crhr2 peptide agonists and uses thereof
A technology for agonists and hormones, which can be used in applications, hormone peptides, specific peptides, etc., and can solve problems such as relative binding affinity, receptor activation degree, and selectivity differences.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
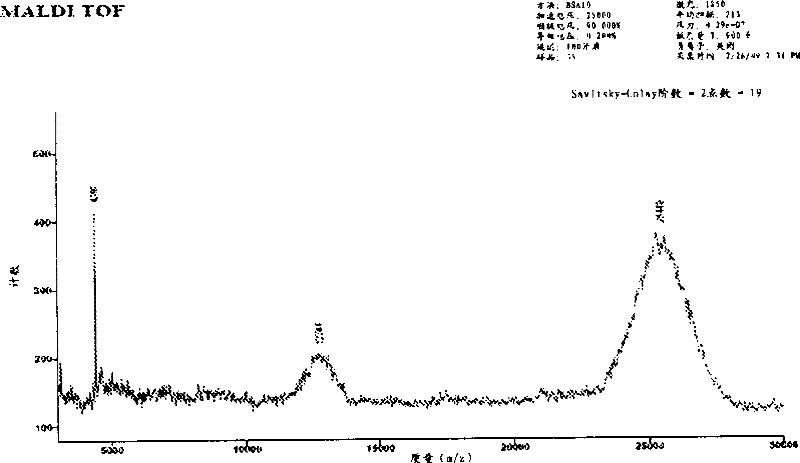
[0168] Example 1: Synthesis and purification of peptides
[0169] The peptide of SEQ ID NO: 29 was prepared by solid-phase peptide synthesis on a Rainin Symphony multi-channel peptide synthesizer (SMPS-110 model) using software version 3.3.0. Resin for peptide amide synthesis (NovaSyn TGR , 440mg, approximately 0.1mmol, degree of substitution 0.23mmol / g, batch number A33379) is a composite material of polyethylene glycol and polystyrene functionalized with acid-labile modified Rink amide linker.
[0170] The amino acid used for synthesis contains the Nα-9-fluorenylmethoxycarbonyl (Fmoc) protecting group at the C-terminus and the following side chain protecting group: arginine (2,2,4,6,7-pentamethyldi Hydrobenzofuran-5-sulfonyl, pbf), aspartic acid (tert-butoxy, OtBu), asparagine (trityl, Trt), glutamine (Trt), cysteine ( Trt), histidine (Trt), lysine (tert-butoxycarbonyl, Boc), serine (tert-butyl, tBu) and threonine (tBu).
[0171] By mixing N-methylpyrrolidone (NMP) p...
example 2
[0175] Example 2: Coupling of peptides with N-ethylmaleimide
[0176] The N-ethylmaleimide end-capping reaction on cysteine residues shown in Scheme 1 was realized according to the following conditions.
[0177] plan 1
[0178]
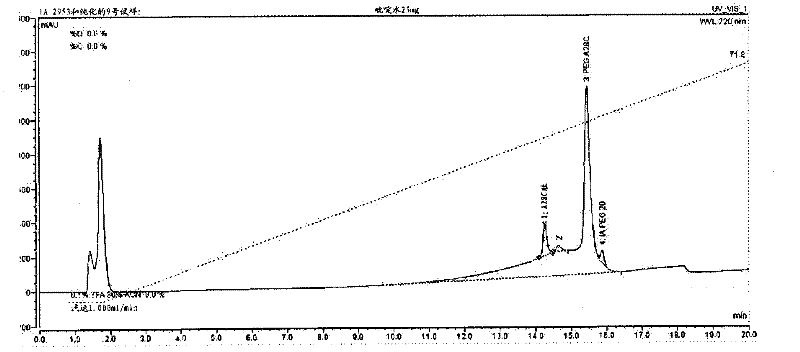
[0179] In a 2.5 mL polypropylene vial, 2.0 mg of the peptide of the invention was dissolved in 1.0 mL of water. Then 20 microliters of 0.1M aqueous N-ethylmaleimide solution was added immediately. The reaction mixture was stirred gently at room temperature for 2 hours. The reaction mixture was purified on a Summit APS (Dionex, CA, U.S.A.) HPLC equipped with a 300 Angstrom pore size Vydac C18 column (10 x 250 mm; Grace Davison, IL, U.S.A.) using the protocol shown in Table 6 below. Final fractions were collected, analyzed by HPLC, and pure fractions were pooled and lyophilized.
[0180] Table 6
[0181] Column:
example 3
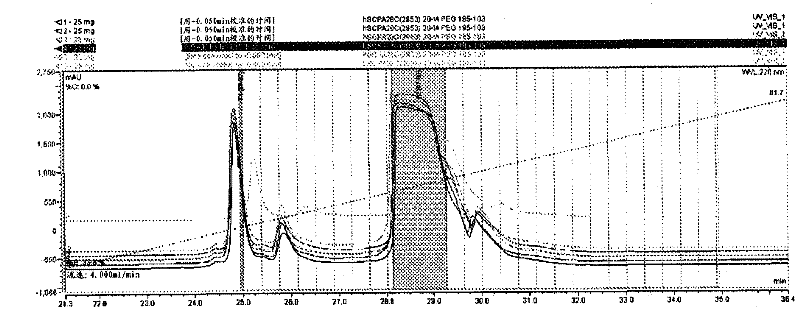
[0182] Example 3: Coupling of Peptides to Iodoacetamide-PEG
[0183] Iodoacetamide-PEG, a 20 kDa polyethylene glycol linear chain with iodoacetamide termini, is present in limiting amounts with the peptide of SEQ ID NO: 29 at slightly alkaline pH, resulting in cysteine modifications such as Specific reactions shown in Scheme 2. Cysteine thiols serve as selective attachment sites for iodoacetamide-PEG. The resulting derivatized α-thiol acetamide linkage is achiral.
[0184] Scenario 2
[0185]
[0186] To a 15 mL Erlenmeyer flask was added 25 mg (5.68 mmol, 1.0 equiv) of the peptide of SEQ ID NO: 1. To the above Erlenmeyer flask was added 140 mg (6.82 mmol, 1.2 equivalents, 95% active) of PEG-20 iodoacetamide produced by Nippon, Oil and Fat (NOF) Corp. (Lot No. M77592). Add 10 mL of water and vortex the solution until all solids are dissolved. To this cloudy solution was added 50 mL of pyridine having a solution pH of about 8.91. After 2 hours, a 20 mL aliquot wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com