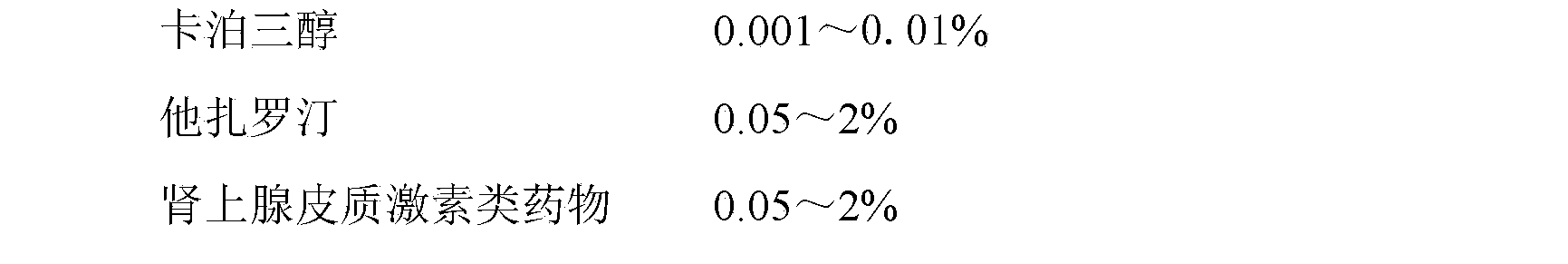Pharmaceutical composition for treating hyperproliferative skin disease and preparation of pharmaceutical composition
A technology for epidermal hyperproliferation and disease, which is applied in the field of pharmacy, can solve the problems of non-persistence and protein kinase signal transduction system defects, etc., and achieves the effects of simple preparation process, uniform drug release and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: Contain the emulsifiable paste of 0.001% calcipotriol
[0065] prescription:
[0066]
[0067] method:
[0068] Heat 6g of stearic acid and 3.5g of glyceryl stearate in a water bath until melted, add 0.001g of calcipotriol, 0.1g of tazarotene, 0.1g of betamethasone dipropionate and 0.1g of vitamin E, and stir to dissolve evenly . In addition, heat 70g of PEG-4000 and 10g of PEG-400 in a water bath to 65°C, add 1g of steareth-20, 5g of propylene glycol and 0.1g of ethylparaben, stir evenly, slowly add to the oil phase, stir until the emulsification is complete, add distilled water to 100g, let cool.
Embodiment 2
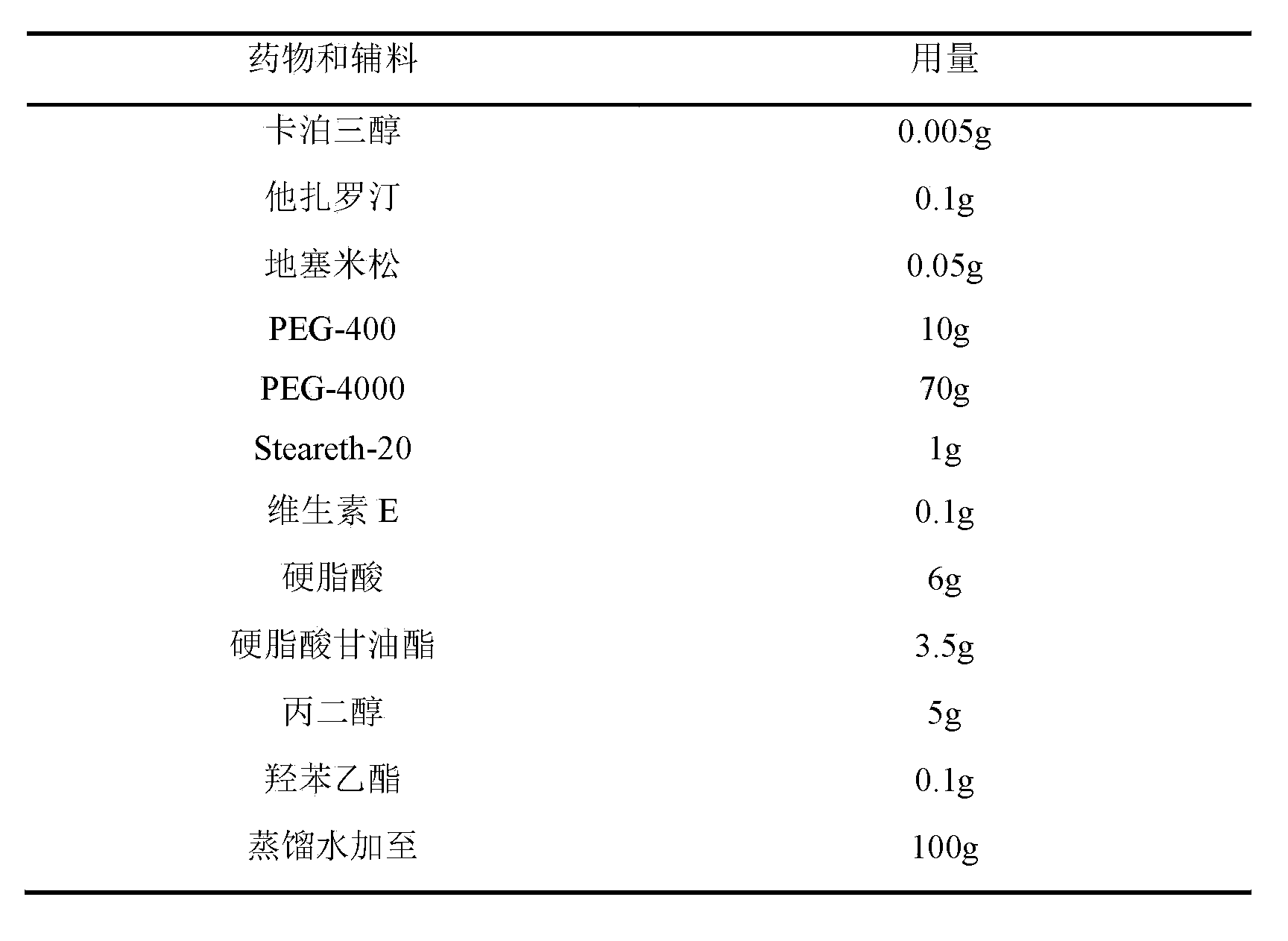
[0069] Embodiment 2: the cream containing 0.005% calcipotriol
[0070] prescription:
[0071]
[0072] method:
[0073] Heat 6g of stearic acid and 3.5g of glyceryl stearate in a water bath until melted, add 0.005g of calcipotriol, 0.1g of tazarotene, 0.1g of betamethasone dipropionate and 0.1g of vitamin E, and stir to dissolve evenly . In addition, heat 70g of PEG-4000 and 10g of PEG-400 in a water bath to 65°C, add 1g of steareth-20, 5g of propylene glycol and 0.1g of ethylparaben, stir evenly, slowly add to the oil phase, stir until the emulsification is complete, add distilled water to 100g, let cool.
Embodiment 3
[0074] Embodiment 3: Contain the emulsifiable paste of 0.01% calcipotriol
[0075] prescription:
[0076]
[0077] method:
[0078] Heat 6g of stearic acid and 3.5g of glyceryl stearate in a water bath until melted, add 0.01g of calcipotriol, 0.1g of tazarotene, 0.1g of betamethasone dipropionate and 0.1g of vitamin E, and stir to dissolve evenly . In addition, heat 70g of PEG-4000 and 10g of PEG-400 in a water bath to 65°C, add 1g of steareth-20, 5g of propylene glycol and 0.1g of ethylparaben, stir evenly, slowly add to the oil phase, stir until the emulsification is complete, add distilled water to 100g, let cool.
[0079] Part II: The dosage range of tazarotene in ointment
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com