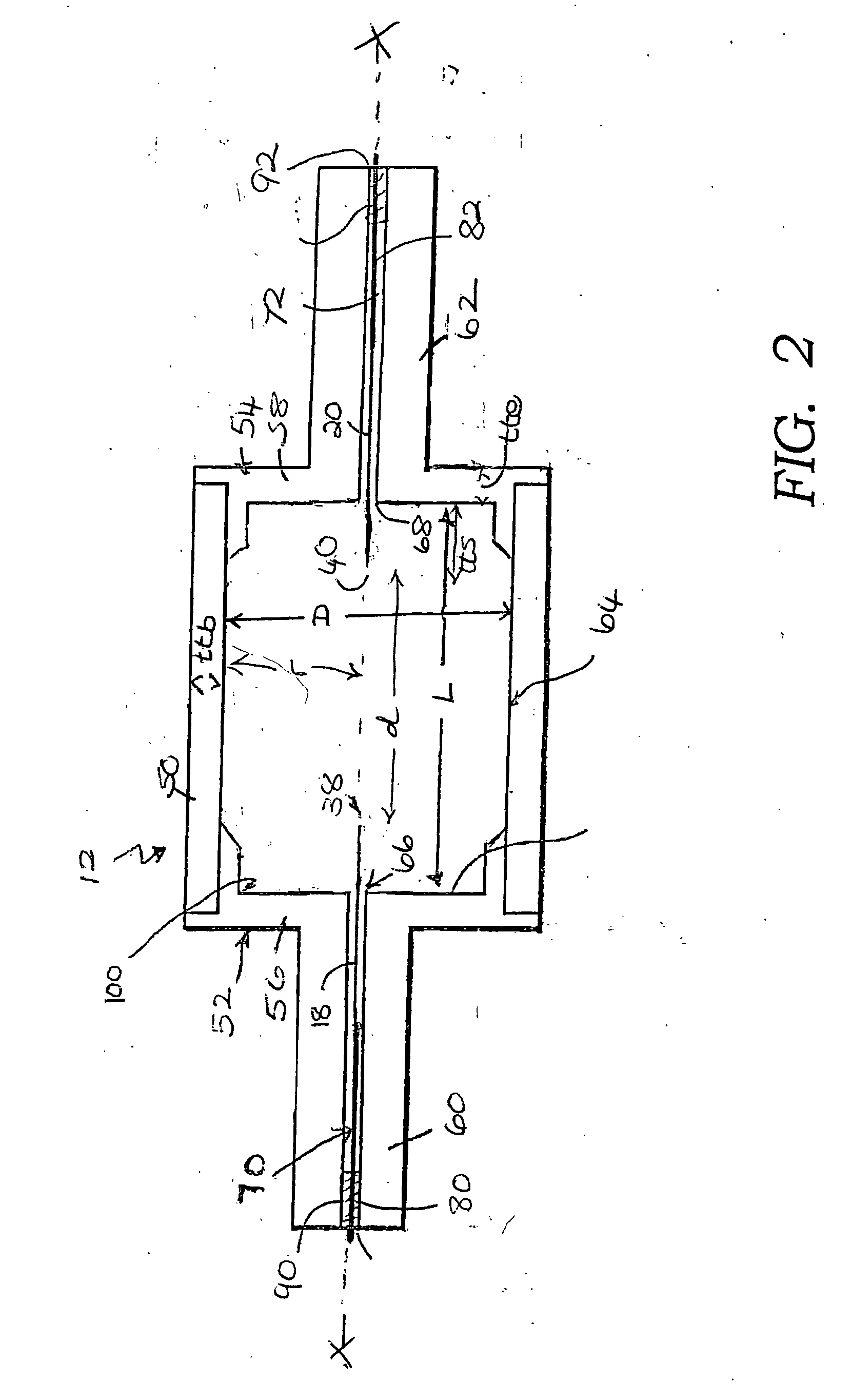
Ceramic metal halide lamp
InactiveUS20060164016A1Good colorImprove efficiencySolid cathode detailsGas discharge lamp detailsAlkaline earth metalRare earth
A metal halide lamp (10) includes a discharge vessel (12) which may be formed of a ceramic material. The vessel defines an interior space (16). An ionizable fill (17) is disposed in the interior space. The ionizable fill includes an inert gas, mercury, and a halide component. The halide component includes an alkali metal halide, an alkaline earth metal halide component, and optionally at least one of a rare earth halide and a Group IIIA halide. The alkaline earth metal halide component includes at least one of a barium halide and a strontium halide. At least one electrode (18, 20) is positioned within the discharge vessel so as to energize the fill when an electric current is applied thereto. The lamp having a wall loading, when energized, which is sufficient to maintain an active tungsten halogen cycle.
Owner:GENERAL ELECTRIC CO
Preparation method of metal silver nanowires with adjustable length and diameter
InactiveCN102328095ALow reaction temperatureLow costPolycrystalline material growthSingle crystal growth detailsAlcoholReaction temperature
The invention discloses a preparation method of metal silver nanowires with adjustable length and diameter. The method comprises the following steps: dissolving silver nitrate in polyhydric alcohol for preparing solution; dissolving an alkali metal halide and a reducing compound in the polyhydric alcohol for preparing the solution; mixing the two types of the solution, and fully reacting under stirring to get the mixed solution; heating the polyhydric alcohol to 120-160 DEG C, dripping into the mixed solution, keeping the temperature and reacting for 15-44 hours; and performing centrifugal separation on reaction solution after the reaction, and washing lower-layer precipitate to get the silver nanowires. According to the preparation method, inert gas does not need to be introduced for protection, the reaction temperature is low, the preparation process is simple, the yield is high, the cost is low, and the insufficiencies of complex preparation procedure, low yield, high cost and the like in the template plate, the seeding method, the traditional polyhydroxy reduction method and the like can be overcome, thereby having important significance for large-batch industrial production and actual application thereof of the silver nanowires.
Owner:UNIV OF JINAN
Energy storage device and method
A composition is provided that includes a ternary electrolyte having a melting point greater than about 150 degree Celsius. The ternary electrolyte includes an alkali metal halide, an aluminum halide and a zinc halide. The amount of the zinc halide present in the ternary electrolyte is greater than about 20 mole percent relative to an amount of the aluminum halide. An energy storage device including the composition is provided. A system and a method are also provided.
Owner:GENERAL ELECTRIC CO
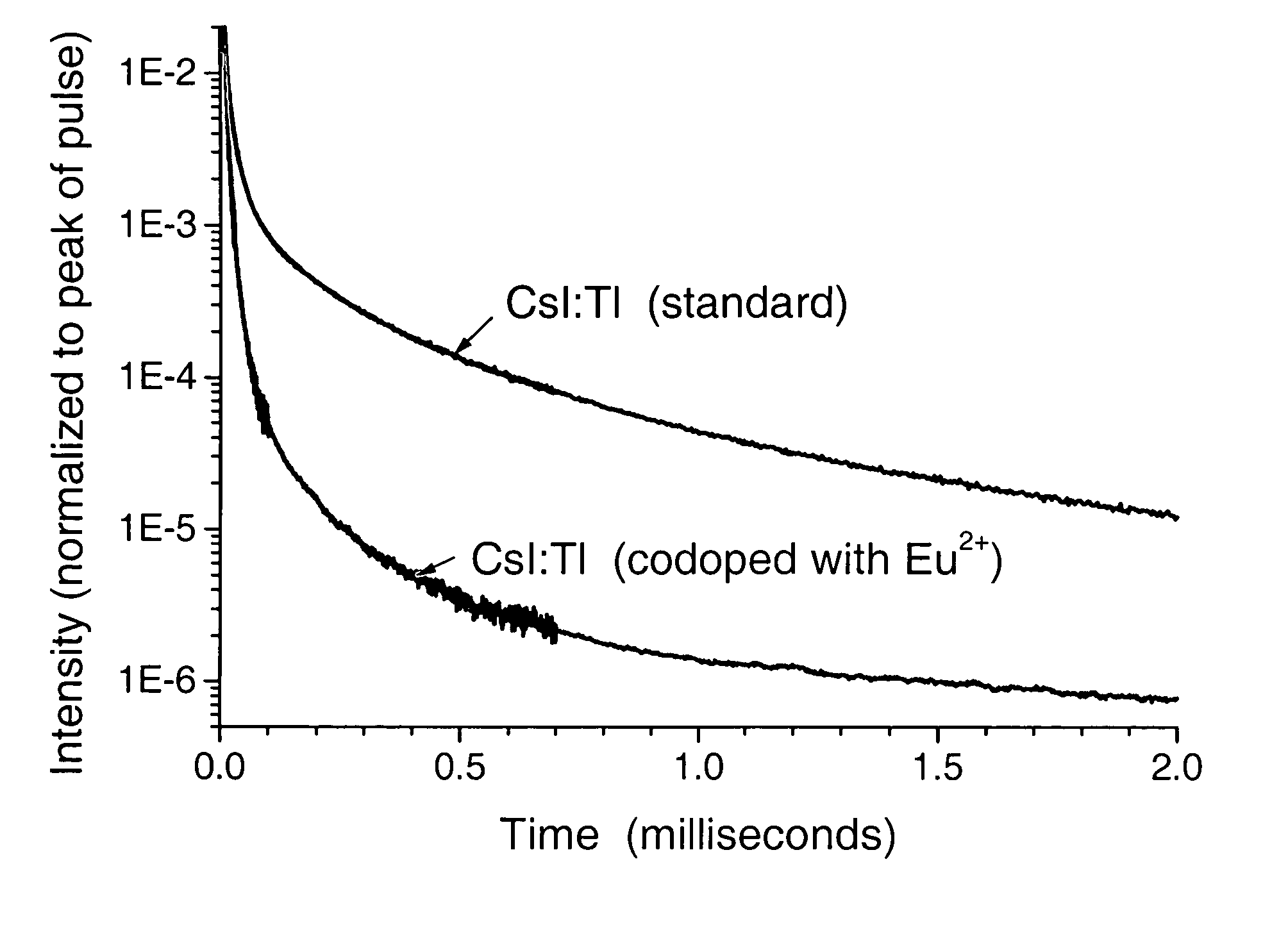
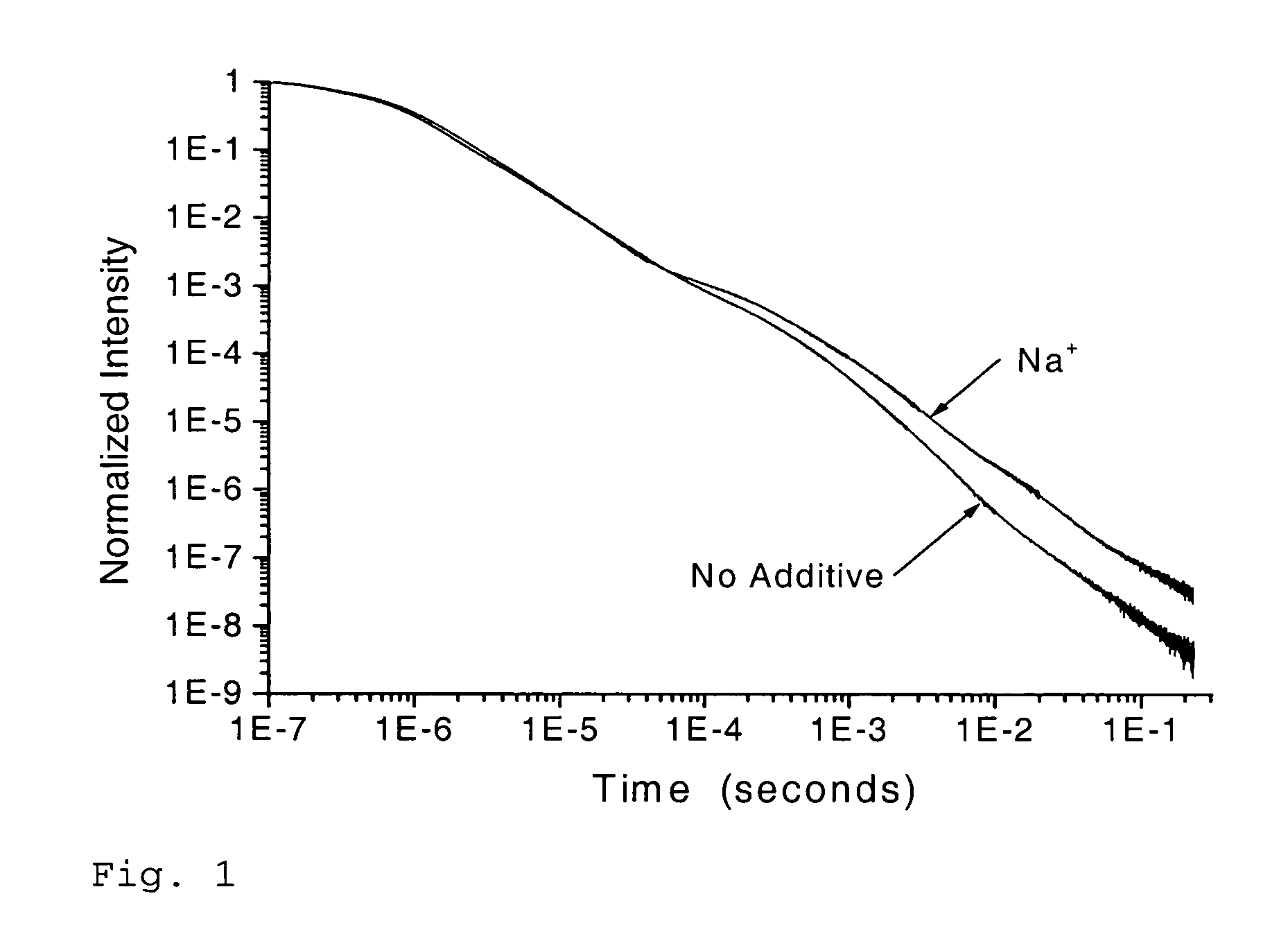
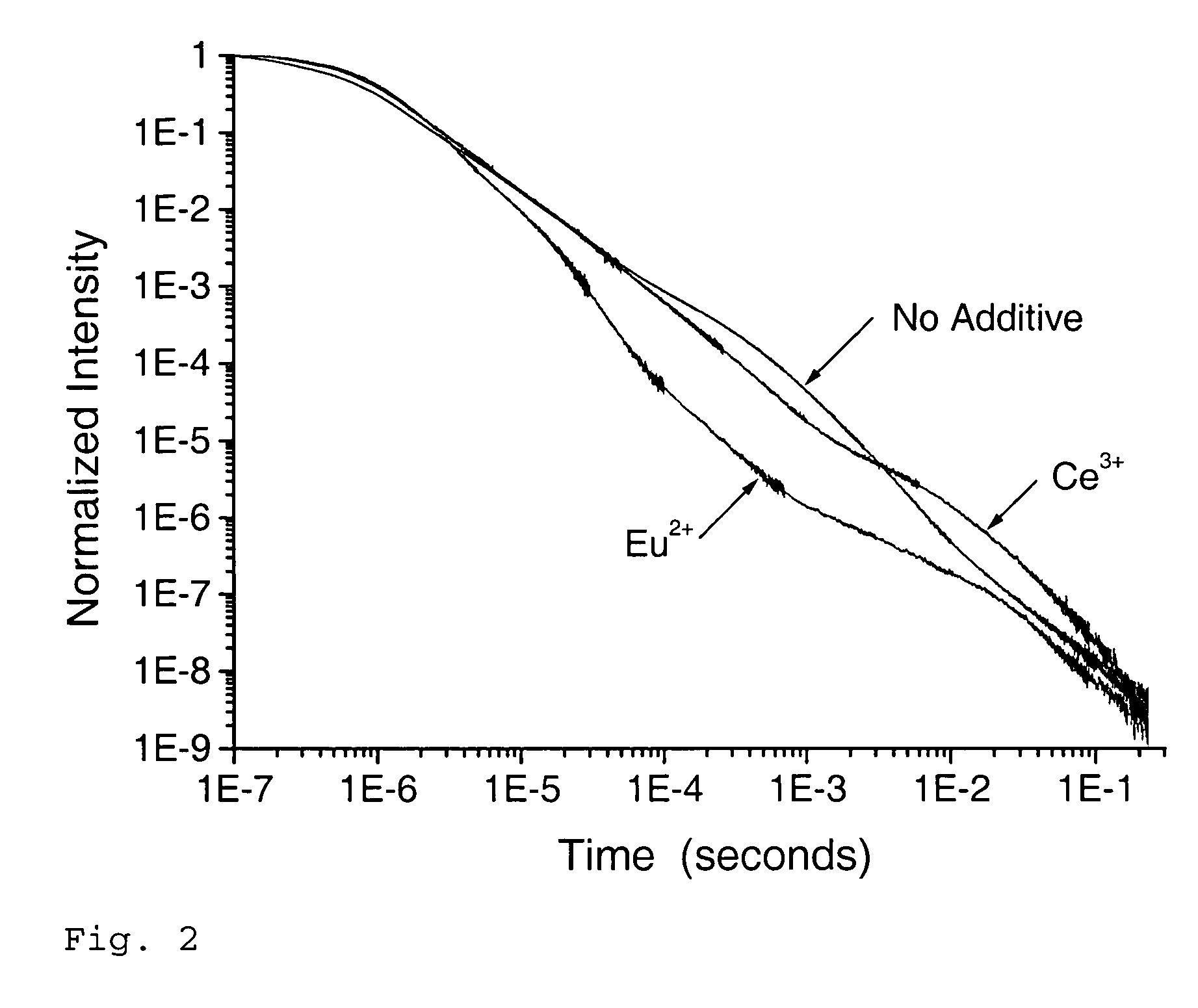
Scintillation materials with reduced afterglow and method of preparation
Scintillation materials of this invention have an alkali halide host material, a (first) scintillation dopant of various types, and a variety of second dopants (co-dopants). In another embodiment, the scintillation materials of this invention have an alkali halide host material, a (first) scintillation dopant of various types, a variety of second dopants (co-dopants), and a variety of third dopants (co-dopants). Co-dopants of this invention are capable of providing a second auxiliary luminescent cation dopant, capable of introducing an anion size and electronegativity mismatch, capable of introducing a mismatch of anion charge, or introducing a mismatch of cation charge in the host material.
Owner:ALEXANDER LEMPICKI
Vacuum evaporation method
InactiveUS20080286461A1Improve efficiencyPreventing phosphorVacuum evaporation coatingSputtering coatingBoiling pointPhosphor
The vacuum evaporation method provides a heating element between an evaporation source of a film-forming material and a substrate; and forms a phosphor layer of an alkali halide-based phosphor on a surface of said substrate by vacuum evaporation while causing the heating element to generate heat at a temperature t (° C.) satisfying Formula (1):T−200≦t<T (1)where T is a boiling point (° C.) of the film-forming material. This method is capable of using the film-forming material making up the phosphor layer with higher efficiency owing to a heating element while preventing the substrate and the phosphor layer from being adversely affected by heat from the heating element.
Owner:FUJIFILM CORP
Process for synthesizing cyclic carbonate
InactiveCN1343668AEasy to separateHigh purityOrganic chemistryEpoxyNitrogenous heterocyclic compound
A process for preparing cyclic carbonate features that catalytic cycloaddition reaction of CO2 on epoxy compound at 100-140 deg.C and 1.5-4.5 MPa for 4-8 hrs under the existance of the catalyst whichis composed of azacyclic compound (halogenated alkylpyridine or halogenated 1,3-dialkyl imidazole) and non-metal halide, and the cocatalyst which is alkali-metal halide or ammonium tetrebutyl bromide. Its advantages are high catalytic activity, smooth reaction condition, easy separation of resultant from catalyst, and cyclic use of catalyst.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for producing difluorophosphate, nonaqueous electrolyte solution, and nonaqueous electrolyte secondary battery
ActiveCN102036912AEasy to manufactureHigh purityFinal product manufactureLi-accumulatorsHydrogen fluorideAlkaline earth metal
A method for producing a high-purity difluorophosphate by simple processes; a method for producing an electrolyte solution using a difluorophosphate obtained by the method; an electrolyte solution; and a secondary battery. Specifically disclosed is a method for producing a difluorophosphate, which comprises the following step (1) or (2). (1) A step wherein (A) at least one substance selected froma group consisting of phosphorus oxo acids, oxo acid anhydrides and oxyhalides is reacted with (B) a hexafluorophosphate in the presence of hydrogen fluoride. (2) A step wherein at least one halide selected from a group consisting of alkali metal halides, alkaline earth metal halides, aluminum halides and onium halides is reacted with difluorophosphoric acid in the presence of a hexafluorophosphate. Also disclosed are a nonaqueous electrolyte solution containing the thus-obtained difluorophosphate, and a nonaqueous electrolyte secondary battery comprising the nonaqueous electrolyte solution.
Owner:STELLA CHEMIFA CORP +2
Composition and energy storage device
In accordance with one aspect of the present invention, a cathode composition is provided that includes at least one transition metal or a transition metal salt, wherein the transition metal is at least one selected from the group consisting of nickel, iron, cobalt, chromium, manganese, molybdenum, and antimony; an alkali metal halide; a salt comprising an alkali metal halide and a metal halide; and a metal polysulfide compound MSn wherein M is a metal and n is an integer equal to or greater than 2. The salt comprising an alkali metal halide and a metal halide has a melting point of less than about 300° C. An energy storage device comprising the electrode composition is also provided.
Owner:GENERAL ELECTRIC CO
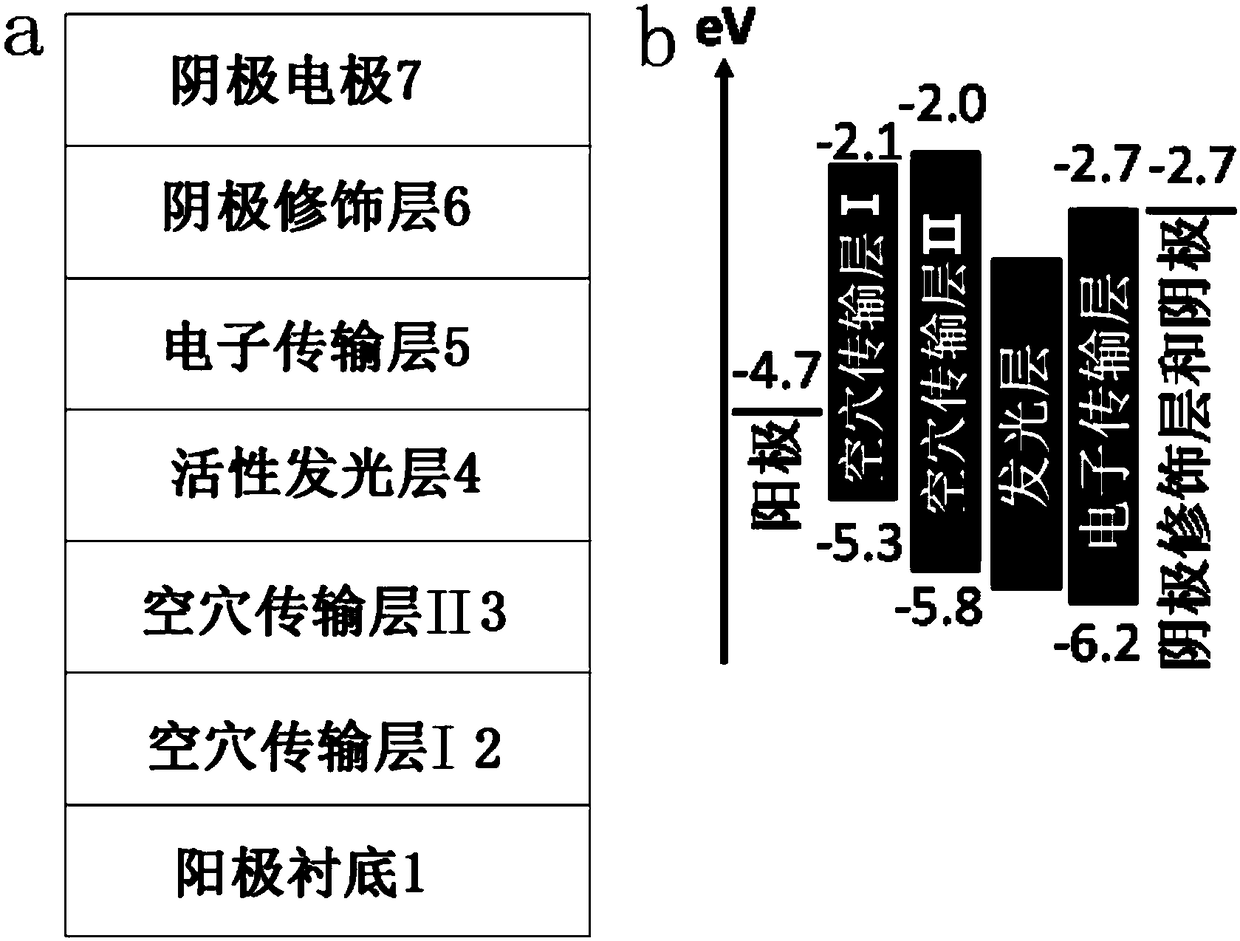
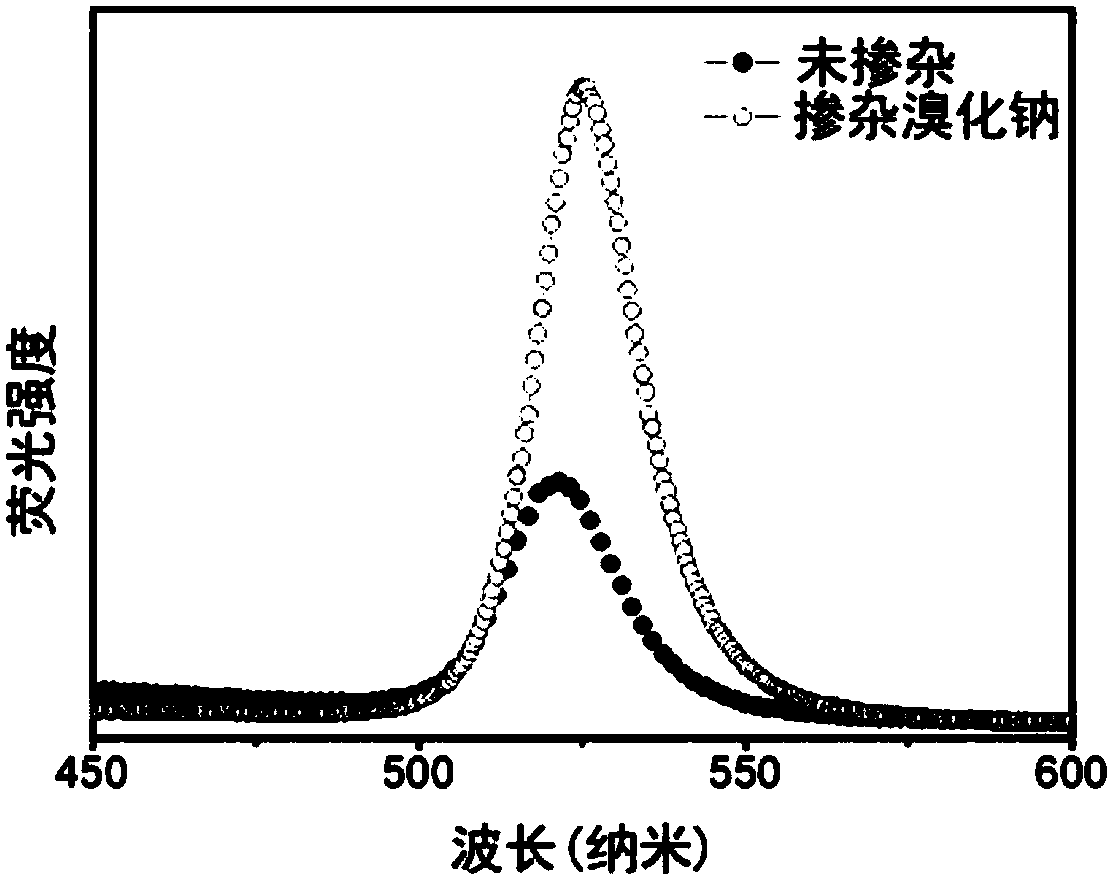
Alkali halide-doped perovskite light-emitting diode and fabrication method thereof
InactiveCN108269940ALow costSimple preparation processSolid-state devicesSemiconductor/solid-state device manufacturingHole transport layerAlkali metal halide
The invention relates to an alkali halide-doped perovskite light-emitting diode. The alkali halide-doped perovskite light-emitting diode comprises a substrate, a hole transmission layer, an active light-emitting layer, an electron transmission layer, an electrode modification layer and an electrode, wherein the thickness of the active light-emitting layer is 5-100 nanometers, the active light-emitting layer comprises perovskite and an alkali halide doped in the perovskite, the molecular formula of the perovskite is one or more of CsPbCl<x>Br<3-x>, CsPbBr<x>I<3-x>, MAPbCl<x>Br<3-x>, MAPbBr<x<I<3-x>, FAPbCl<x>Br<3-x> and FAPbBr<x>I<3-x>, x is equal to 0, 1, 2 or 3, and the alkali halide is one or more of LiCl, NaCl, KCl, RbCl, LiBr, NaBr, KBr, RbBr, LiI, NaI, KI and RbI. The invention also provides a fabrication method of the alkali halide-doped perovskite light-emitting diode. The fabrication method comprises the steps of forming the hole transmission layer or the electron transmissionlayer o the substrate; modifying an alkali halide-containing perovskite precursor solution used as the active light-emitting layer on the hole transmission layer of the electron transmission layer; sequentially forming the electron transmission layer, a negative electrode modification layer and a negative electrode on the active light-emitting layer or sequentially forming the hole transmission layer, a positive electrode modification layer and a positive electrode on the active light-emitting layer; and performing package.
Owner:SUZHOU UNIV
Composition and energy storage device
A cathode composition is provided. The cathode composition includes at least one electroactive metal, wherein the electroactive metal is at least one selected from the group consisting of titanium, vanadium, niobium, molybdenum, nickel, iron, cobalt, chromium, manganese, silver, antimony, cadmium, tin, lead and zinc; a first alkali metal halide; an electrolyte salt comprising a reaction product of a second alkali metal halide and a metal halide, wherein the electrolyte salt has a melting point of less than about 300 degrees Centigrade; and a metal chlorosulfide compound having a formula (I) M1M2p+1SnCl4+3p−2n wherein “M1” is a metal selected from group IA of the periodic table, “M2” is a metal selected from group IIIA of the periodic table, “p” is 0 or 1, and “n” is equal to or greater than 0.5. An article and an energy storage device comprising the cathode composition is provided. A method of forming the energy storage device is provided.
Owner:GENERAL ELECTRIC CO
Electrochemical cell, electrode composition thereof and method for making same
A composition of the positive electrode comprises at least one electroactive metal, at least one iodide of at least one transition metal, a first alkali metal halide, and an electrolyte salt having a melting point of less than about 300° C. The at least one electroactive metal is selected from the group consisting of titanium, vanadium, niobium, nickel, cobalt, chromium, manganese, silver, antimony, cadmium, tin, lead, iron, and zinc. An electrochemical cell and a method for making an electrochemical cell are also presented.
Owner:GENERAL ELECTRIC CO
Process for producing nickel oxyhydroxide by electrolytic oxidation
InactiveUS7407521B2Advantages in terms of working environment and costElectrolysis componentsPrimary cellsElectrolysisNickel oxide hydroxide
A process for production of nickel oxyhydroxide by electrolytic oxidation of nickel hydroxide in the presence of an alkali metal halide. The nickel oxyhydroxide is produced by adding nickel hydroxide particles to an aqueous solution of an alkali metal halide and stirring the mixture to prepare a nickel hydroxide slurry, and then converting a portion of the nickel hydroxide to nickel oxyhydroxide by electrolytic oxidation in the presence of the alkali metal halide.
Owner:TANAKA CHEM +1
Composition and energy storage device
A cathode composition is provided. The cathode composition includes at least one electroactive metal, wherein the electroactive metal is at least one selected from the group consisting of titanium, vanadium, niobium, molybdenum, nickel, iron, cobalt, chromium, manganese, silver, antimony, cadmium, tin, lead and zinc; a first alkali metal halide; an electrolyte salt comprising a reaction product of a second alkali metal halide and a metal halide, wherein the electrolyte salt has a melting point of less than about 300 degrees Centigrade; and a metal chlorosulfide compound having a formula (I) M1M2p+1SnCl4+3p-2n wherein “M1” is a metal selected from group IA of the periodic table, “M2” is a metal selected from group IIIA of the periodic table, “p” is 0 or 1, and “n” is equal to or greater than 0.5. An article and an energy storage device comprising the cathode composition is provided. A method of forming the energy storage device is provided.
Owner:GENERAL ELECTRIC CO
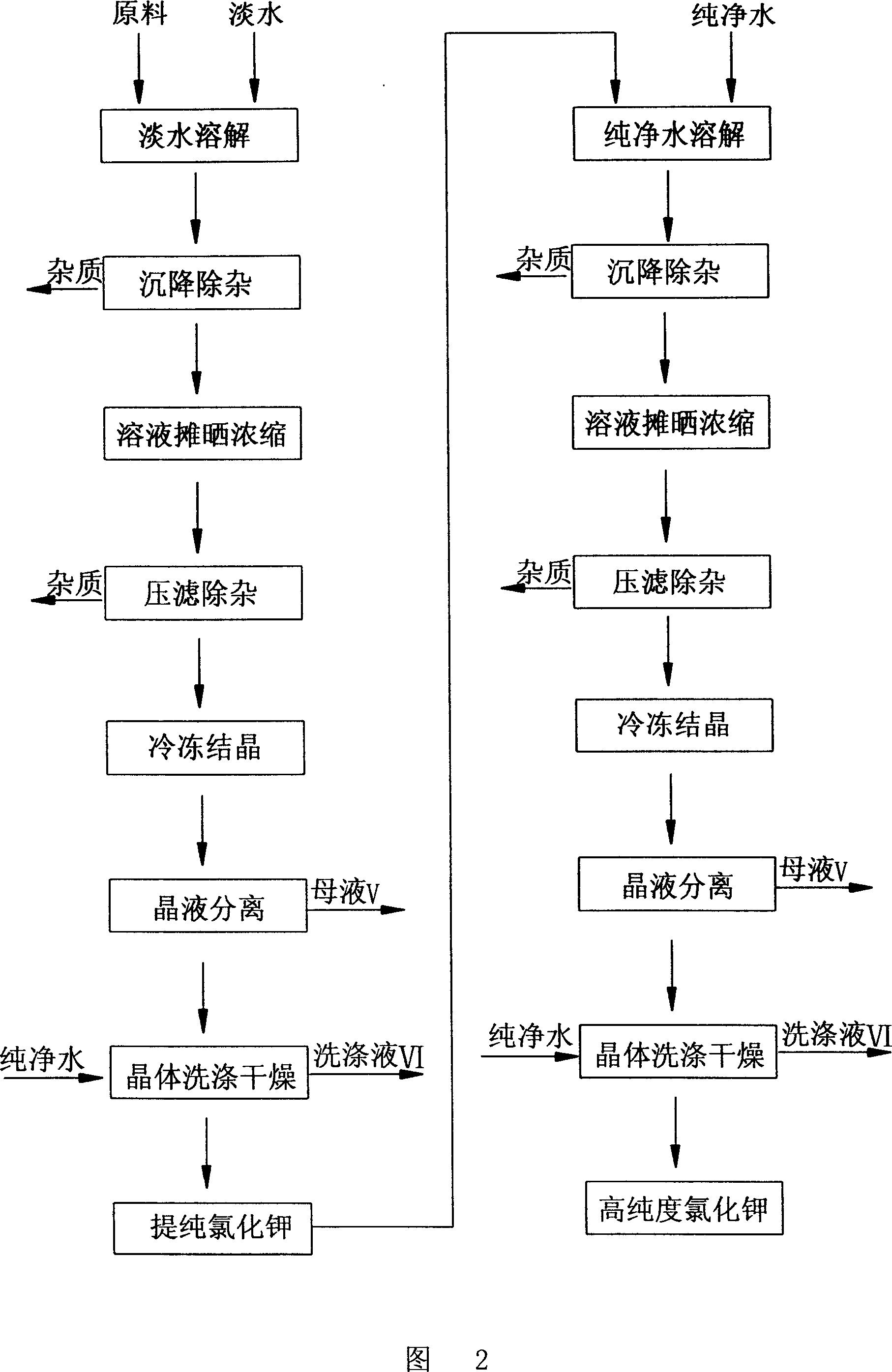
Process for producing high-purity potassium chloride
This invention relates to a producing technology for pure KCl, which takes KCl with the purity below 98% as the raw material characterizing in including steps of dissolving in fresh water, sedmentation and filtration, concentrating and removing trash, reducing the temperature and crystallizing and separation of liquid and crystals to get pure KCl over 99%, another method includes: dissolving in fresh water, sedmentation and filtration, reducing the temperature and crystallizing and separation of liquid and crystals and the whole production applies total blocking out continuous mode.
Owner:高崧耀
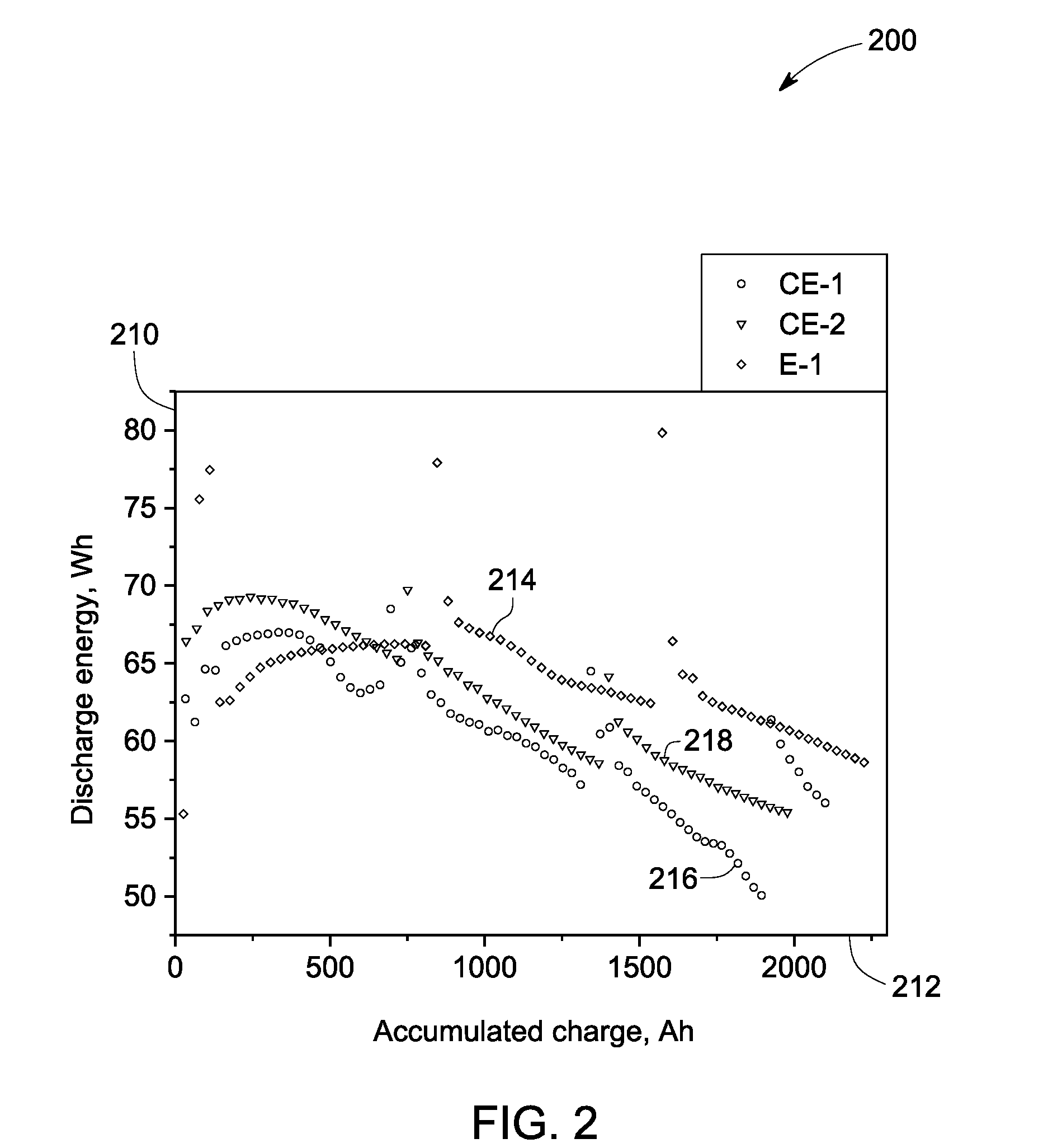
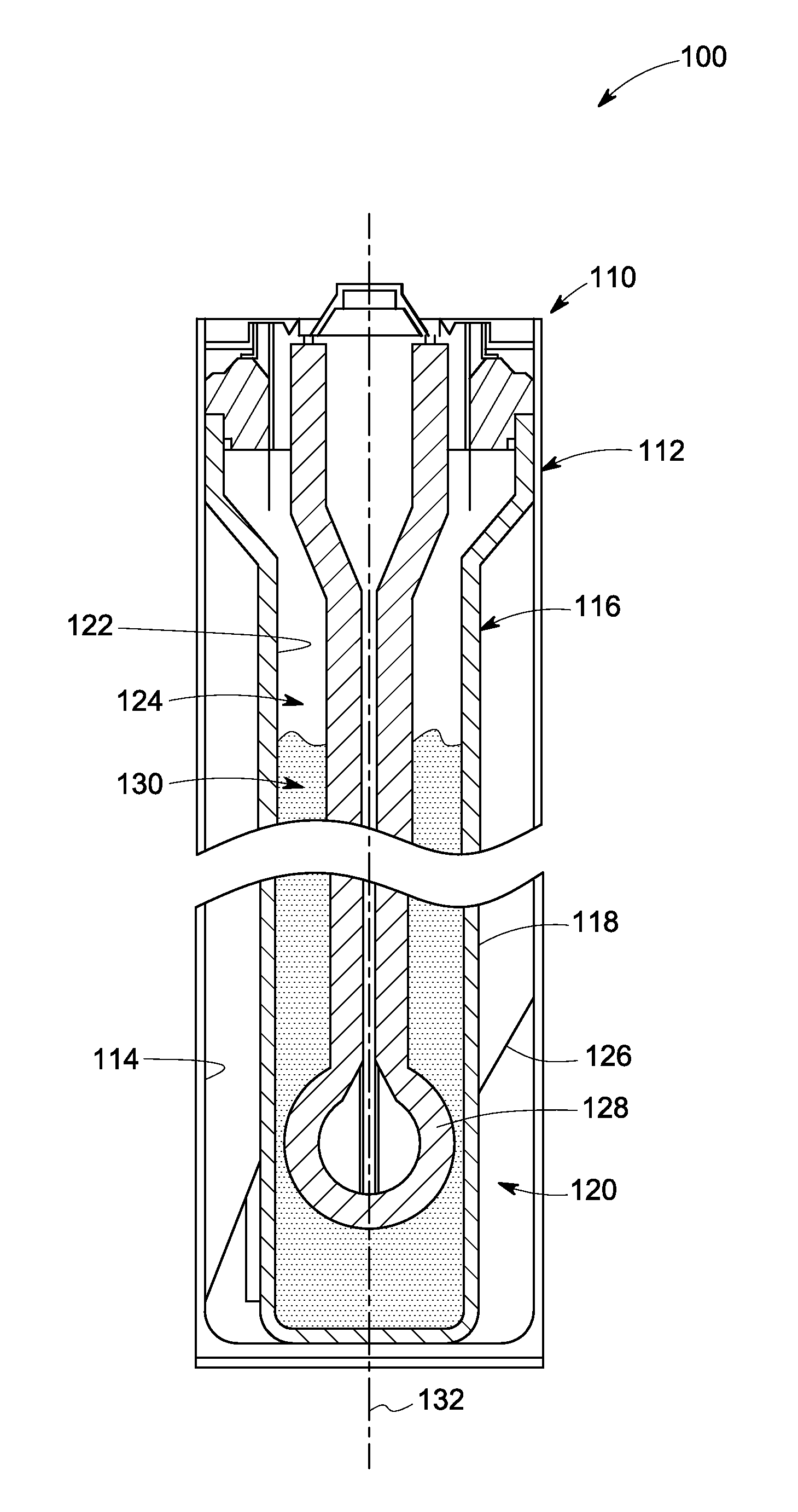
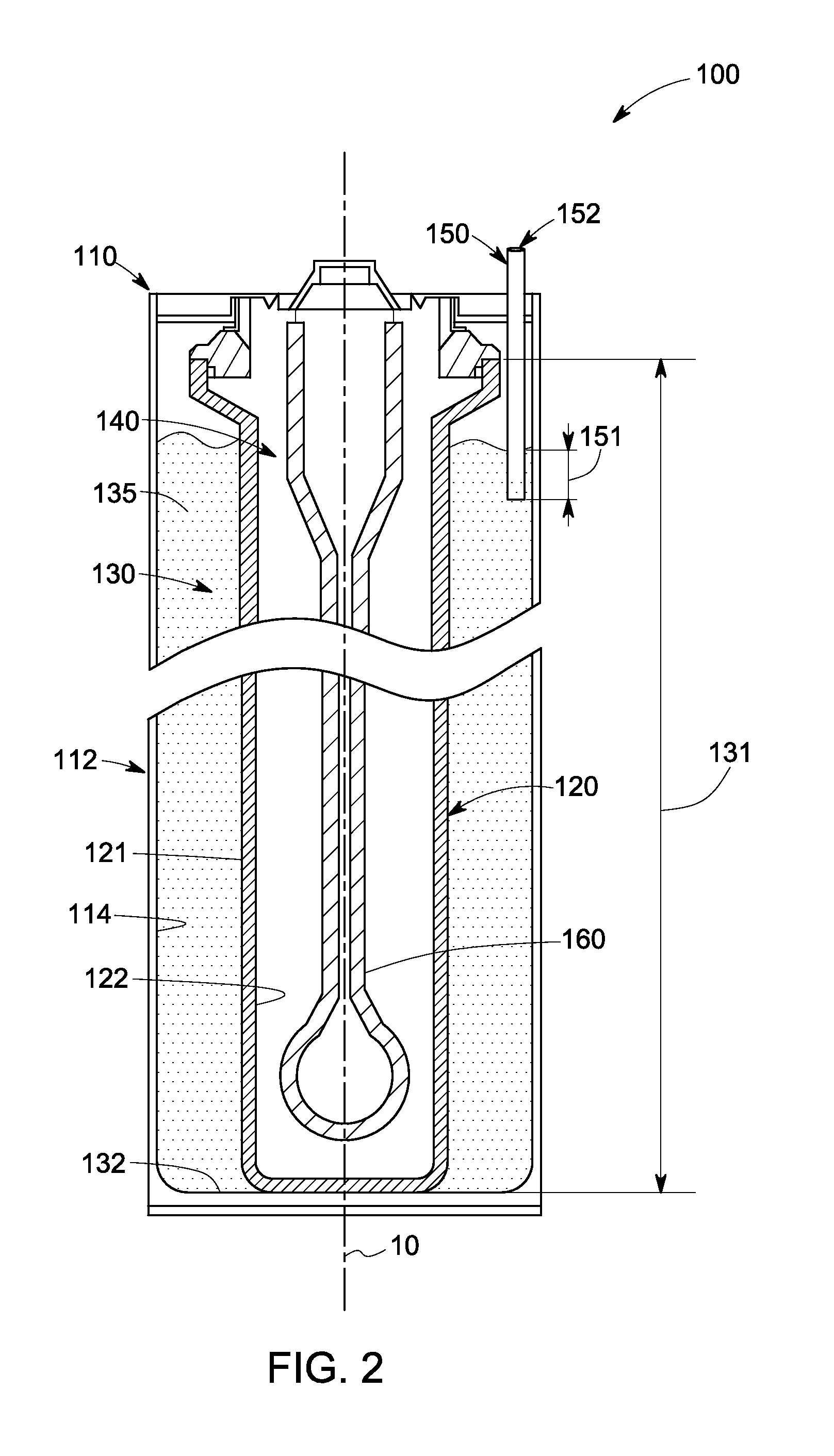
Electrochemical cells and related methods
An electrochemical cell is presented. The electrochemical cell includes an elongated ion-conducting separator defining at least a portion of a first compartment; a positive electrode composition disposed in the first compartment, the positive electrode composition comprising at least one electroactive metal, at least one alkali metal halide, and at least one electrolyte. A positive current collector is further disposed in the first compartment such that a portion of the positive current collector extends into the positive electrode composition, and a primary dimension of the extended portion of the positive current collector is less than about 20% of a primary dimension of the first compartment. A related method for the preparation of an electrochemical cell is also presented.
Owner:GENERAL ELECTRIC CO
Vapor deposition apparatus and method of vapor deposition making use thereof
InactiveUS20090098280A1Apparent advantageVacuum evaporation coatingSputtering coatingGas phaseLanthanide
A vapor deposition apparatus comprises as a vaporization assembly a container in form of a boat or crucible and a support for vapor depositing phosphor or scintillator material thereupon from raw materials present in said container, wherein said boat or crucible internally comprises an assembly of two perforated covers or lids, one of which is an outer lid (also called first lid) more close to the said support and the other cover is an inner lid (also called second lid) more close to the bottom of the said crucible; and wherein perforations present in said outer lid represent a total surface exceeding the total surface of perforations present in said inner lid more close to the bottom of the said crucible and wherein in said vapor deposition apparatus the said raw materials or the bottom of the said crucible cannot be directly seen through said perforations from any point of said support; thereby providing the manufacturing of a radiation image storage phosphor layer on a support or substrate, by a vapor depositing step of raw materials of an alkali metal halide salt and a lanthanide dopant salt or a combination thereof in order to ensure vapor deposition of a binderless needle-shaped storage phosphor layer in the said vapor deposition apparatus, so that a ratio between the total surface of perforations in said inner lid more close to the bottom of crucible and the total surface of perforations in said outer lid more close to the support is not more than 1.0.
Owner:T2PHARMA GMBH
Si surface cleaning for semiconductor circuits
ActiveUS7994066B1Semiconductor/solid-state device manufacturingSemiconductor devicesSurface cleaningEngineering
A method is disclosed for the cleaning of a Si surface at low temperatures. Oxide on the Si surface is brought into contact with Ge, which then sublimates off the surface. The Ge contamination remaining after the oxide removal is cleared away by an exposure to an alkali halide. The disclosed cleaning method may by used in semiconductor circuit fabrication for preparing surfaces ahead of epitaxial growth.
Owner:CISCO TECH INC
Novel imidazoline compound corrosion inhibitor and preparation method thereof
The invention provides a novel imidazoline compound corrosion inhibitor and belongs to the field of metal corrosion inhibition. The corrosion inhibitor aims at solving the problems that most oxidation film type corrosion inhibitors and precipitating film type corrosion inhibitors contain heavy metal or the phosphorus element, are large in toxicity and severely pollute the environment. The effective components of the corrosion inhibitor are formed by compounding, by mass, 10% to 30% of thiourea derivatives, 5% to 30% of alkali halide, 30% to 80% of imidazoline-ammonium-salt derivatives and 0 to 15% of urotropin. Raw materials or components of the novel imidazoline compound corrosion inhibitor prepared through the process are simple and easy to obtain and free of or low in toxicity, the characters of molecules of all the components are greatly different, the radii of the molecules of all the components are greatly different as well, and the complementary performance is high; all the components can be adsorbed to the metal surface after being compounded to form a dense monomolecular film; and the corrosion inhibitor has the beneficial effects of being good in universality, small in use amount, high in efficiency, low in toxicity, good in stability, simple in preparation process, low in cost and the like.
Owner:SHENZHEN GCD PETROLEUM ADDITIVE CO +1
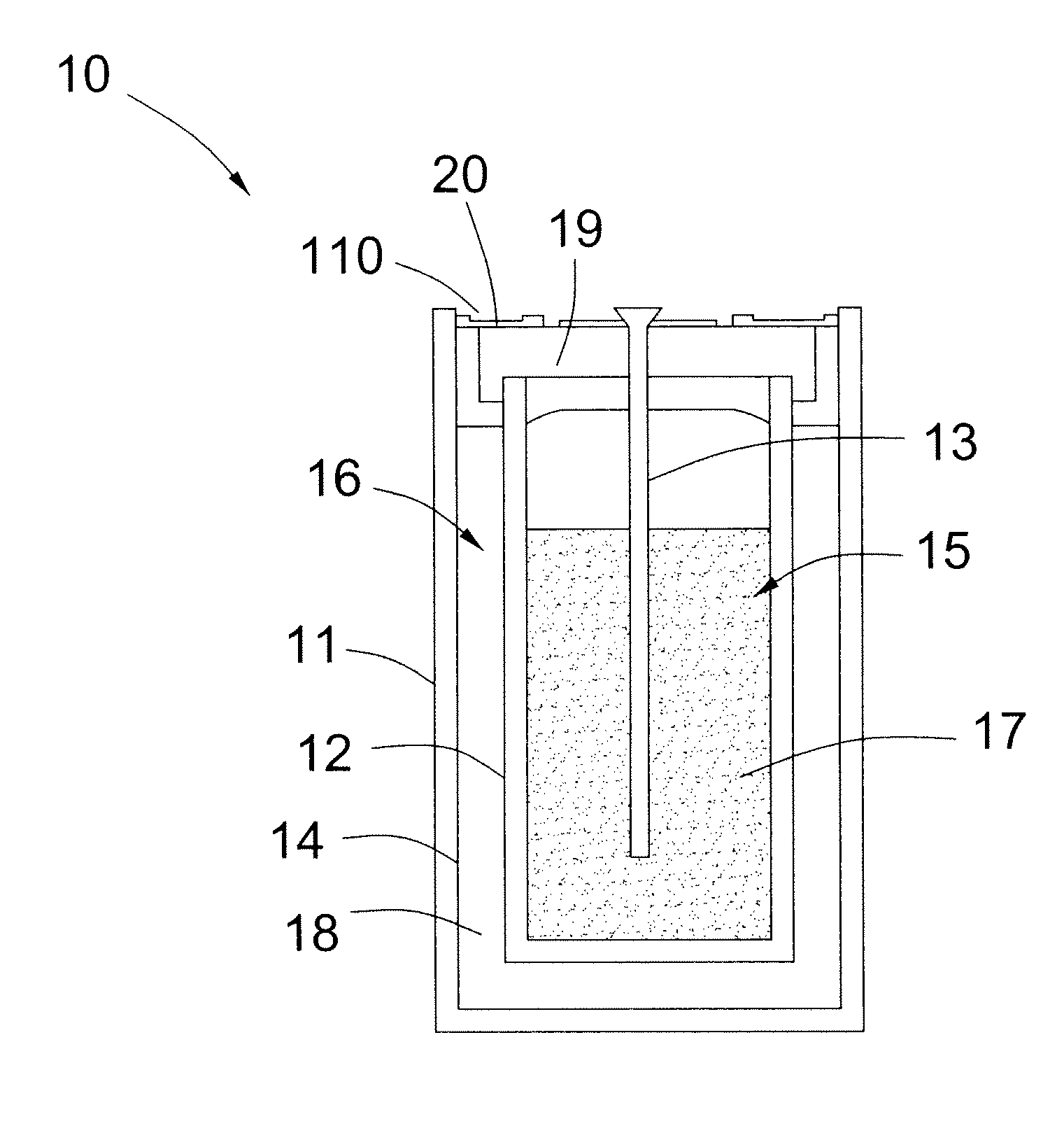
Energy storage device and associated method
ActiveUS20100086834A1Reduce pressureFinal product manufactureSecondary cellsAlkali metal halideEnergy storage
An energy storage device is provided that includes a separator having a first surface and a second surface. The first surface defines at least a portion of a cathodic chamber, and the second surface defines an anodic chamber. The cathodic chamber includes an alkali metal halide that forms an ion that is capable of conducting through the separator. The anodic chamber has a volume that is filled with a consumable fluid. The amount of the consumable fluid is greater than 90 percent by volume of the anodic chamber volume. Furthermore, the consumable fluid is reactive with an ionic species of the alkali metal halide. A method of sealing the energy storage device is also provided.
Owner:GENERAL ELECTRIC CO
Process for production of polyarylene sulfide
There is provided a method for producing a poly(arylene sulfide) in which a dihalo-aromatic compound and an alkali metal halide are polymerized by heating in an organic amide solvent, wherein the cooling time for the polymerization reaction system is significantly reduced. After the polymerization process, there is provided a cooling process for cooling the polymerization reaction system comprising a liquid phase containing the product poly (arylene sulfide) and the organic amide solvent and a vapor phase containing a gas component (A); and in the cooling process, the gas component (A) in the vapor phase is cooled while the content of a low boiling gas component (A1), which has a lower boiling point than water and exists in the gas component (A), is reduced in the vapor phase of the polymerization system.
Owner:KUREHA KAGAKU KOGYO KK
Ceramic metal halide lamp
InactiveUS7268495B2Easy maintenanceImprove performanceSolid cathode detailsGas discharge lamp detailsAlkaline earth metalRare earth
A metal halide lamp (10) includes a discharge vessel (12) which may be formed of a ceramic material. The vessel defines an interior space (16). An ionizable fill (17) is disposed in the interior space. The ionizable fill includes an inert gas, mercury, and a halide component. The halide component includes an alkali metal halide, an alkaline earth metal halide component, and optionally at least one of a rare earth halide and a Group IIIA halide. The alkaline earth metal halide component includes at least one of a barium halide and a strontium halide. At least one electrode (18, 20) is positioned within the discharge vessel so as to energize the fill when an electric current is applied thereto. The lamp having a wall loading, when energized, which is sufficient to maintain an active tungsten halogen cycle.
Owner:GENERAL ELECTRIC CO
Process for synthesizing dibutyl carbonate
InactiveCN1569811AEasy to separateImprove conversion ratePreparation from organic carbonatesMolecular sieveAlkali metal halide
The invention discloses a process for synthesizing dibutyl carbonate through the catalysis of load type catalyst, wherein alkali metal hydroxide, alkali metal halides, alkali metal carbonates are loaded onto active aluminium oxide, activated charcoal, or molecular sieve carrier through vacuum dipping method, thus obtaining solid catalyst.
Owner:NINGXIA UNIVERSITY
Method for recovering potassium chloride from abandon mine of salt lake
This invention discloses a method for recovering KCl from waste ore of salt lake. The method comprises: utilizing waste ore of salt lake as the raw material, dissolving in solvent, removing the solid phase, adjusting Mg content, clarifying the precipitate, and solarizing to obtain high-purity carnallite. The method expands the exploitation range of potassium ore, and extends the service life of salt lake.
Owner:陈天强 +1
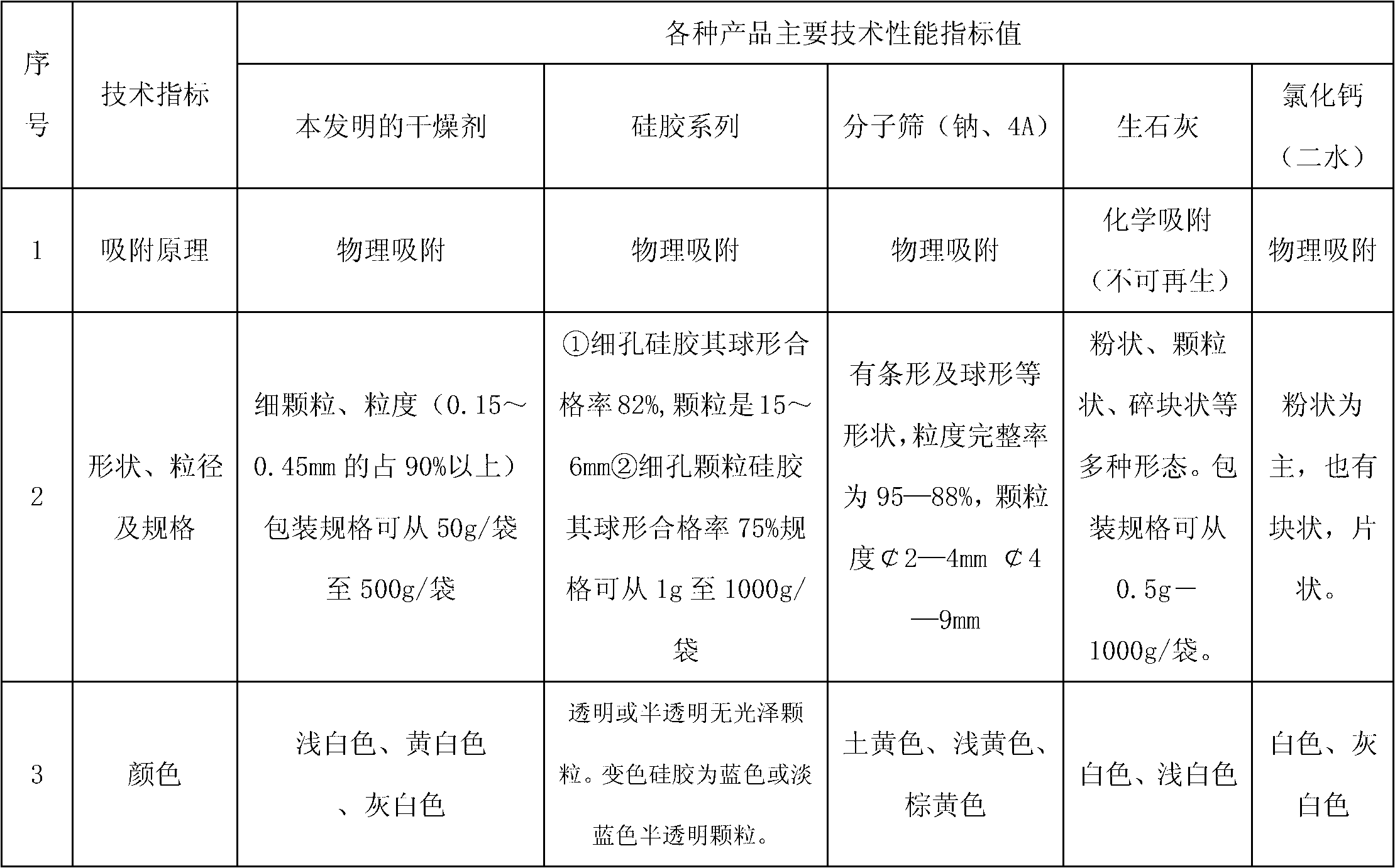
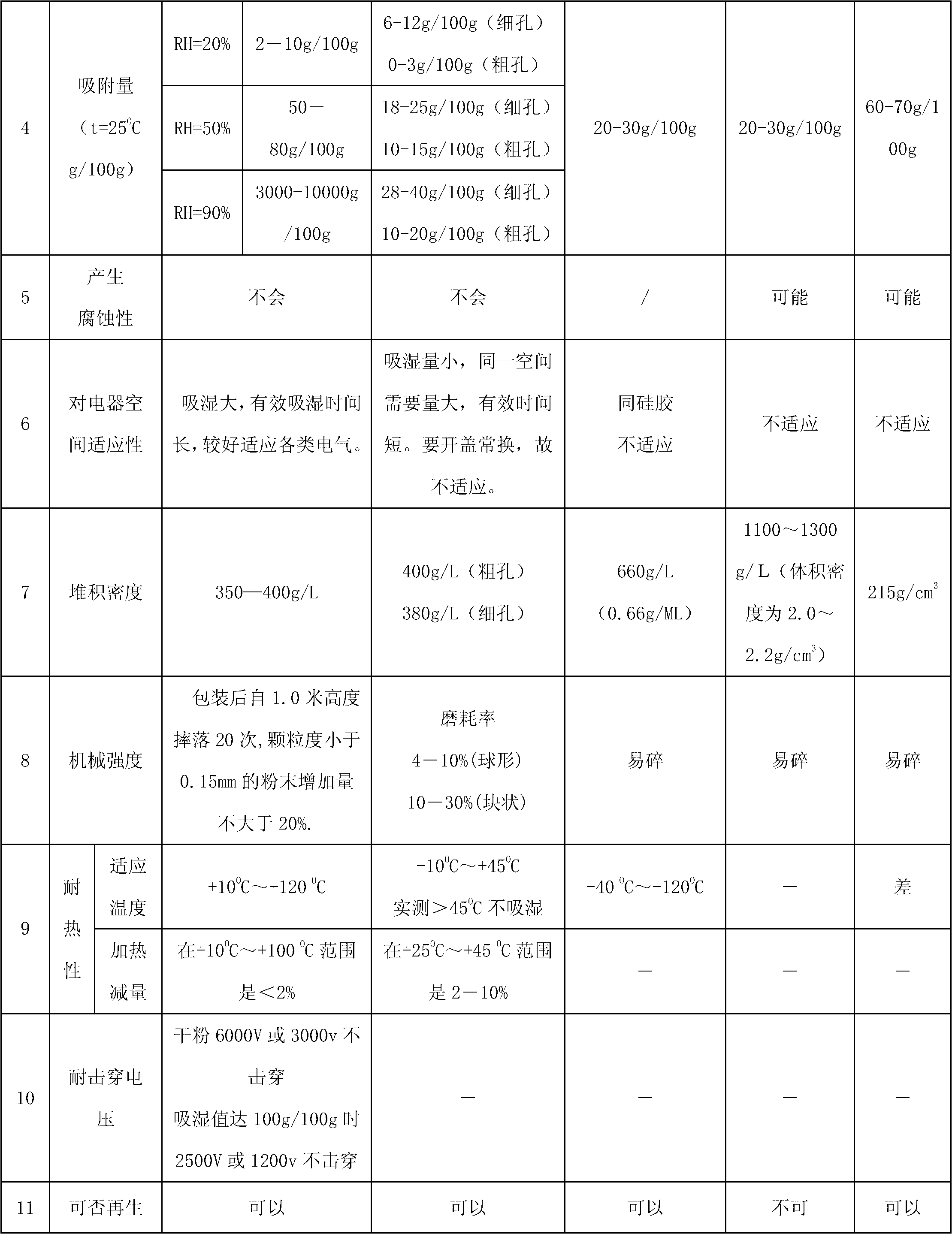
Drying agent for mine underground electric apparatus and preparation method thereof
ActiveCN102698573ANot corrosiveNo tendency to explodeDispersed particle separationLow voltageAlkali metal halide
The invention discloses a drying agent for an mine underground electric apparatus and a preparation method of the drying agent, belonging to the field of drying agent, wherein macromolecule water absorbent, compound of silicon or calcium and alkali halide or modified starch are mixed so as to prepare the powdery draying agent according to part by weight; the macromolecule water absorbent is 35-70 parts; the compound of silicon or calcium is 25-55 parts; the alkali halide or a mixture of the alkali halide and the modified starch is 10-15 parts; the grain size of the macromolecule water absorbent is 0.20-0.40mm; and the drying agent is powdery. According to the invention, raw material is wide in source and is purchased easily on the market; the drying agent is used for high / low-voltage power supply appliances worked on a mine underground high-humidity condition (RH=80-100%); the dry powder resists 6000V voltage or does not be broken down under voltage of 3000V; when moisture rate reaches to 1g / g, the dry powder resists 2500V voltage or does not be broken down under voltage of 1200V.
Owner:徐州意创化工科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com