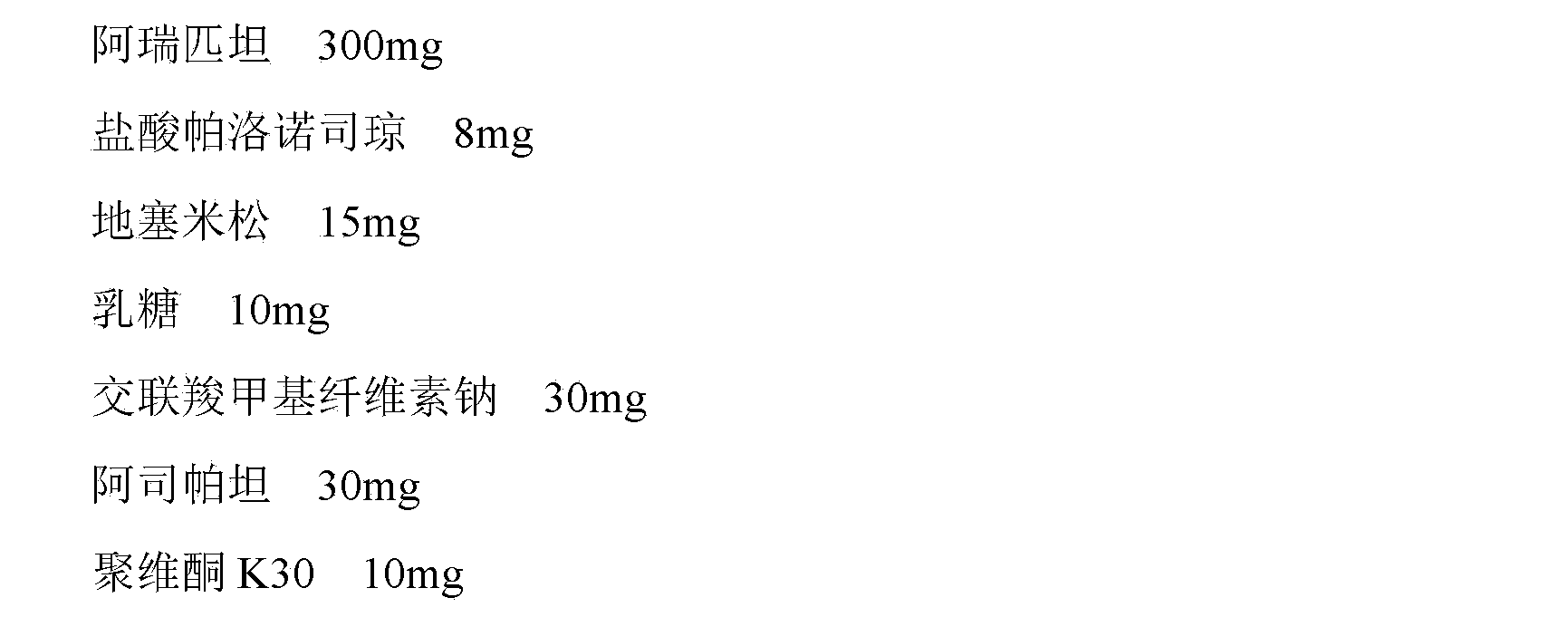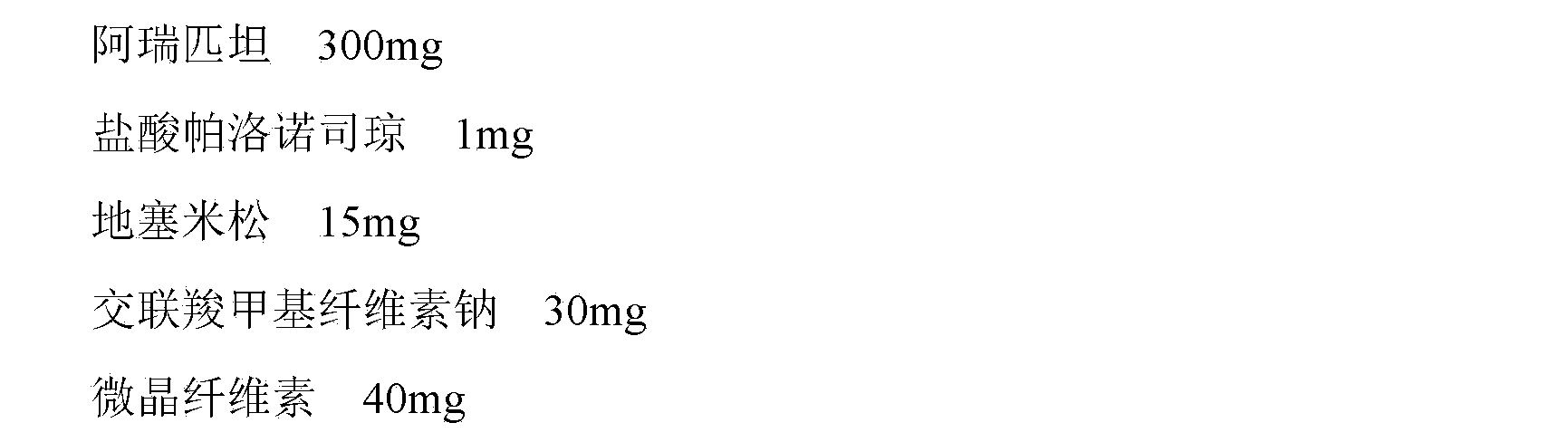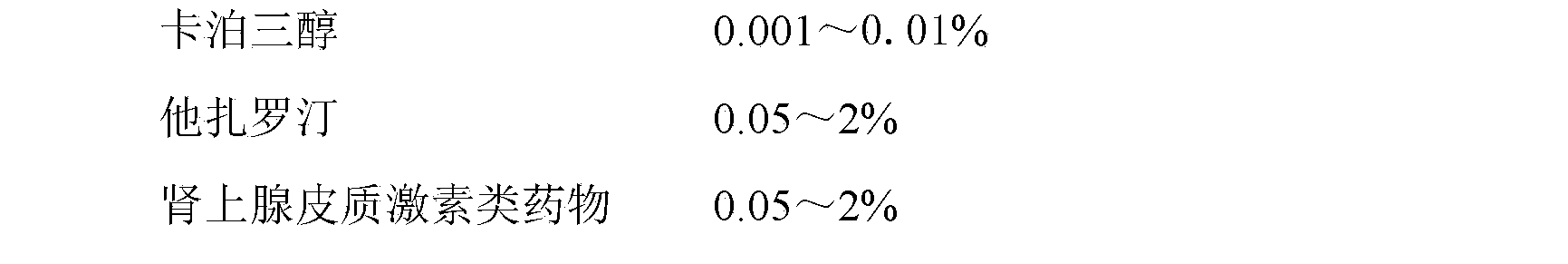Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Adrenocortical hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In humans and other animals, the adrenocortical hormones are hormones produced by the adrenal cortex, the outer region of the adrenal gland. These polycyclic steroid hormones have a variety of roles that are crucial for the body’s response to stress (for example, the fight-or-flight response), and they also regulate other functions in the body. Threats to homeostasis, such as injury, chemical imbalances, infection, or psychological stress, can initiate a stress response. Examples of adrenocortical hormones that are involved in the stress response are aldosterone and cortisol. These hormones also function in regulating the conservation of water by the kidneys and glucose metabolism, respectively.
Method for extracting compounds with pregnane mother nucleus structure from compositions
ActiveCN104198609AEfficient extractionEfficient removalComponent separationSolventComponents of crude oil
The invention particularly relates to a method for extracting compounds with a pregnane mother nucleus structure from compositions, belonging to the technical field of analytical chemistry. According to the method, inorganic salt is added into acetonitrile or an acetonitrile water solution which serves as a basic solvent so as to improve oil-water partition coefficients, the temperature is lowered to accelerate separation of auxiliary materials / matrixes in the compositions, and the separation of immiscible components such as oil and water is accelerated by centrifugation. The method has the beneficial effects that the universality is wide, the compounds can be effectively extracted without being damaged, and the subsequent analysis and detection cannot be interfered by the used solvent; the obtained solution can be directly utilized for carrying out high performance liquid phase analysis, and an obtained atlas has clean base lines, symmetric peak shapes and high number of theoretical plates; meanwhile, a sample solution is relatively stable, and more related substances can be detected; the method can be widely applied to the quality control of the compositions containing adrenocortical hormone components.
Owner:CHONGQING HUAPONT PHARMA
Medicinal composition for treating emesis
ActiveCN103520725AImprove complianceDigestive systemHeterocyclic compound active ingredientsCancer RadiotherapyDrug
The invention provides a medicinal composition containing a neurokinin 1(NK-1) receptor antagonist, a 5-hydroxytryptamine 3 (5HT-3) receptor antagonist and adrenocortical hormone. The medicinal composition adopts a NK-1 receptor antagonist, a 5HT-3 receptor antagonist and adrenocortical hormone as active components, is added with certain auxiliaries with specific spices and proportion to prepare and develop an oral preparation according to a technical means specified in the invention. The medicinal composition can be used for preventing and treating nausea and emesis caused by cancer radiotherapy, chemotherapy and operation. The invention aims to provide a medicinal composition preparation which can prevent and treat emesis more conveniently by utilizing the NK-1 receptor antagonist, the 5HT-3 receptor antagonist and adrenocortical hormone, thereby improving the compliance of a patient.
Owner:HAISCO PHARMA GRP INC
Traditional Chinese medicine decoction for treating female endocrine disorders and preparation method thereof
InactiveCN103495055AReturn to normal functional statusGood treatment effectFish material medical ingredientsEndocrine system disorderSalvia miltiorrhizaNervous system
The invention discloses a traditional Chinese medicine decoction for treating female endocrine disorders and a preparation method thereof, belonging to the field of traditional Chinese medicines. The active ingredients of the medicine are prepared from the following raw material medicines in parts by weight: 40-60g of mulberry, 25-32g of astragalus, 20-27g of dogwood, 14-18g of root of rehmannia, 15-20g of the seed of Chinese dodder, 12-17g of Chinese angelica, 10-16g of salvia miltiorrhiza, 9-13g of medlar, 10-15g of cortex albiziae, 11-15g of honeysuckle, 8-12g of fallopia japonica, 7-10g of verbena officinalis, 8-11g of radix ophiopogonis, 6-10g of lalang grass rhizome, 5-10g of Chinese yam, 3-6g of fructus cnidii, 4-7g of donkey-hide gelatin, 2-4g of sea horse and 1-2g of poria cocos. The selected medicines are compatible in composition, multiple traditional Chinese medicinal materials are elaborately selected and matched aiming at characterizations of qi and blood stagnation, pathogenic toxin invasion and qi-blood deficiency causing endocrine disorders, yin nourishing treatment is performed according to aspects of excess, deficiency, yin, yang, qi and blood, the nervous system is regulated, a normal function state of the organism is restored, and the traditional Chinese medicine decoction has an excellent treatment effect on abnormal endocrine disorder symptoms of sex hormone, adrenocortical hormone, insulin and thyroid gland.
Owner:许颖
Application of Loureirin B in preparation of Kv1.3 channel blocker
InactiveCN104523663AClear ingredientsQuality is easy to controlMetabolism disorderAntipyreticDiseaseSide effect
The invention discloses an application of Loureirin B in the preparation of a Kv1.3 channel blocker and provides a new drug approach for treating autoimmune diseases. The Loureirin B contains clear components and quality of the Loureirin B is controllable. The Loureirin B can effectively inhibit a Kv1.3 potassium channel, thus effectively treating various autoimmune diseases. Through various verification means of patch clamp experiment, calcium imaging techniques, ELISA assay and manufacturing of animal models of autoimmune encephalitis, rheumatoid arthritis and the like, it is found that the Loureirin B has a good therapeutical effect on autoimmune diseases at the overall level, can effectively overcome side effects of traditional therapeutics such as adrenocortical hormone and the like and can maintain normal protective immune responses of patients.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Preparation method of (R)-budesonide
The invention relates to a preparation method of adrenocortical hormone agents, and particularly discloses a preparation method of (R)-budesonide. 11beta,21-dihydroxy-16alpha,17- [(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione is taken as an initial raw material, and the budesonide (R-isomer) is obtained through exchange and splitting. The preparation method of the (R)-budesonide is simple in process, high in yield and suitable for industrial production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
External use medicine composition and preparing method thereof
InactiveCN104338118ASmooth appearanceDelicate appearancePeptide/protein ingredientsDermatological disorderEffective treatmentAstressin-B
The invention belongs to the technical field of medicine compositions, and discloses an external use medicine composition used for treating alopecia. The external use medicine composition comprises annular adrenocorticotrophic hormone release factor (CRF) antagonism peptide astressin B in effective treatment amount or acceptable salt on the pharmacy. The external use medicine composition is in a gel dosage form preferably and suitable for clinic use and industrialization production. In addition, the invention further discloses a method for preparing the external use medicine composition and application of the astressin B in effective treatment amount or the acceptable salt on the pharmacy to preparing the external use medicine composition used for treating the alopecia. According to the external use medicine composition, the problem of the alopecia for a long time can be effectively treated; and the external use medicine composition is safe, reliable, free of obvious skin irritants and free of sensitization.
Owner:HYBIO PHARMA
Methods for alleviating symptoms associated with menopause using sensory regimen
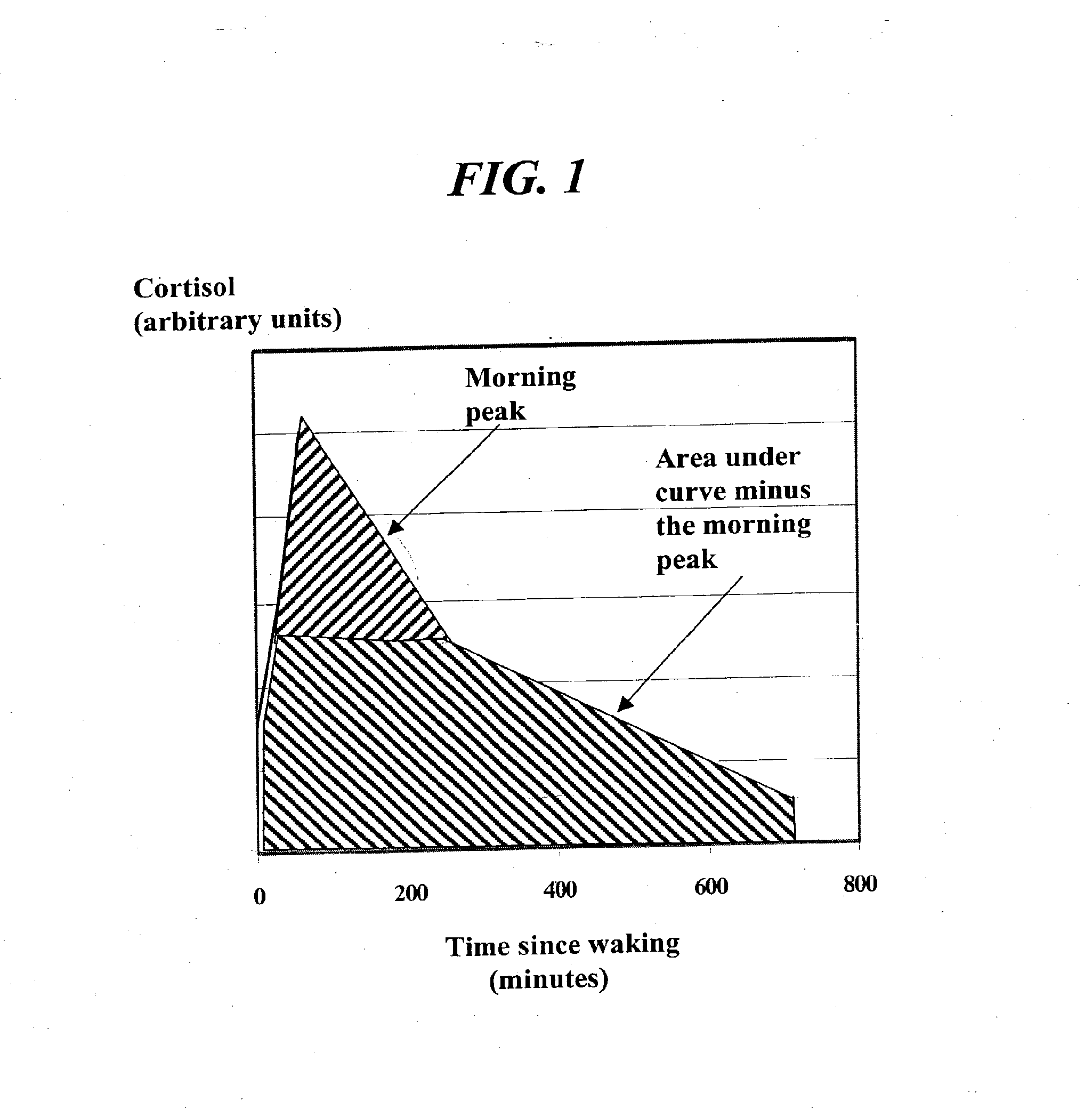
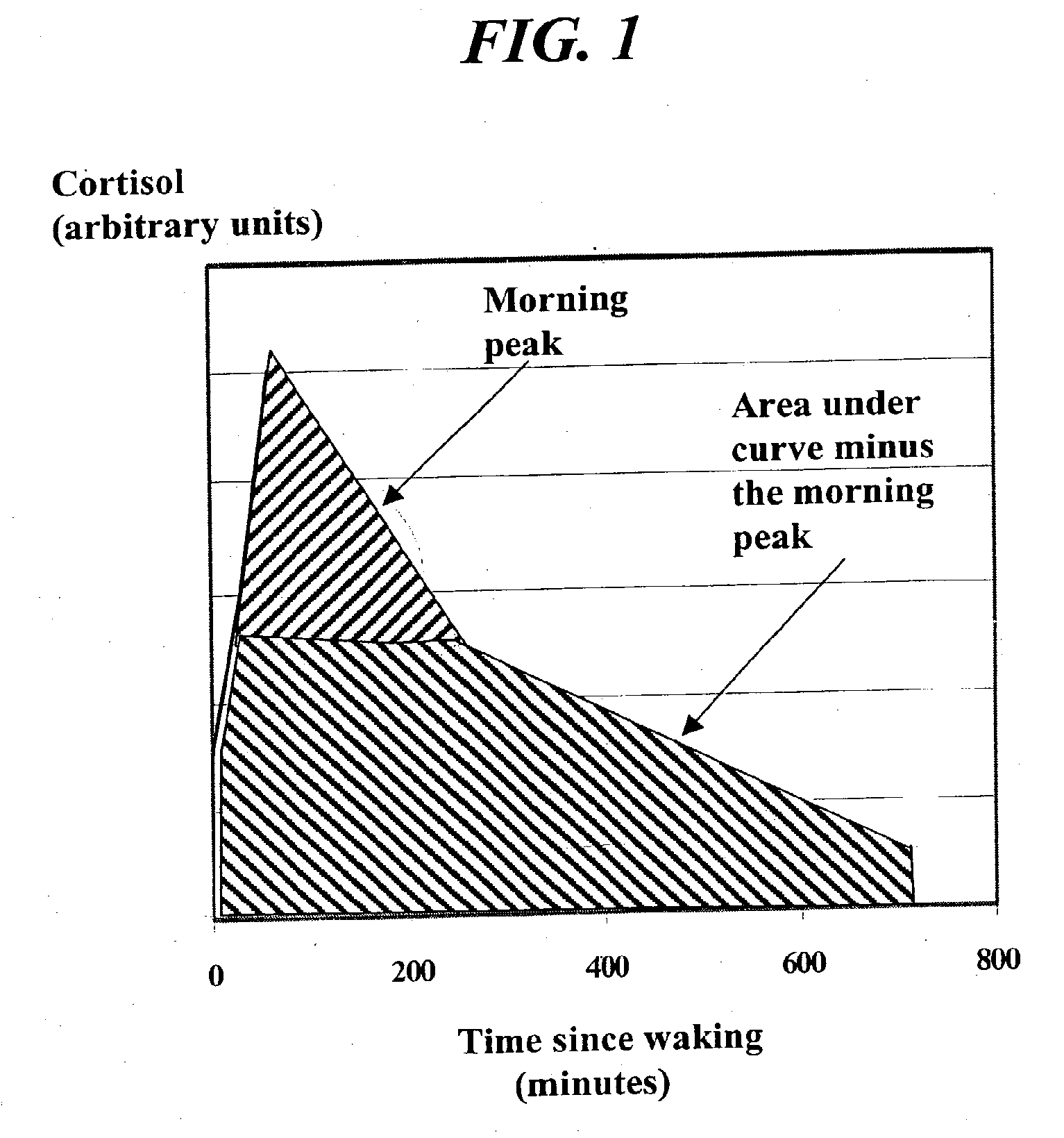
The invention relates to a method for alleviating on or more of the symptoms associated with menopause in a woman in the peri-menopause or menopause stage, said method comprising the step of administering a sensory regimen in an amount effective to downregulate the activity of the HPA axis of said woman; wherein said HPA axis comprises: a) levels of adrenocortical hormone present as a function of time in said woman; b) a total daily amount of adrenocortical hormone; c) an integrative measure of morning peak adrenocortical hormone; and d) an onset of sleep threshold; wherein said sensory regimen is selected from the group consisting of auditory stimuli, visual stimuli, tactile stimuli, gustatory stimuli, olfactory stimuli, and combinations thereof.
Owner:WIEGAND BENJAMIN +1
Specification of functional cranial placode derivatives from human pluripotent stem cells
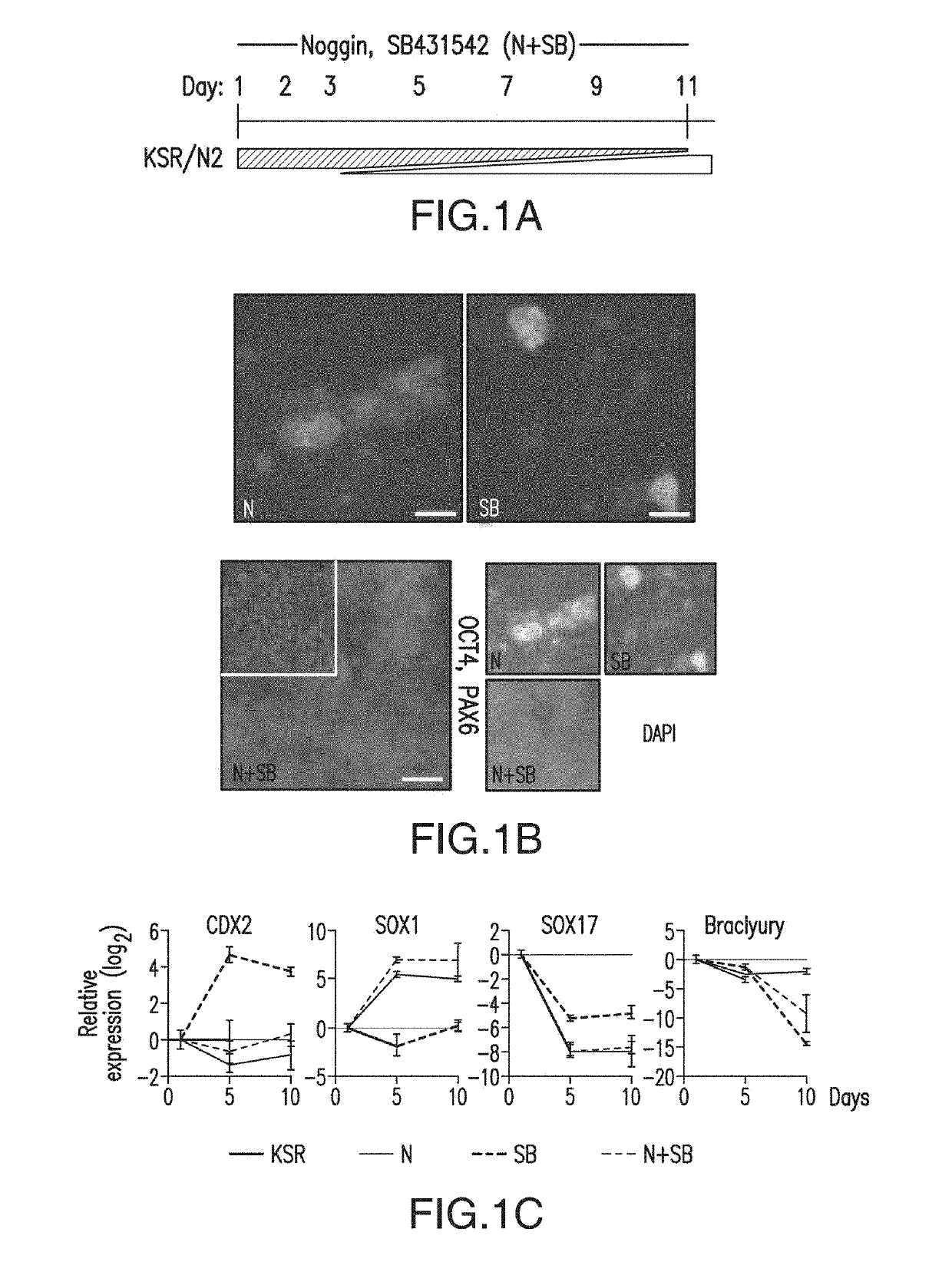
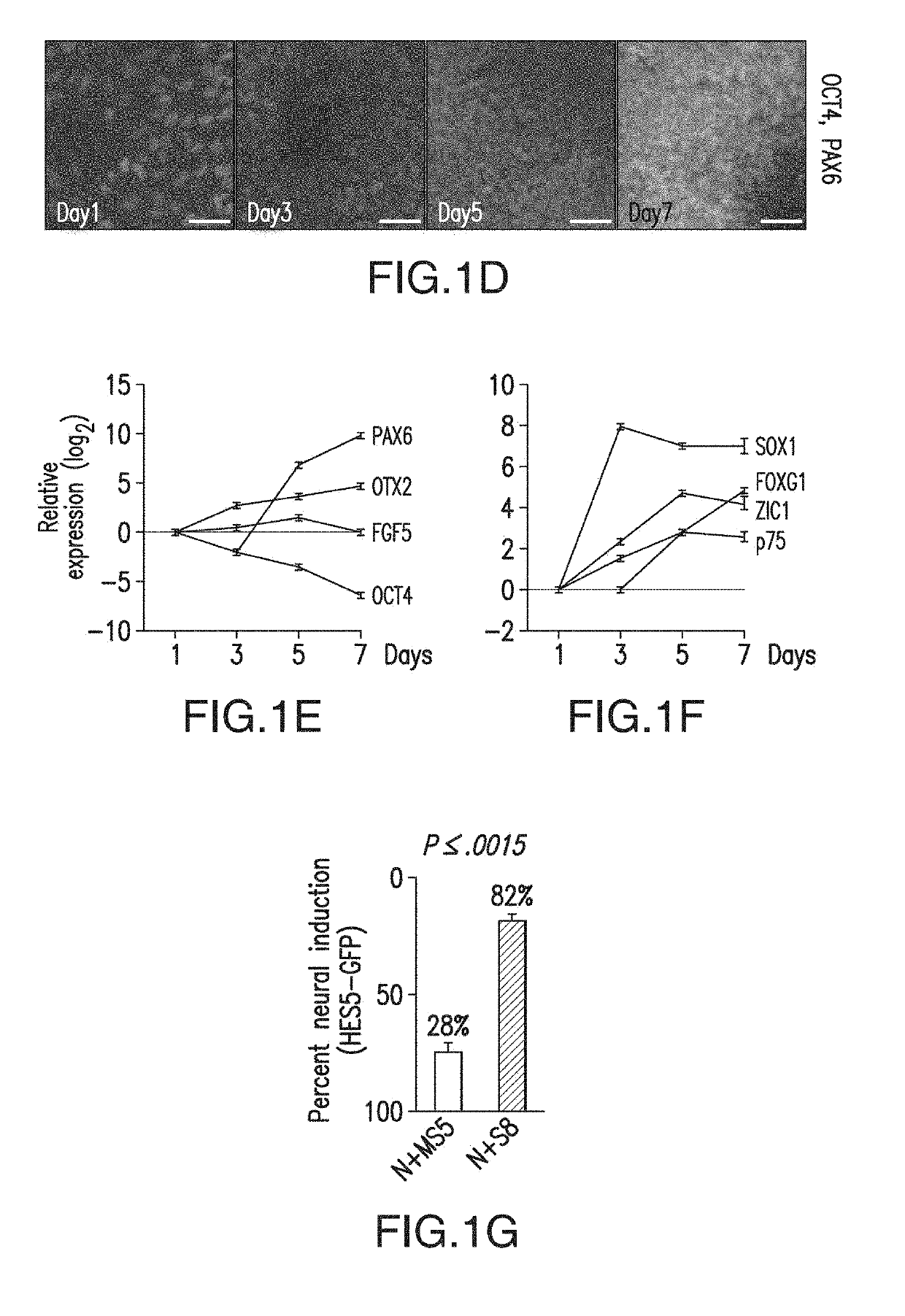
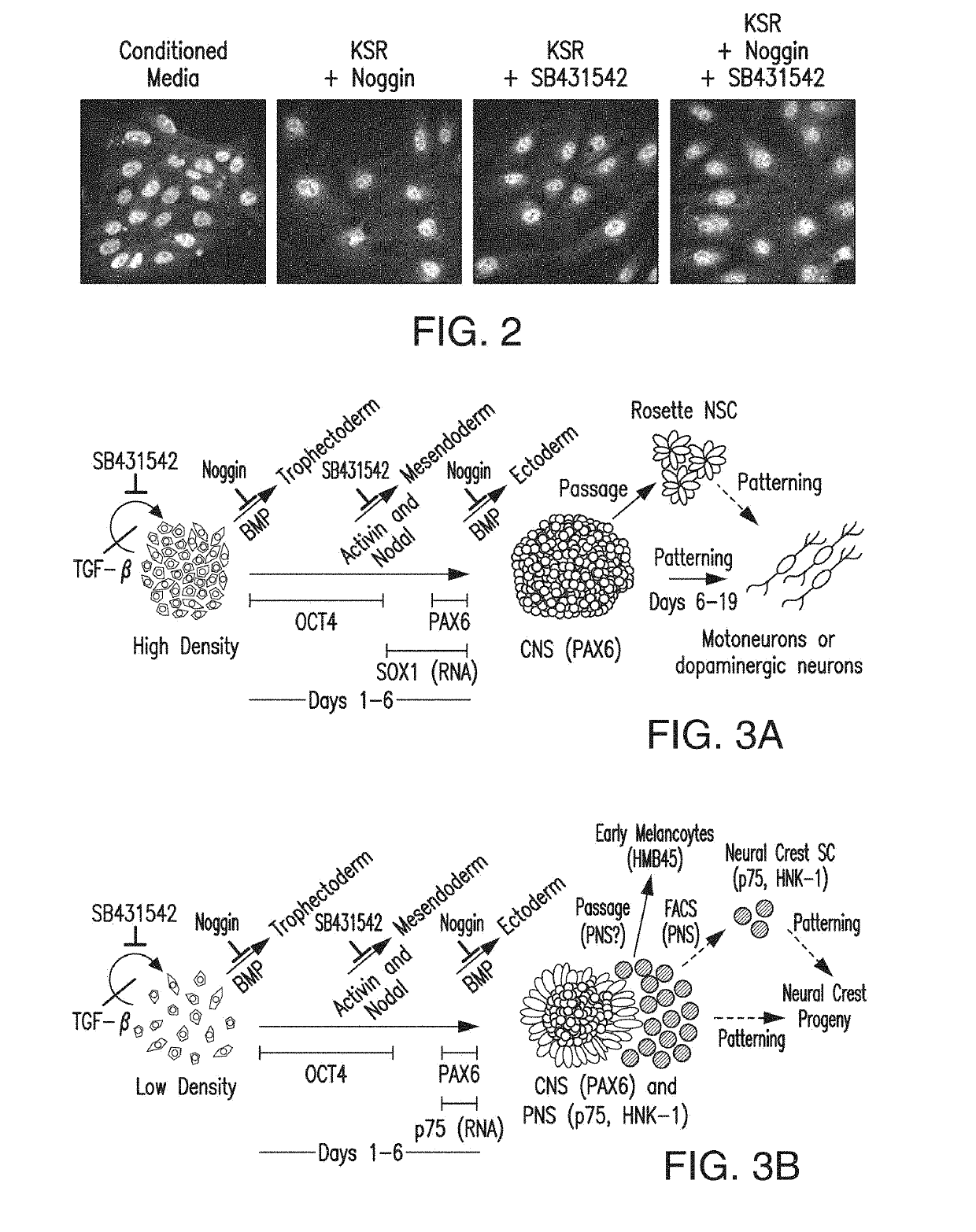
ActiveUS10273452B2Reduce functionInhibit phosphorylationSenses disorderNervous disorderInduced pluripotent stem cellLens Fiber
Cranial placodes are embryonic structures essential for sensory and endocrine organ development. The efficient derivation of cranial placodes from human pluripotent stem cells is disclosed where the timed removal of the BMP inhibitor Noggin, a component of the dual-SMAD inhibition strategy of neural induction, triggers placode induction at the expense of CNS fates. Further fate specification at the pre-placode stage enables the selective generation of placode-derived trigeminal ganglia capable of in vivo engraftment, mature lens fibers and anterior pituitary hormone-producing cells that upon transplantation produce hormones including, but not limited to, human growth hormone and adrenocortiocotropic hormone in vivo. Alternatively, anterior pituitary hormone-producing cells are generated in cell culture systems in vitro.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Method for induction of t cells from pluripotent stem cells
ActiveUS20170326175A1Efficient production of CD8-positiveEfficient productionCulture processMammal material medical ingredientsVitamin CInduced pluripotent stem cell
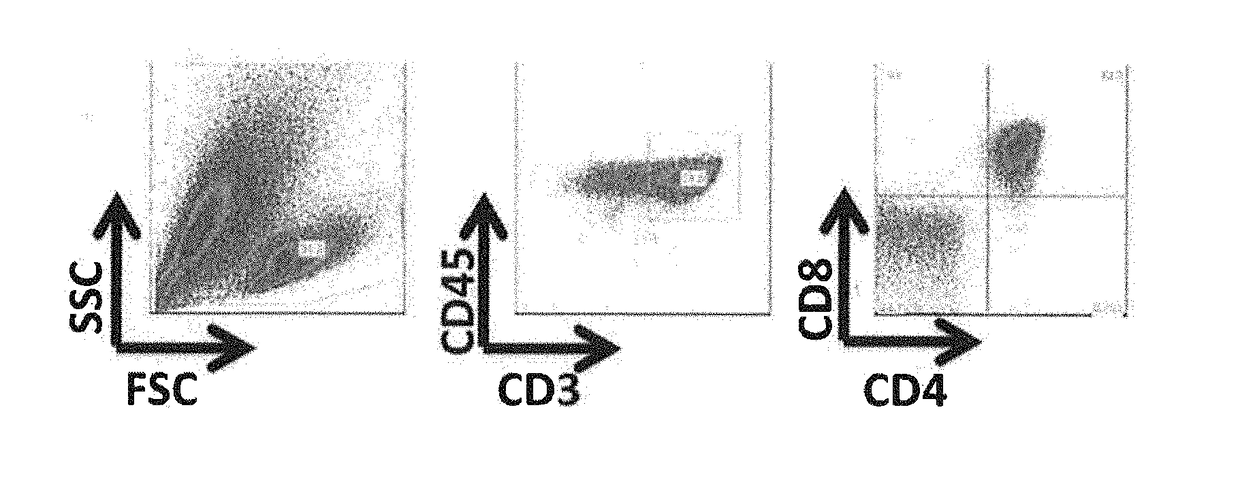
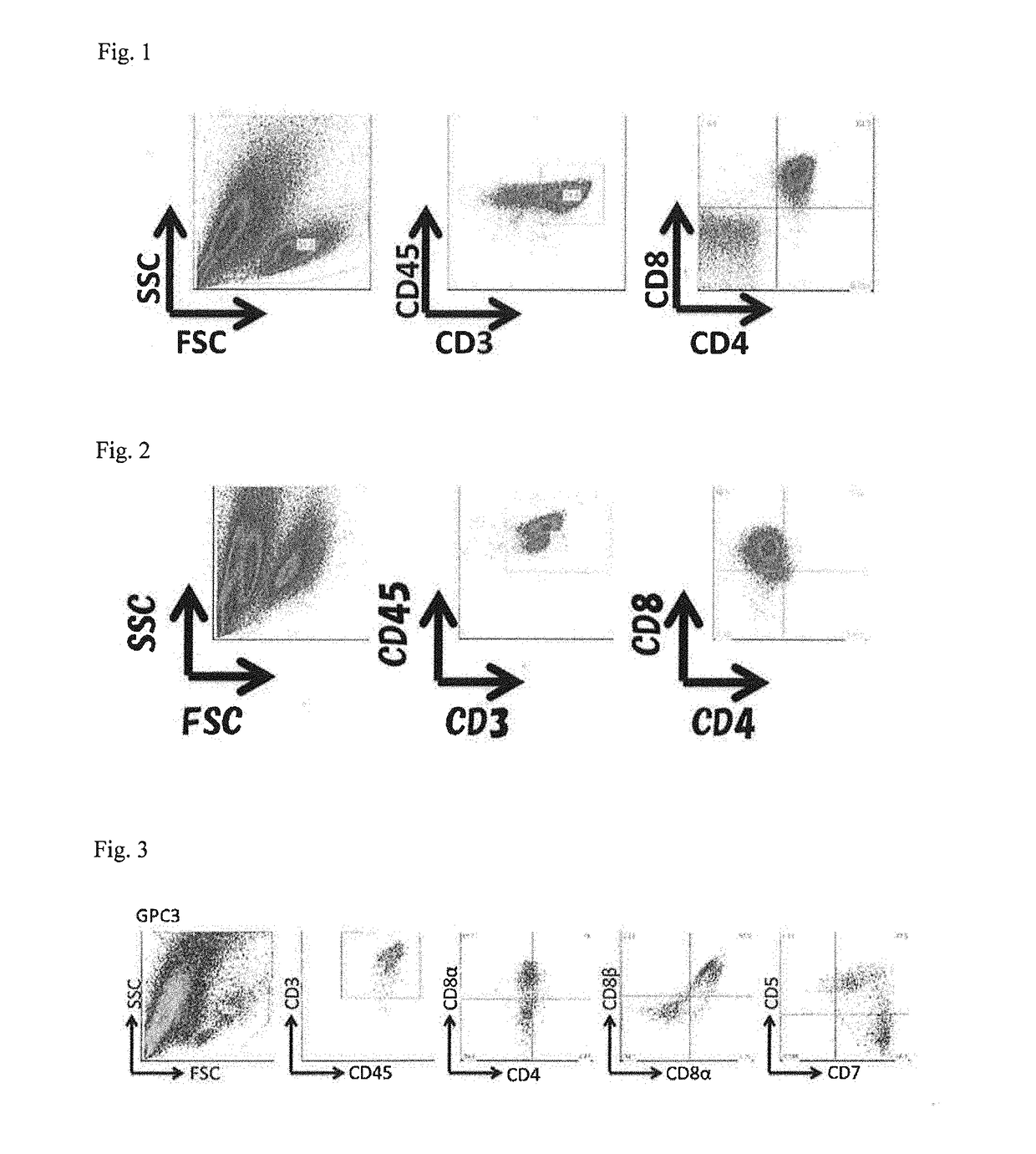
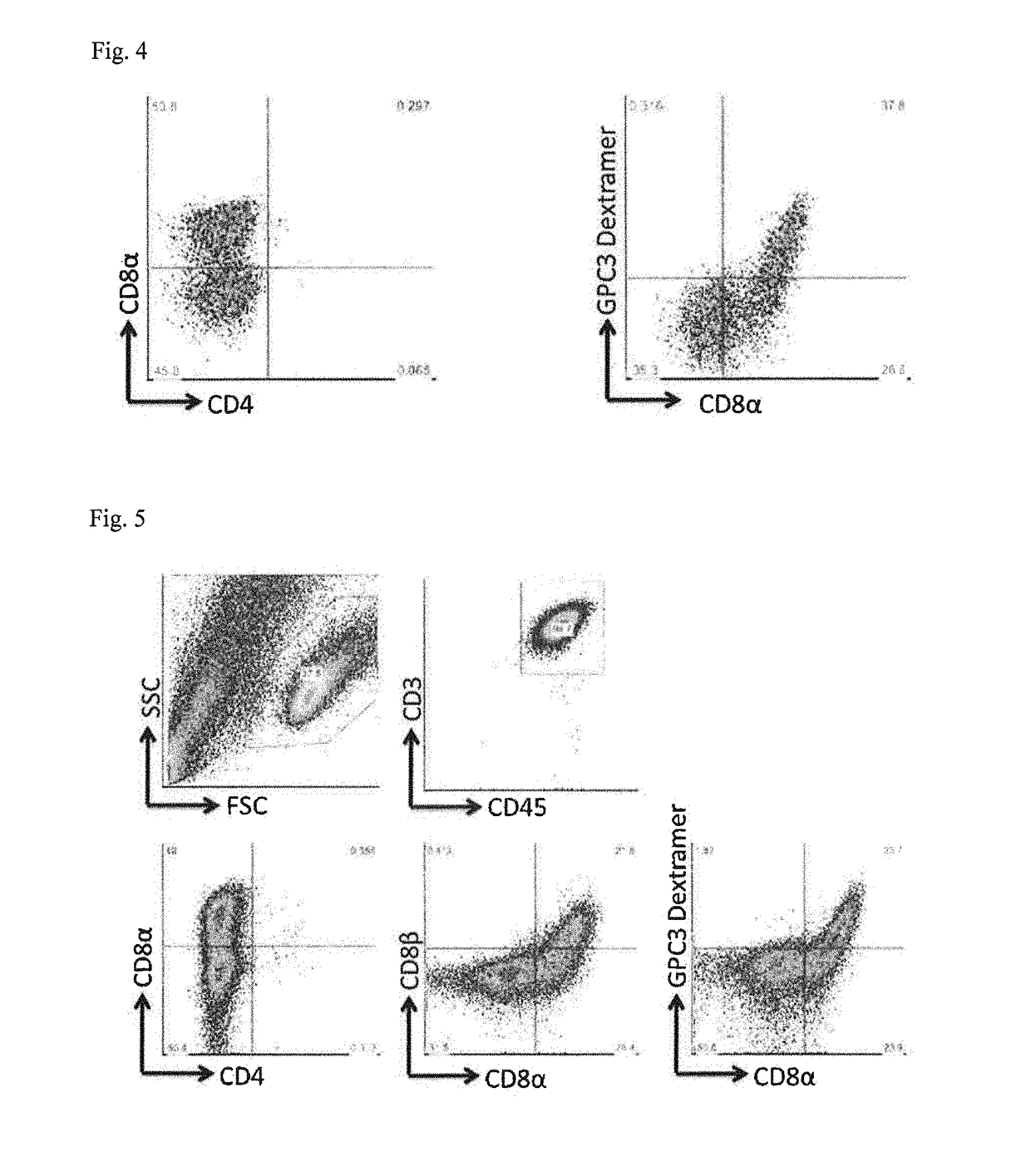
The present invention provides a method for efficiently inducing CD8-positive T cells by adding vitamin C to the medium in the steps of induction of the CD8-positive T cells from pluripotent stem cells. The present invention also provides a method for efficiently inducing CD8-positive T cells by performing culture in a medium supplemented with an adrenocortical hormone agent in the step of induction of the CD8-positive T cells from CD4 / CD8 double-positive T cells.
Owner:KYOTO UNIV
Natural medicine composition for treating involutional depression and non-classical depression and use of natural medicine composition
InactiveCN106692236ASignificant specificity effectResolve side effectsNervous disorderCapsule deliverySerum markersInterleukin 2
The invention discloses a natural medicine composition for treating involutional depression and non-classical depression and use of the natural medicine composition, and belongs to the technical field of research of natural medicine formulas in compatibility dosage effect and use of the natural medicine. The composition consists of the components in percentage by mass: 70%-97.5% of hippophae rhamnoides fruit oil and 2.5%-30% of pseudo-ginseng stem and leaf total saponins. The natural medicine composition disclosed by the invention is reasonable in compatibility and safe in dosage, is free from any side effects on human bodies, can solve the problems that a conventional medicine has side effects and poor treatment effects when being used for treating climacterium and involutional depression, and has a significant regulation effect on serum marker indexes, namely adenylate cyclase-cyclic adenosine monophosphate and cyclic guanosine monophosphate, and reproductive hormonesm and target acceptors of various impact factors, such as follicular generation promoting hormones, pitocin, dihydrotestosterone hormones, female hormones, adrenocorticotropin hormones, cytokine interleukin-6, interleukin-8 cytokine and interleukin-2, so as to achieve a treatment effect on treatment of the involutional depression and the non-classical depression diseases.
Owner:SHAANXI TIANKUI BIOMEDICAL TECH
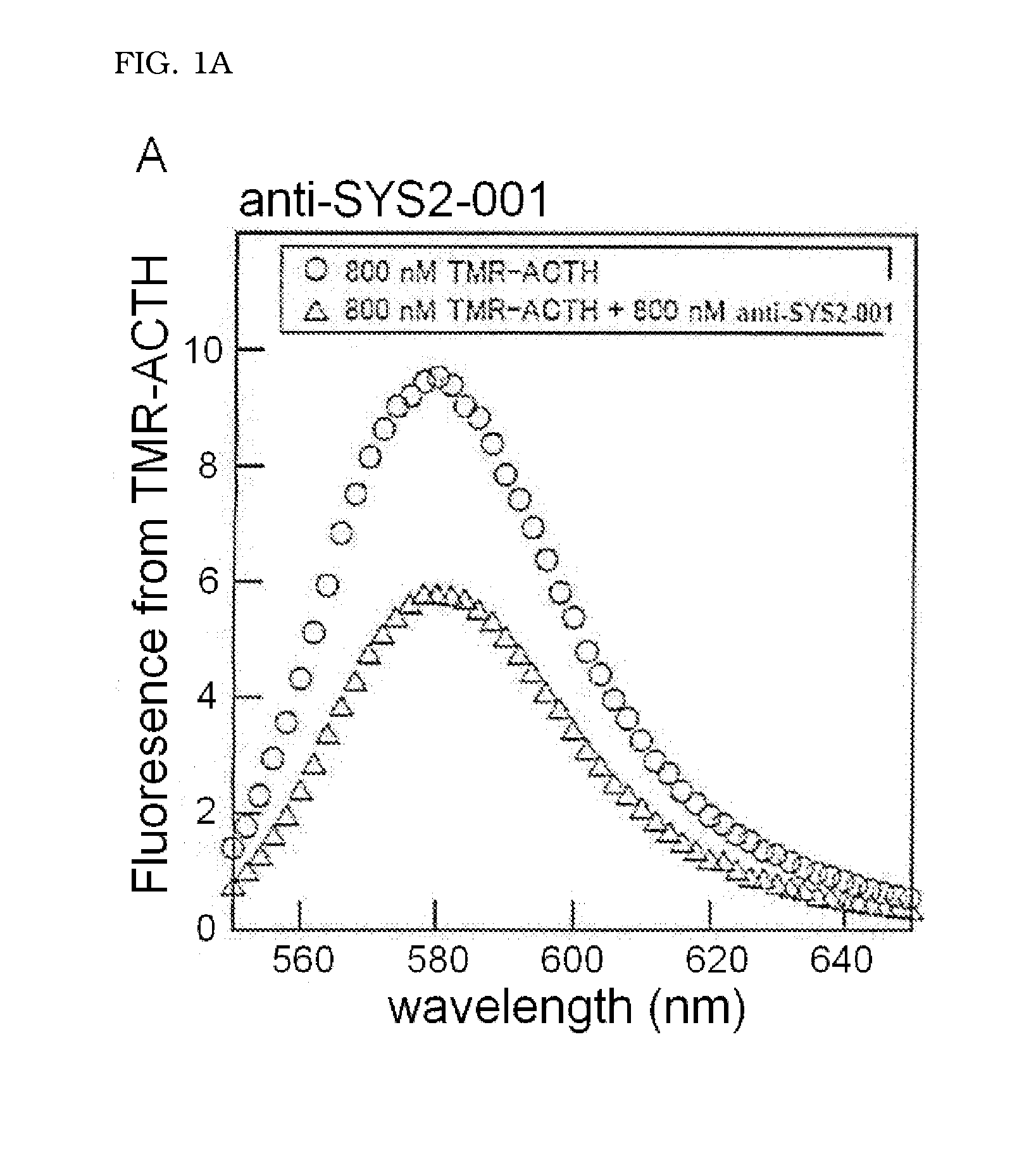
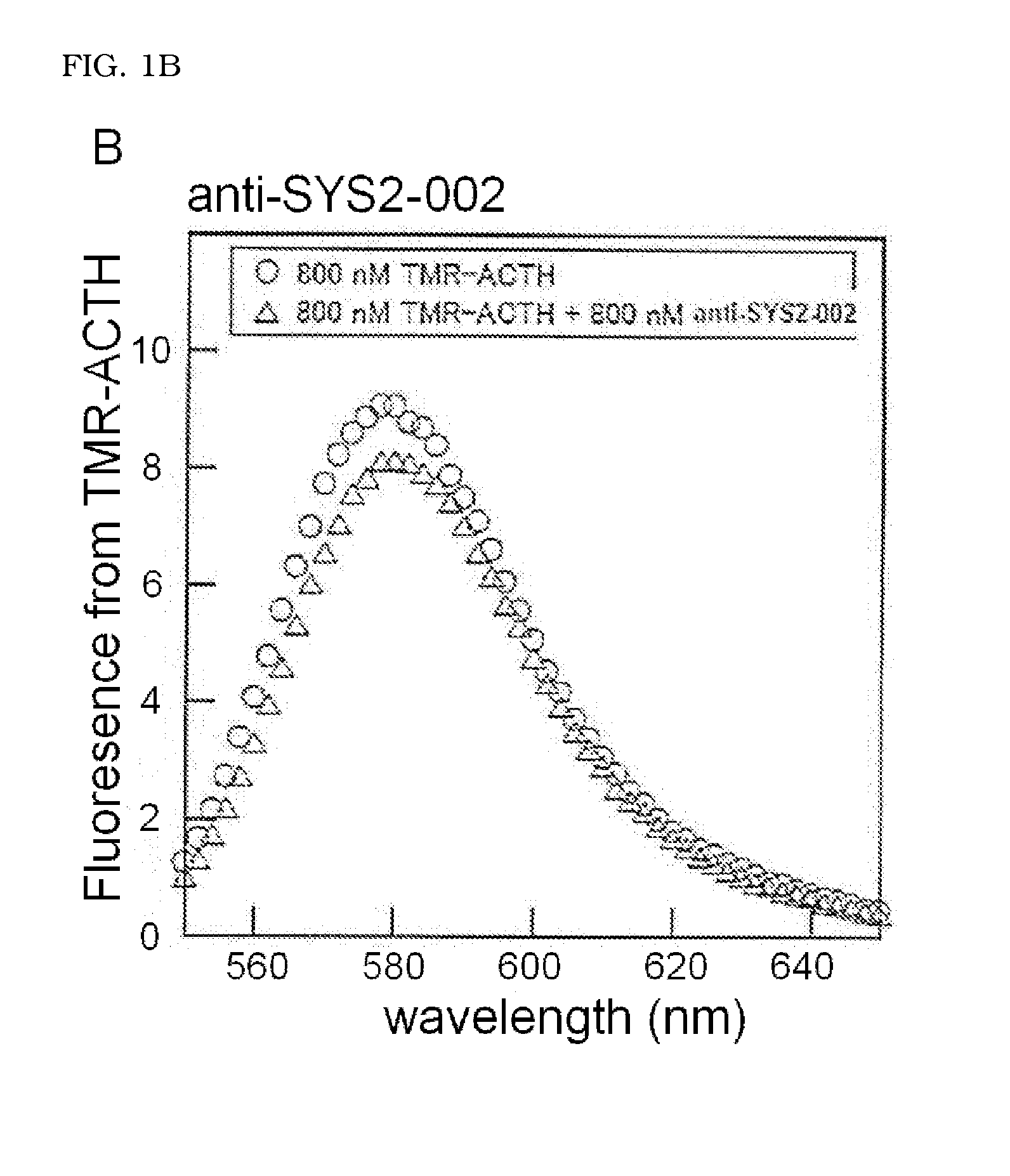
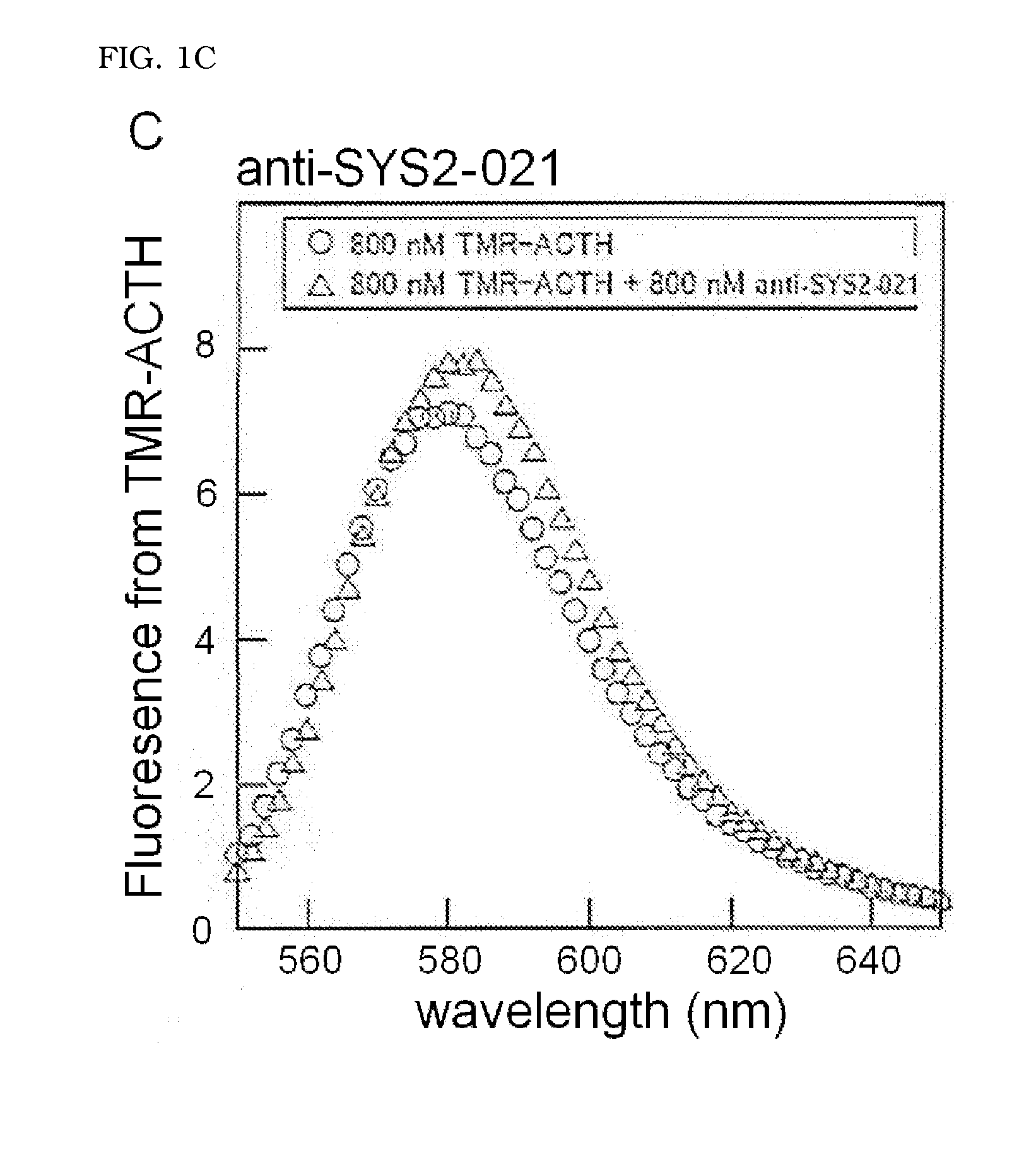
Molecule capable of binding to adrenocorticotropic hormone, and use thereof
ActiveUS20140322821A1Peptide/protein ingredientsMaterial analysis by observing effect on chemical indicatorChemistryAdrenocorticotropic hormone
The present invention relates to a molecule capable of binding to adrenocorticotropic hormone (ACTH) with high affinity. The present invention also relates to use of the molecule for detection and / or purification of ACTH.
Owner:SYSMEX CORP
Combined application of statin and adrenocortical hormone medicaments to treatment of chronic subdural hematoma
ActiveCN106729721APromote hematoma absorptionReduce hematomaNervous disorderHeterocyclic compound active ingredientsChronic subdural hematomaSide effect
The invention discloses combined application of statin and adrenocortical hormone medicaments to treatment of chronic subdural hematoma. Through combined application of the medicaments, hematoma absorption can be accelerated remarkably, the curative effect of other oral statins is improved greatly, and other side effects are avoided; moreover, the dosage of the adrenocortical hormone medicament is one tenth of the dosage of hormone, and the incidence of relevant complications caused by hormone treatment is lowered greatly. Through the technical scheme of combined application of the medicaments, a good curative effect is achieved, and wide application prospect and clinical significance are achieved.
Owner:张建宁 +2
Anti-dairy cow transport stress pharmaceutical composition
ActiveCN102716313AIncrease blood sugar concentrationAvoid damageMetabolism disorderDigestive systemAnti stressOyster
The invention aims to provide an anti-dairy cow transport stress pharmaceutical composition, which comprises the following crude drugs in parts by weight: 80-100g of spina date seed, 25-30g of Poria cocos, 15-20g of polygala tenuifolia, 15-20g of rhizoma anemarrhenae, 20-30g of jasmine, 10-20g of Scutellaria baicalensis, 5-10g of ossa draconis and 10-20g of oyster. Through experimental studies, the composition provided by the invention is capable of effectively preventing the blood sugar concentration of dairy cows under the stress state from rising and restraining the rise of the concentration of adrenocortical hormone and the concentration of lactic dehydrogenase, so, hearts and livers are prevented from being damaged. The anti-dairy cow transport stress pharmaceutical composition is convenient to use, i.e., the anti-dairy cow transport stress pharmaceutical composition only needs to be blended into a feed in an amount converted from the weights of the dairy cows. The anti-dairy cow transport stress pharmaceutical composition is expected to be developed to become a novel anti-stress pharmaceutical preparation product used before the dairy cow is transported.
Owner:HEBEI UNIV OF ENG
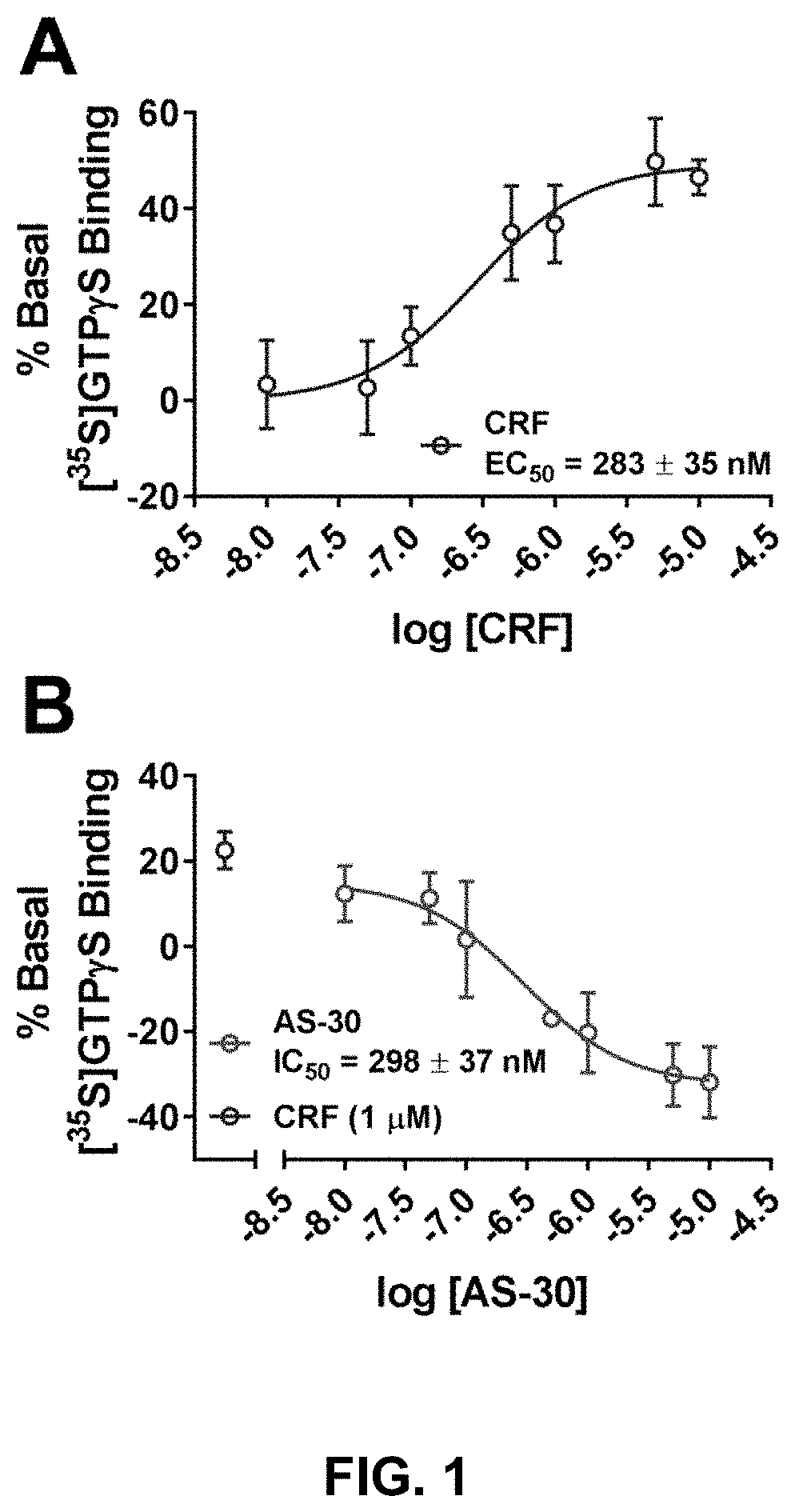
Compositions and methods for the modulation of the corticotropin releasing factor binding protein and the treatment of alcohol use disorder
ActiveUS11278527B2Organic chemistryHeterocyclic compound active ingredientsAspartic acid receptorsSubstance abuser
Stress responses involve corticotropin releasing factor (CRF), the two cognate receptors (CRF1 and CRF2) and the CRF-binding protein (CRFBP). Utilizing a novel cell-based assay, a C-terminal CRFBP fragment [CRFBP(10 kD)] was found to potentiates CRF-intracellular Ca2+ release, demonstrating that CRFBP possesses excitatory roles in addition to the inhibitory role established by the N-terminal fragment of CRFBP [CRFBP(27 kD)]. This interaction was CRF2-specific, as CRF1 responses were not potentiated by CRFBP(10 kD). As there were currently no small molecule ligands available that selectively interact with either CRFBP or CRF2, a cell-based assay was miniaturized, wherein CRFBP(10 kD) was fused as a chimera with CRF2α, that allowed us to a perform a high-throughput screen (HTS) of approximately 350,000 small molecules. This resulted in the identification of negative allosteric modulators (NAMs) of the CRFBP(10 kD)-CRF2 complex that blunt CRF-induced potentiation of N-Methyl-D-aspartic acid receptor (NMDAR)-mediated synaptic transmission in dopamine neurons in the ventral tegmental area (VTA). These results provide the first evidence of specific roles for CRF2 and CRFBP in the modulation of neuronal activity and suggest that NMDARs in the VTA may be a target for the treatment of stress and substance abuse disorders such as alcohol use disorder.
Owner:BROWN UNIVERSITY
Method of affecting sleep and sleep-related behaviors
A method of affecting sleep and sleep-related behaviors of a mammal having a diurnal rhythm, by reducing the basal activity of the hypothalamus-pituitary-adrenal axis by administering an effective amount of a sensory regimen is disclosed. Such reduction may be accomplished by reducing at least one of the following: a. the average total daily amount of adrenocortical hormone; or b. the average total daily amount adrenocortical hormone minus the integrative measure of morning peak adrenocortical hormone. Preferably, such reduction also includes reducing at least one of the following: c. the level of adrenocortical hormone 4 hours to 8 hours after waking; d. the level of adrenocortical hormone in the period of time preceding bedtime; or e. the level of adrenocortical hormone below the onset of sleep threshold.
Owner:MCCULLOCH LAURA +1
Bergamot mulberry leaf wolfberry tea and preparation method thereof
InactiveCN103798435BHeat-clearing and detoxifyingAntibacterialPre-extraction tea treatmentCitrus medicaBlood sugar
The invention discloses fingered citron folium mori and wolfberry tea and a preparation method thereof. The tea is characterized by comprising the following raw materials in parts by weight: 13-20 parts of folium mori, 10-20 parts of chayote leaves, 7-10 parts of green tea, 3-5 parts of wolfberries, and 4-6 parts of liquorice. The tea combines essences of the folium mori, the chayote leaves and the green tea, and also comprises the wolfberries and the liquorice, and thus the tea is scientific in compatibility of medicines. The tea has the good taste and the pleasant tea aroma, is easy to prepare, has no pollution, is a all natural product, and has effects of increasing immunity, regulating adrenocortical hormone, lowering blood sugar, resisting bacteria, lowering blood pressure, resisting tumors, resisting ageing, curing stomachache, and the like.
Owner:朱世超
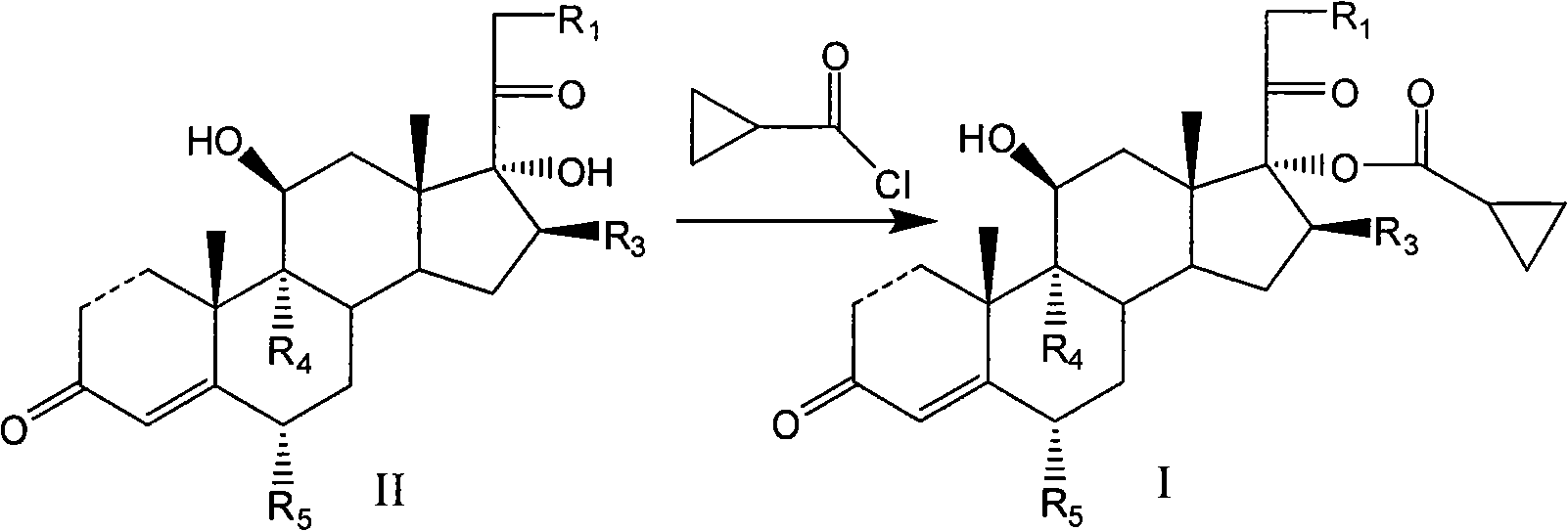
Cyclopropyl pregnene compound and application thereof
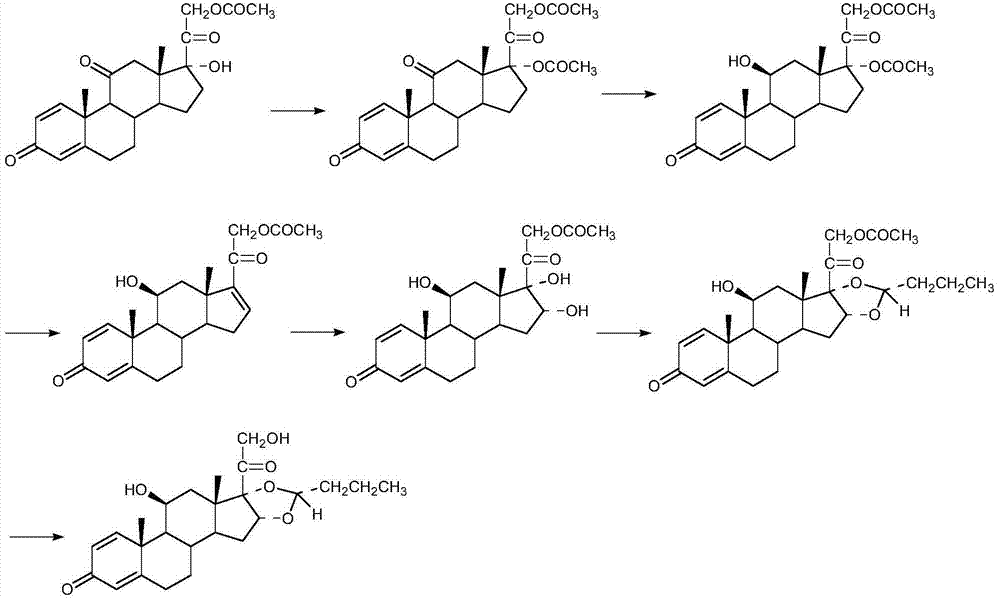
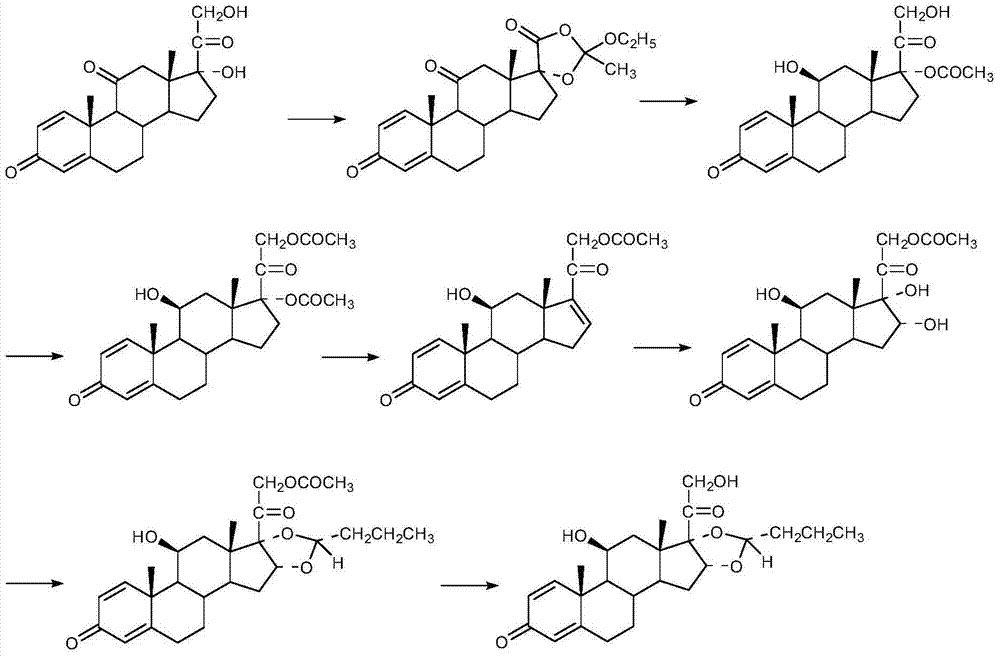
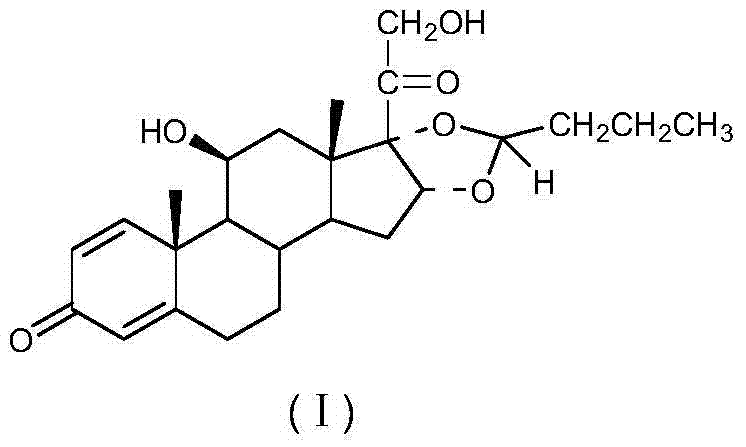
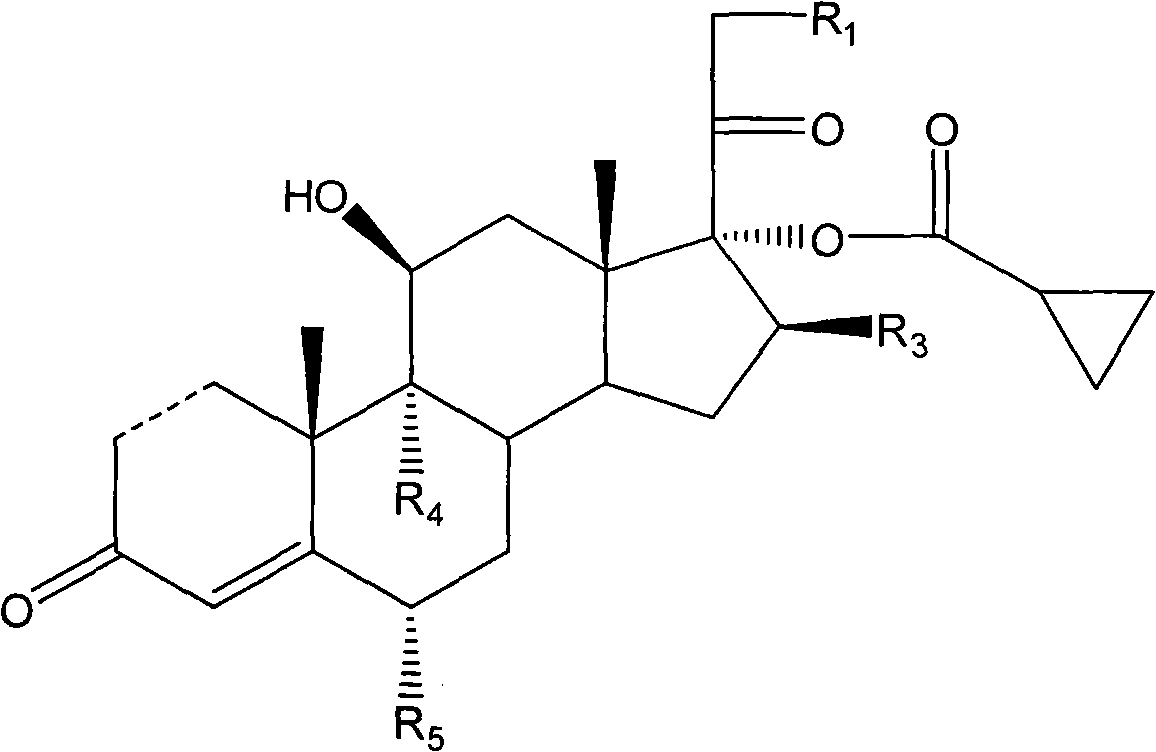
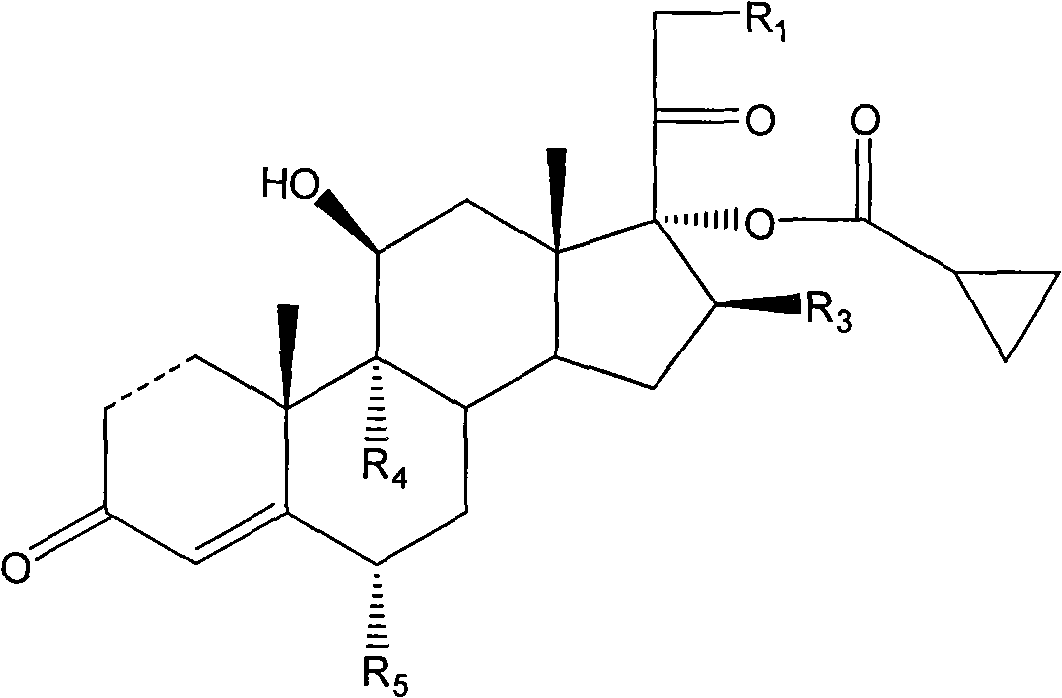
The invention discloses a cyclopropyl pregnene compound and application thereof, and discloses a novel adrenocortical hormone compound shown in a formula (I), a medicine composition containing the compound shown in the formula (I) as an active component and one or more pharmaceutic adjuvants, and application of the compound shown in the formula (I) or physiologically acceptable salt or solvate thereof as a medicine for treating of the diseases of mammal especially human, especially local inflammation.
Owner:TIANJIN JINYAO GRP
Traditional Chinese medicine decoction for treating female endocrine dyscrasia and preparation method thereof
InactiveCN105833065AReturn to normal functional statusGood treatment effectDispersion deliveryFish material medical ingredientsSalvia miltiorrhizaNervous system
The invention discloses a traditional Chinese medicine decoction for treating female endocrine dyscrasia and a preparation method thereof and belongs to the field of traditional Chinese medicines. The decoction includes the following raw material medicines, by weight: 40-60 g of mulberries, 25-32 g of astragalus membranaceus, 20-27 g of cornus officinalis, 14-18 g of radix rehmanniae, 15-20 g of semen cuscutae, 12-17 g of angelica sinensis, 10-16 g of salviae miltiorrhizae, 9-13 g of fructus lycii, 10-15 g of cortex albiziae, 11-15 g of honeysuckles, 8-12 g of polygonum cuspidatum, 7-10 g of verbena officinalis, 8-11 g of radix ophiopogonis, 6-10 g of lalang grass rhizome, 5-10 g of Chinese yam, 3-6 g of fructus cnidii, 4-7 g of donkey-hide gelatin, 2-4 g of sea horses, and 1-2 g of poria cocos. The formula of the decoction considers both unusualness and tradition. On the basis of qi and blood stasis, evil toxic invasion, qi and blood deficiency and the like symptoms which cause the endocrine dyscrasia, the decoction nourishes yin according to the aspects of excess, deficiency, yin, yang, qi and blood, regulates nervous system, recovers normal function and status of body, and has excellent treatment effect on the endocrine dyscrasia of sex hormone, adrenocortical hormone, insulin and thyroxine.
Owner:许颖
Pharmaceutical composition for treating hyperproliferative skin disease and preparation of pharmaceutical composition
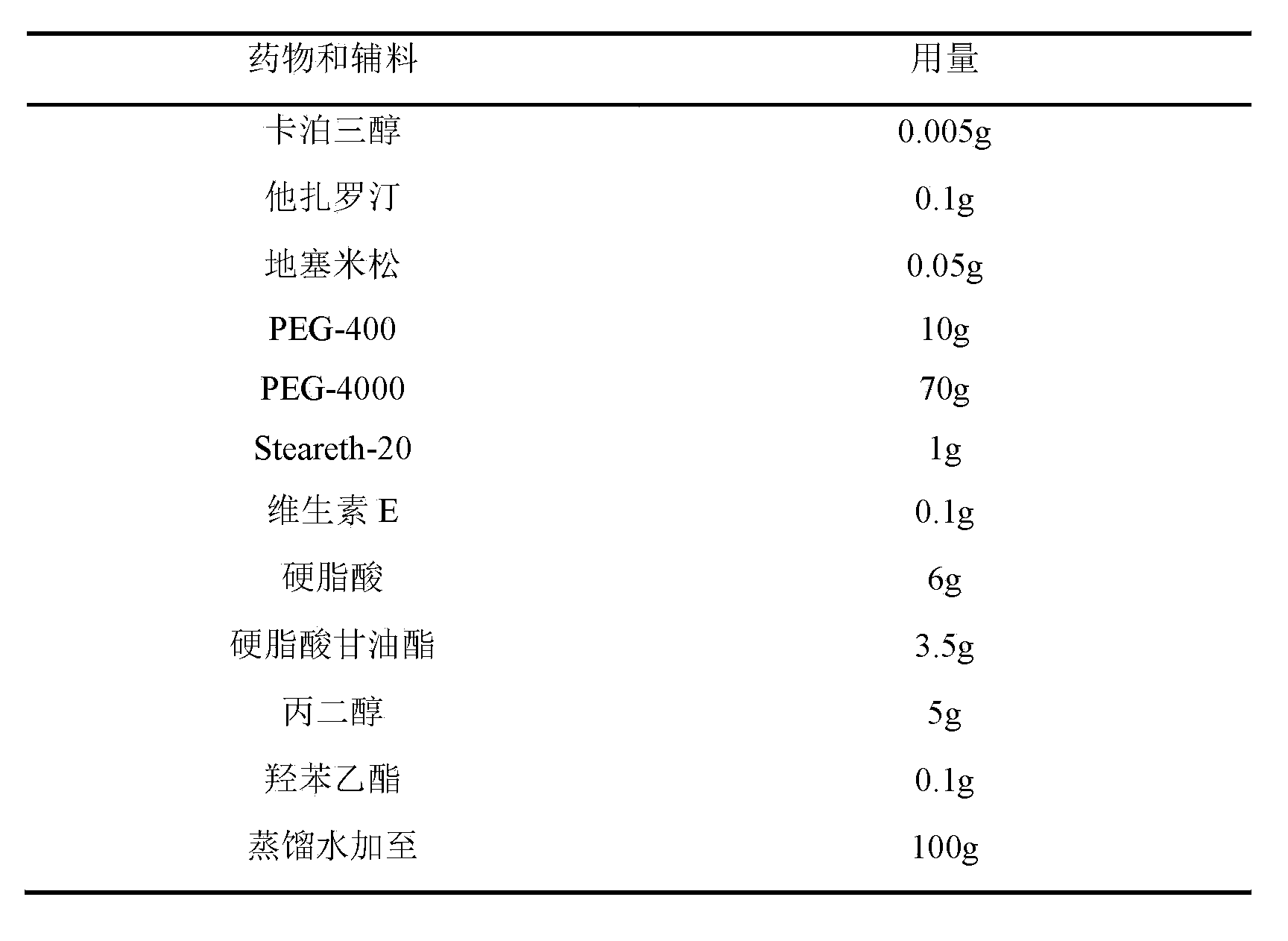
ActiveCN102988987BGood synergyImprove efficacyOrganic active ingredientsAerosol deliveryDiseaseTazarotene
The invention belongs to the field of pharmacy and particularly relates to a pharmaceutical composition for treating hyperproliferative skin disease and a preparation of the pharmaceutical composition. The pharmaceutical composition comprises the following components by weight percent: 0.001-0.01% (W / W) of calcipotriene, 0.05-2% (W / W) of tazarotene and 0.05-2% (W / W) of adrenocortical hormone.
Owner:XIAN LIBANG PHARMA
Chinese patent drug capable of replacing adrenocortical hormone to relieve nephritis and nephropathy
InactiveCN102940758APromote recovery of metabolic functionNo side effectsInanimate material medical ingredientsUrinary disorderSide effectOyster
The invention discloses a Chinese patent drug capable of replacing adrenocortical hormone to relieve nephritis and nephropathy. The Chinese patent drug comprises 150 parts of astragali radix, 30 parts of bighead atractylodes rhizome, 15 parts of poria cocos, 30 parts of crude dragon bone, 30 parts of raw oyster, 30 parts of concha haliotidis, 15 parts of cuttlebone, 15 parts of radix rubiae, 12 parts of crinis trachycarpi, 30 parts of radix rehmanniae, 20 parts of rhizoma dioscoreae, 20 parts of semen euryales and 15 parts of loranthus parasiticus. The Chinese patent drug comprises various effective ingredients, has remarkable curative effects on urine protein, occult blood and various discomfort of edema, high blood pressure, swirl, headache, backache, nausea, anorexia and the like caused by nephropathy, adopts pure traditional Chinese medicines, and is free of hormone, toxic and side effect, adverse reaction, rapid in effect and low in cost.
Owner:刘美海
Medicament for treating olfaction disorder
ActiveUS9931380B2Regenerating the olfactory epitheliumAmeliorate olfactory disturbanceSenses disorderPeptide/protein ingredientsBULK ACTIVE INGREDIENTCvd risk
Owner:KUBOKI AKIHITO +1
Biscuit for children
InactiveCN109258746AHigh nutritional valueSuitable for growth and development needsDough treatmentBakery productsNutritive valuesSodium bicarbonate
The invention discloses a biscuit for children. The biscuit for children comprises the following raw materials in parts by weight: 20-50 parts of refined wheat powder, 20-30 parts of pumpkin powder, 10-15 parts of hydrolyzed milk, 10-20 parts of fruit and vegetable juice, 15-25 parts of protein powder, 5-10 parts of butter, 1-2 parts of sodium bicarbonate, 3-10 parts of yeast, 10-20 parts of maplesugar and 2-5 parts of salt. The butter and hydrolyzed milk are mixed and stirred for 10 minutes, are added with eggs, the salt, the maple sugar and the fruit and vegetable juice, and then are stirred for 5 minutes to obtain a mixed solution. The pumpkin powder and the maple sugar are added to process the biscuit for children, and the pumpkin has high nutritive value, contains abundant zinc, participates in synthesis of nucleic acid and protein in human bodies, is an inherent ingredient of adrenocortical hormone, is an important substance for growth and development of human bodies, and is exactly suitable for growth and development of the children.
Owner:江苏天凤源食品有限公司
crhr2 peptide agonists and uses thereof
InactiveCN102272151APeptide/protein ingredientsMetabolism disorderDiseaseCorticotropin-releasing hormone receptor
The present invention relates to novel peptides that are selective corticotropin releasing hormone receptor type 2 (CRHR2) agonists and compositions thereof for the treatment, amelioration or inhibition of cardiovascular conditions, including but not limited to heart failure. The novel peptide agonists preferably comprise modifications that include pegylated peptides. Furthermore, the present invention also relates to methods for the treatment and prevention of a disease or disorder related to CRHR2 activity.
Owner:JANSSEN PHARMA NV
Anti-dairy cow transport stress pharmaceutical composition
ActiveCN102716313BIncrease blood sugar concentrationAvoid damageMetabolism disorderDigestive systemAnti stressOyster
The invention aims to provide an anti-dairy cow transport stress pharmaceutical composition, which comprises the following crude drugs in parts by weight: 80-100g of spina date seed, 25-30g of Poria cocos, 15-20g of polygala tenuifolia, 15-20g of rhizoma anemarrhenae, 20-30g of jasmine, 10-20g of Scutellaria baicalensis, 5-10g of ossa draconis and 10-20g of oyster. Through experimental studies, the composition provided by the invention is capable of effectively preventing the blood sugar concentration of dairy cows under the stress state from rising and restraining the rise of the concentration of adrenocortical hormone and the concentration of lactic dehydrogenase, so, hearts and livers are prevented from being damaged. The anti-dairy cow transport stress pharmaceutical composition is convenient to use, i.e., the anti-dairy cow transport stress pharmaceutical composition only needs to be blended into a feed in an amount converted from the weights of the dairy cows. The anti-dairy cow transport stress pharmaceutical composition is expected to be developed to become a novel anti-stress pharmaceutical preparation product used before the dairy cow is transported.
Owner:HEBEI UNIV OF ENG
Adrenocorticotropic hormone quality control material and preparation method thereof
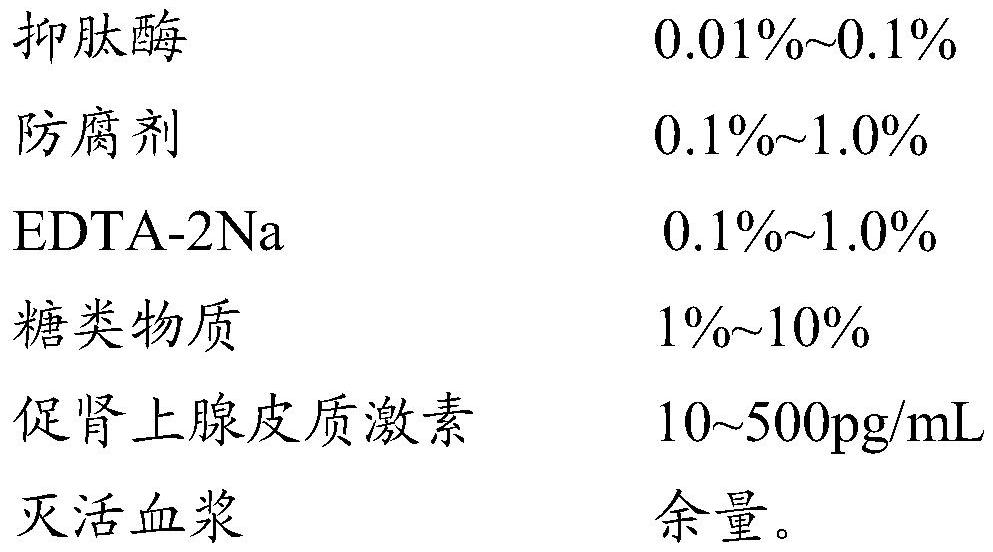
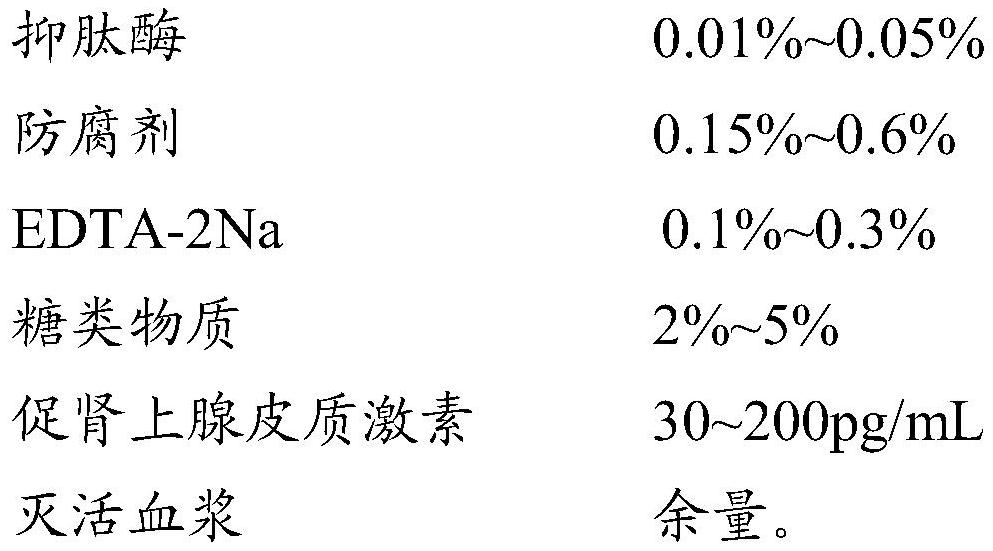
PendingCN112540171AReduce matrix effectImprove stabilityBiological testingReference solutionsAprotininAdrenal gland
The invention relates to the technical field of quality control, particularly to an adrenocorticotropic hormone quality control product and a preparation method thereof. The quality control product comprises the following raw materials and auxiliary materials: 0.01-0.1% of aprotinin, 0.1-1.0% of a preservative, 0.1-1.0% of EDTA-2Na, 1-10% of carbohydrates, 10-500 pg / mL of adrenocorticotropic hormone and the balance of inactivated plasma. By adopting a plasma inactivation process and an added formula, the stability of the adrenocorticotropic hormone quality control product can be improved.
Owner:郑州标源生物科技有限公司
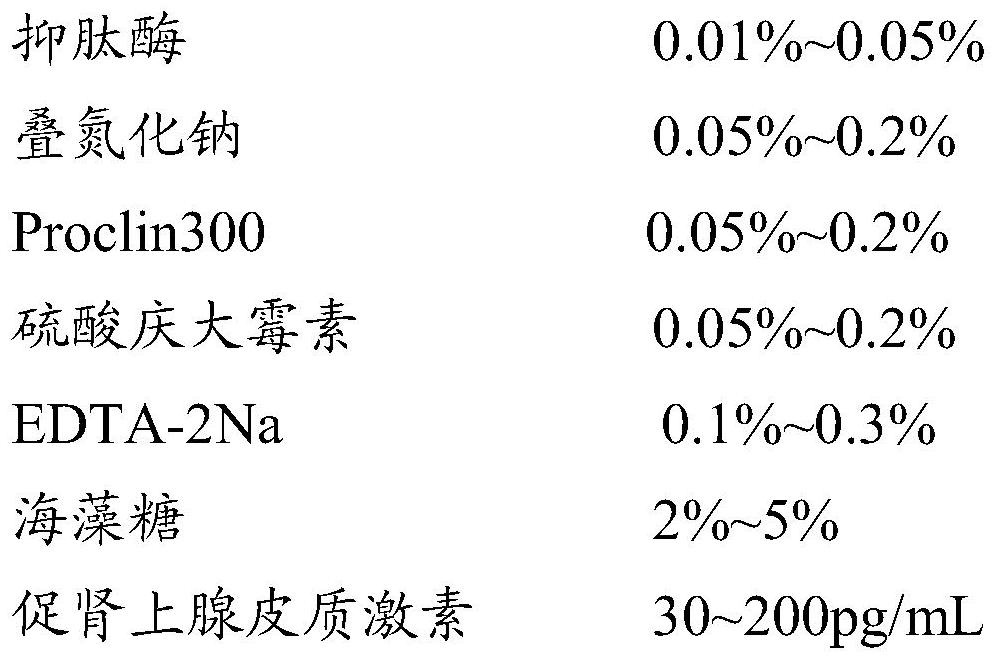
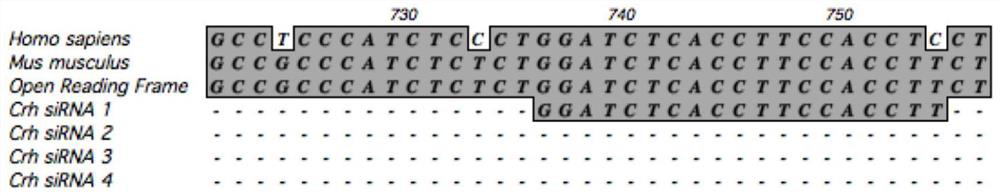
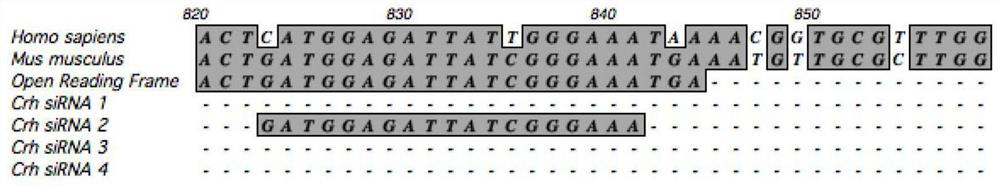
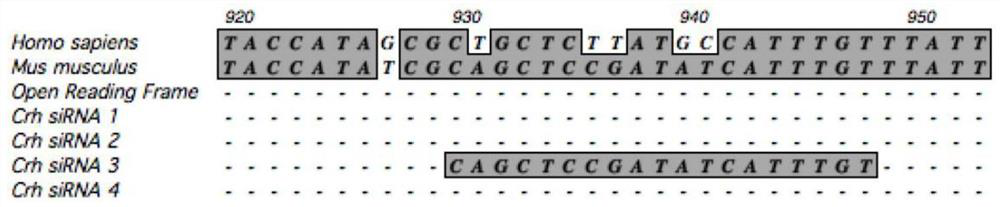
SiRNA of targeted CRH gene and application of siRNA in treatment of neuropathic pain
PendingCN113186188AGood treatment effectHigh clinical application valueOrganic active ingredientsNervous disorderTherapeutic effectNeurological injury
The invention belongs to the technical field of biological medicine, and discloses three groups of siRNAs targeting human and mouse CRH genes and application thereof in treatment of neuropathic pain. The three groups of siRNAs targeting the CRH genes (adrenocorticotropic hormone-releasing hormone) are designed and synthesized, the three groups of siRNAs are intrathecally injected into a neuropathic pain model mouse during in-vivo experiments. Molecular biology experiments show that the two groups of siRNAs can block CRH expression increase induced by nerve injury in mouse dorsal root ganglion (DRG) in vivo, and show that the two groups of siRNAs can effectively reduce CRH gene expression, but the effect of the second group is more obvious. Pain behavioral detection shows that the neuropathic pain can be effectively relieved through intrathecal injection of the second group of CRH siRNA. The invention provides non-chemical modification and chemical modification nucleic acid molecules of the targeted CRH gene, has a better treatment effect on neuropathic pain, provides a new thought and experimental basis for clinical research and development of new analgesic drugs, and has important clinical application value.
Owner:NANTONG UNIVERSITY
A medicinal and edible homologous composition for alcohol use disorder and its application
ActiveCN113521195BEasy to shapeInhibition decreasedNervous disorderDigestive systemAlcohol abuse disorderAdrenocortical hormone
The invention discloses a medicine and food homologous composition for alcohol use disorder and an application thereof, belonging to the technical field of health food. The composition of the invention is composed of dendrobium, polygonatum and kudzu root. The composition of the invention has significant protective effect on alcoholic liver injury in mice, including improving the morphology and structure of liver cells, improving liver function and liver lipid metabolism. On the other hand, the composition of the present invention can improve depression and anxiety-like reactions after alcohol withdrawal, reduce the elevation of corticotropin-releasing hormone, corticotropin and corticosterone caused by alcohol withdrawal; inhibit alcohol withdrawal Causes a decrease in the monoamine transmitters dopamine and norepinephrine.
Owner:DALIAN UNIV OF TECH +1
Compositions and methods for the modulation of the corticotropin releasing factor binding protein and the treatment of alcohol use disorder
ActiveUS20210308107A1Organic chemistryHeterocyclic compound active ingredientsAspartic acid receptorsSubstance abuser
Stress responses involve corticotropin releasing factor (CRF), the two cognate receptors (CRF1 and CRF2) and the CRF-binding protein (CRFBP). Utilizing a novel cell-based assay, a C-terminal CRFBP fragment [CRFBP(10 kD)] was found to potentiates CRF-intracellular Ca2+ release, demonstrating that CRFBP possesses excitatory roles in addition to the inhibitory role established by the N-terminal fragment of CRFBP [CRFBP(27 kD)]. This interaction was CRF2-specific, as CRF1 responses were not potentiated by CRFBP(10 kD). As there were currently no small molecule ligands available that selectively interact with either CRFBP or CRF2, a cell-based assay was miniaturized, wherein CRFBP(10 kD) was fused as a chimera with CRF2α, that allowed us to a perform a high-throughput screen (HTS) of approximately 350,000 small molecules. This resulted in the identification of negative allosteric modulators (NAMs) of the CRFBP(10 kD)-CRF2 complex that blunt CRF-induced potentiation of N-Methyl-D-aspartic acid receptor (NMDAR)-mediated synaptic transmission in dopamine neurons in the ventral tegmental area (VTA). These results provide the first evidence of specific roles for CRF2 and CRFBP in the modulation of neuronal activity and suggest that NMDARs in the VTA may be a target for the treatment of stress and substance abuse disorders such as alcohol use disorder.
Owner:BROWN UNIVERSITY
Medicament for treating olfaction disorder
ActiveUS20160243200A1Promote regenerationRegenerating the olfactory epitheliumSenses disorderPeptide/protein ingredientsCvd riskBULK ACTIVE INGREDIENT
The object of the present invention is to provide an olfactory disturbance therapeutic agent that is effective in repairing or regenerating olfactory epithelium that suffered damages such as scratches and can be administered for a long time.The object of the present invention is achieved by an olfactory disturbance therapeutic agent including at least one active ingredient that is selected from among a group consisting of insulin, insulin analogs, and insulin secretagogues. Preferably, the olfactory disturbance therapeutic agent is used for people with olfactory disturbance who have, or are at risk of having, impaired insulin secretion or insulin resistance, or for people who could suffer an iatrogenic increase in blood sugar level or a disturbance in a balance of adrenocortical hormone stemming from a long-term use of steroid as olfactory disturbance treatment.
Owner:KUBOKI AKIHITO +1
Method of affecting sleep and sleep-related behaviors
A method of affecting sleep and sleep-related behaviors of a mammal having a diurnal rhythm, by reducing the basal activity of the hypothalamus-pituitary-adrenal axis by administering an effective amount of a sensory regimen is disclosed. Such reduction may be accomplished by reducing at least one of the following: a. the average total daily amount of adrenocortical hormone; or b. the average total daily amount adrenocortical hormone minus the integrative measure of morning peak adrenocortical hormone. Preferably, such reduction also includes reducing at least one of the following: c. the level of adrenocortical hormone 4 hours to 8 hours after waking; d. the level of adrenocortical hormone in the period of time preceding bedtime; or e. the level of adrenocortical hormone below the onset of sleep threshold.
Owner:MCCULLOCH LAURA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com