Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Release factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
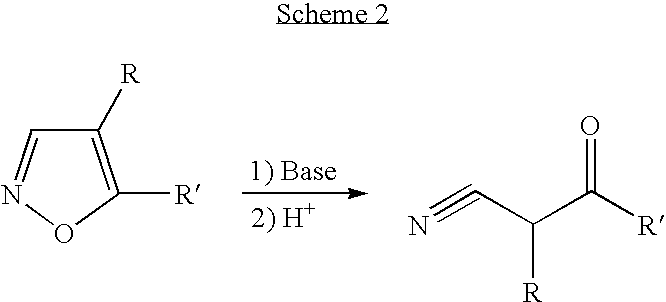
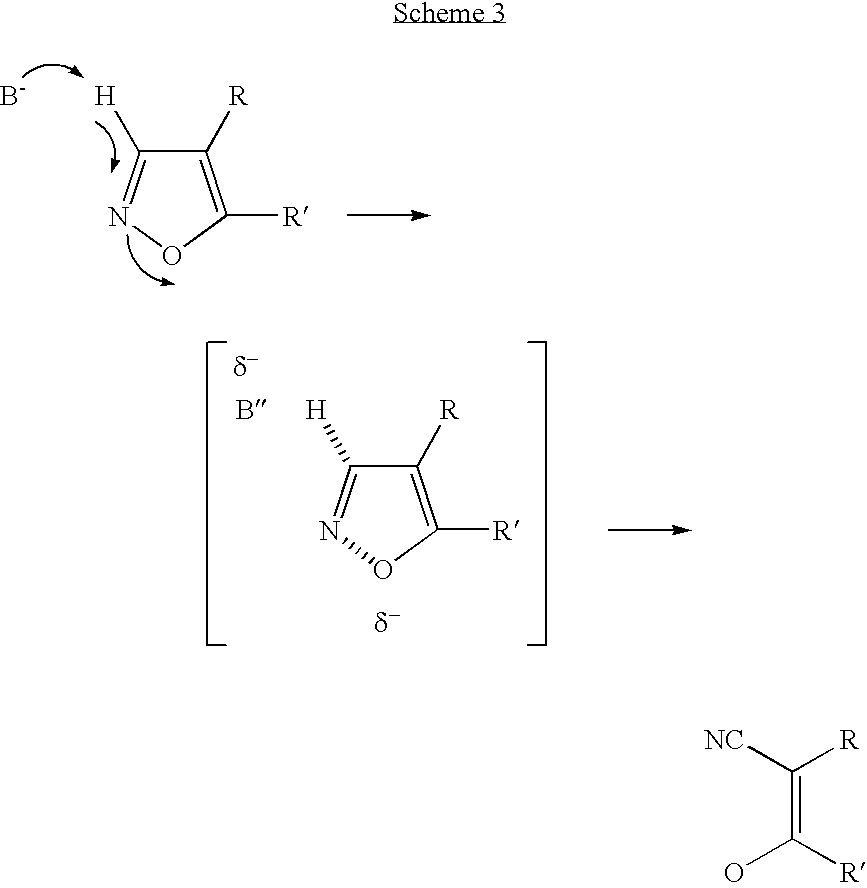
A release factor is a protein that allows for the termination of translation by recognizing the termination codon or stop codon in an mRNA sequence. They are named so because they release new peptides from the ribosome.
Long lasting growth hormone releasing factor derivatives
InactiveUS7268113B2Prolong half-life in vivoPeptide/protein ingredientsMuscular disorderHalf-lifeIn vivo
This invention relates to growth hormone releasing factor (GRF) derivatives. In particular, this invention relates to GRF peptide derivatives having an extended in vivo half-life, for promoting the endogenous production or release of growth hormone in humans and animals.
Owner:CONJUCHEM
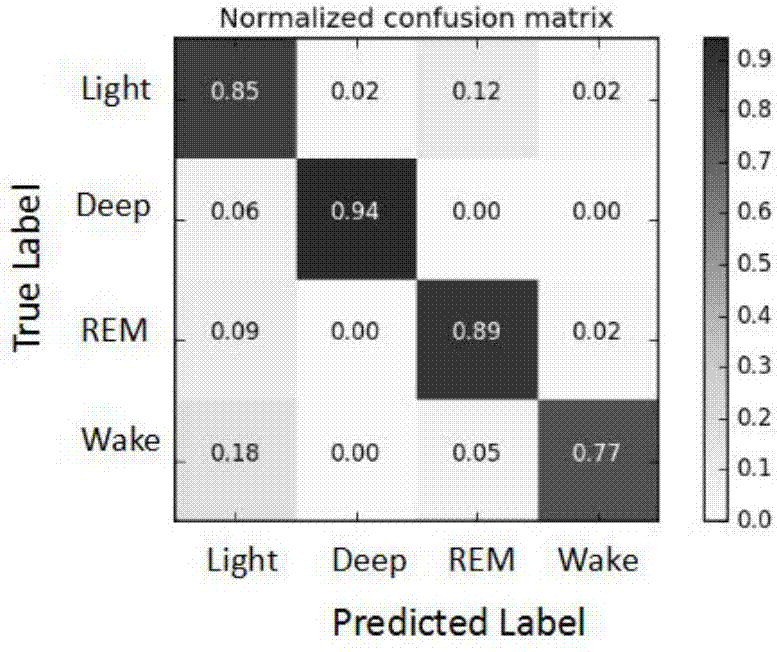
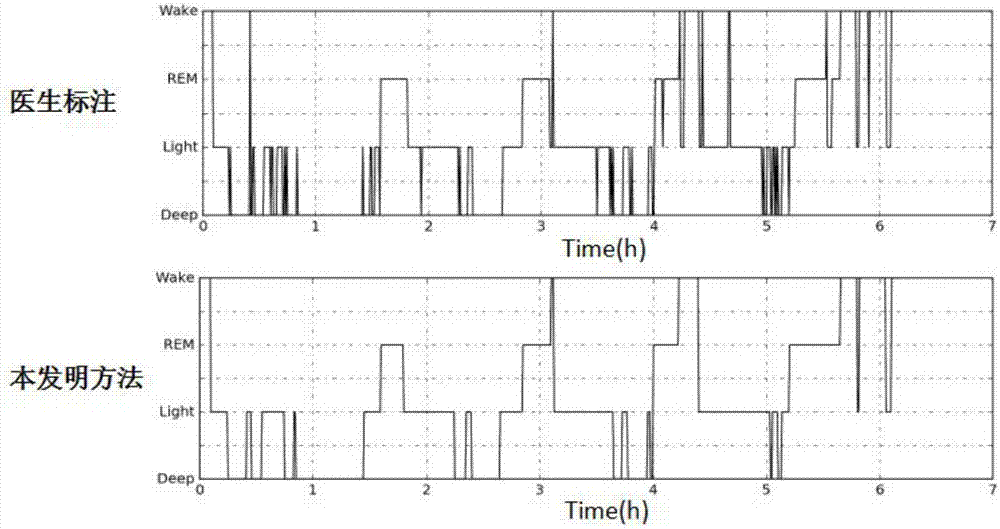
Automatic sleep staging method of single-lead electroencephalogram
ActiveCN107495962APortableMeet needsDiagnostic recording/measuringSensorsDiseaseConditional random field
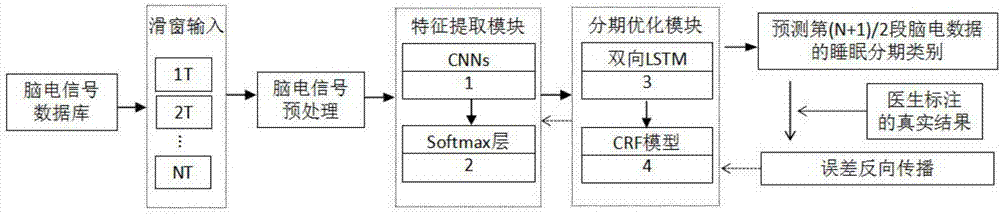
The invention discloses an automatic sleep staging method of a single-lead electroencephalogram. A training model comprises a feature extraction module and a staging optimization module, the feature extraction module comprises CNNs (convolutional neural networks) (1) and an Softmax layer (2), the staging optimization module comprises bi-directional LSTM (long-short term memory) recurrent neural networks (3) and a CRF (corticotropin releasing factor) conditional random field model (4), and the CNNs (1), the Softmax layer (2), the LSTM recurrent neural networks (3) and the CRF conditional random field model (4) are sequentially connected. The method only needs the single-lead sleep electroencephalogram, portable and comfortable sleep monitoring requirements are met, temporal and spatial characteristics of the electroencephalogram are sufficiently excavated according to the convolutional neural networks and the recurrent neural networks, the method has dynamic learning capacity and can adapt to great changed environments of diseases, the staging optimization module sufficiently considers relation between the front and the back of N 30s of electroencephalogram data, and the staging accuracy and the generalization ability of the model are improved.
Owner:PEKING UNIV
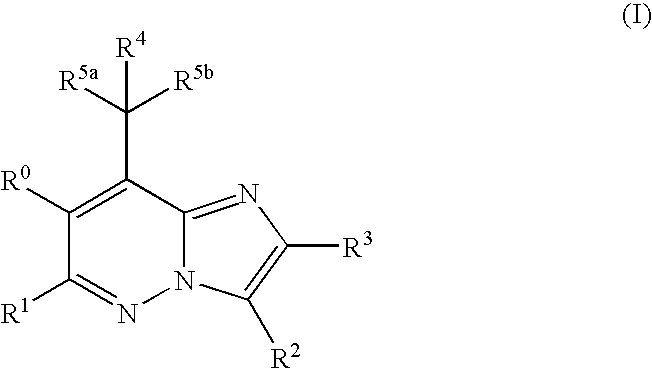
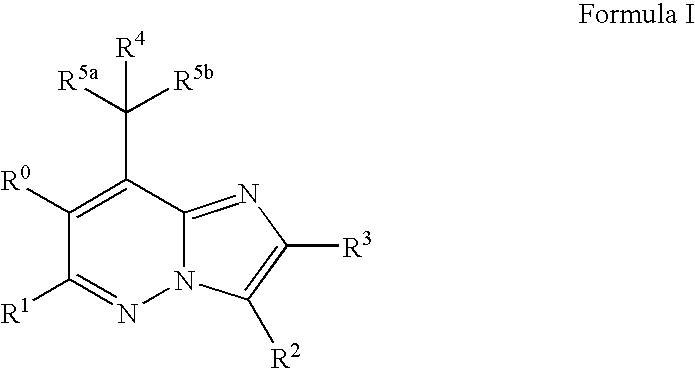
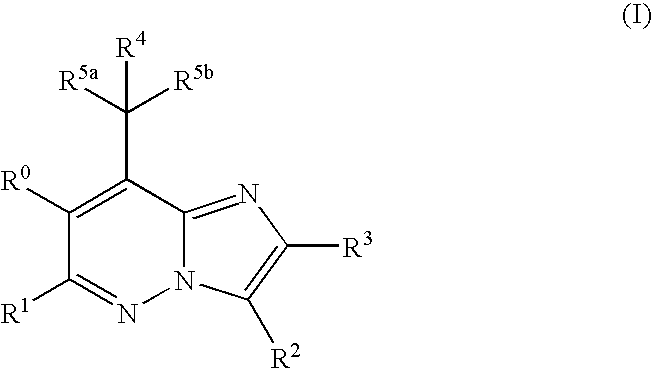
Imidazopyridazine compounds
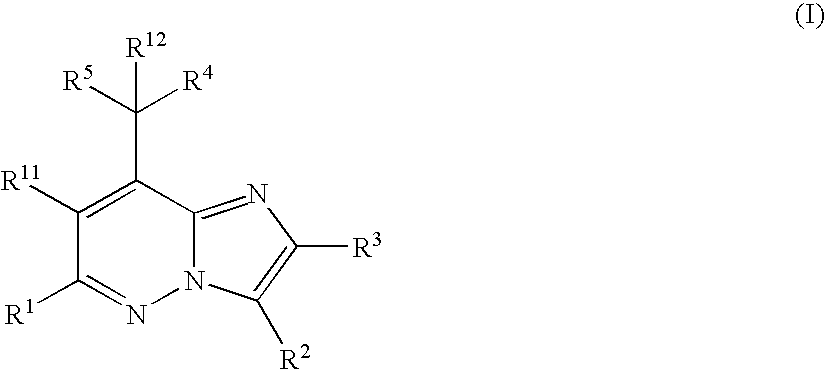
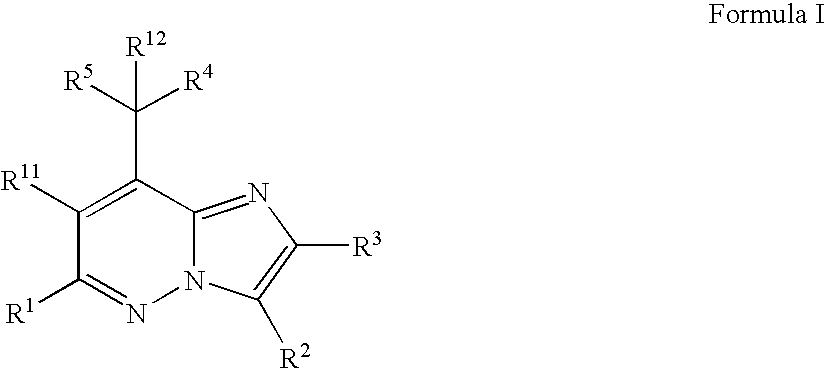
The present invention relates to novel substituted imidazo[1,2-b]pyridazine compounds of Formula (I): pharmaceutical compositions thereof, and the use such compounds as corticotropin releasing factor 1 (CRF1) receptor antagonists in the treatment of psychiatric disorders and neurological diseases.
Owner:ELI LILLY & CO
Methods for improved in vitro protein synthesis with proteins containing non standard amino acids
ActiveUS20160060301A1Improves chaperone levelImproves translation functionPeptide-nucleic acidsBacteriaBiopolymerAmino acid
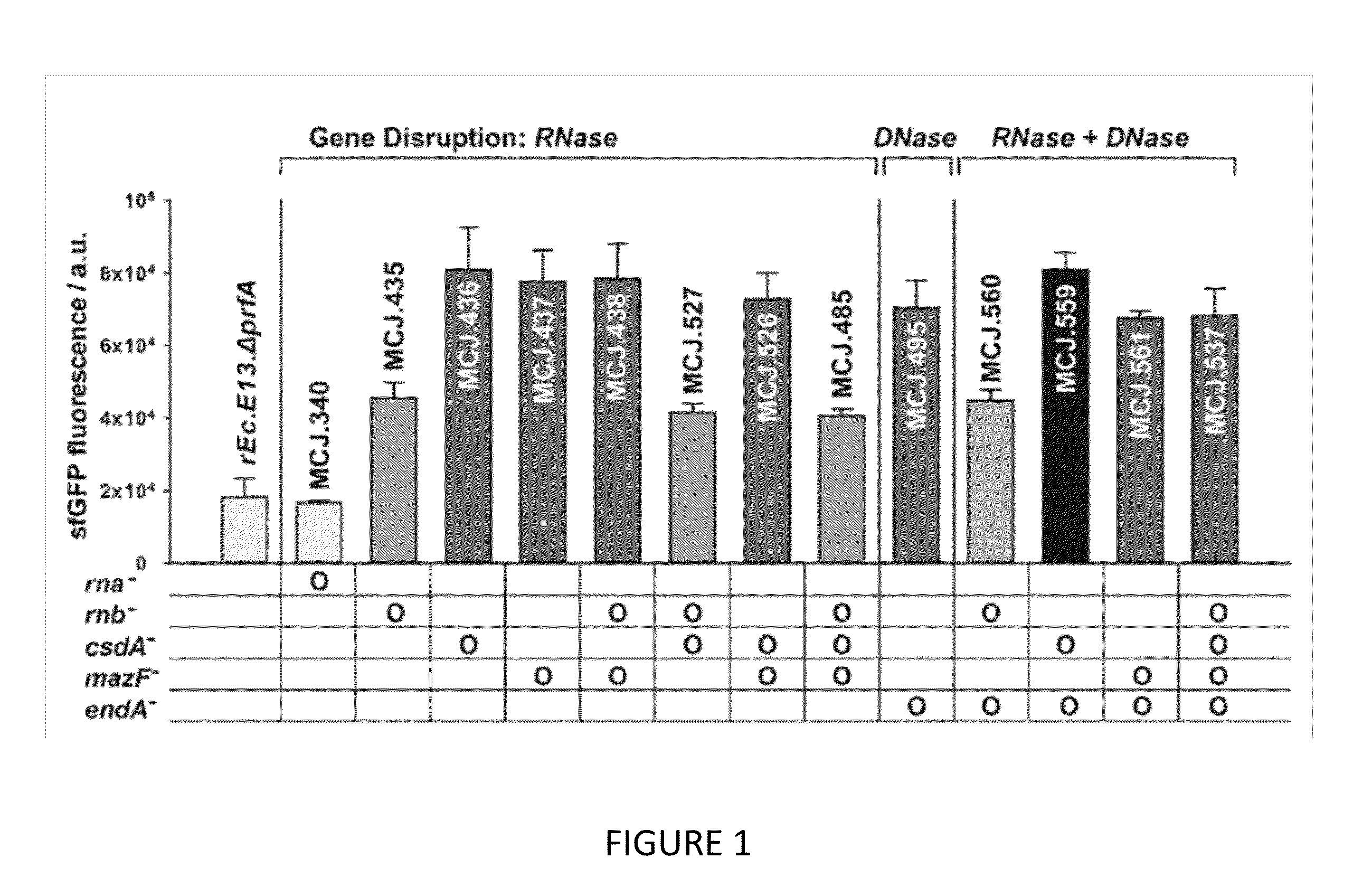
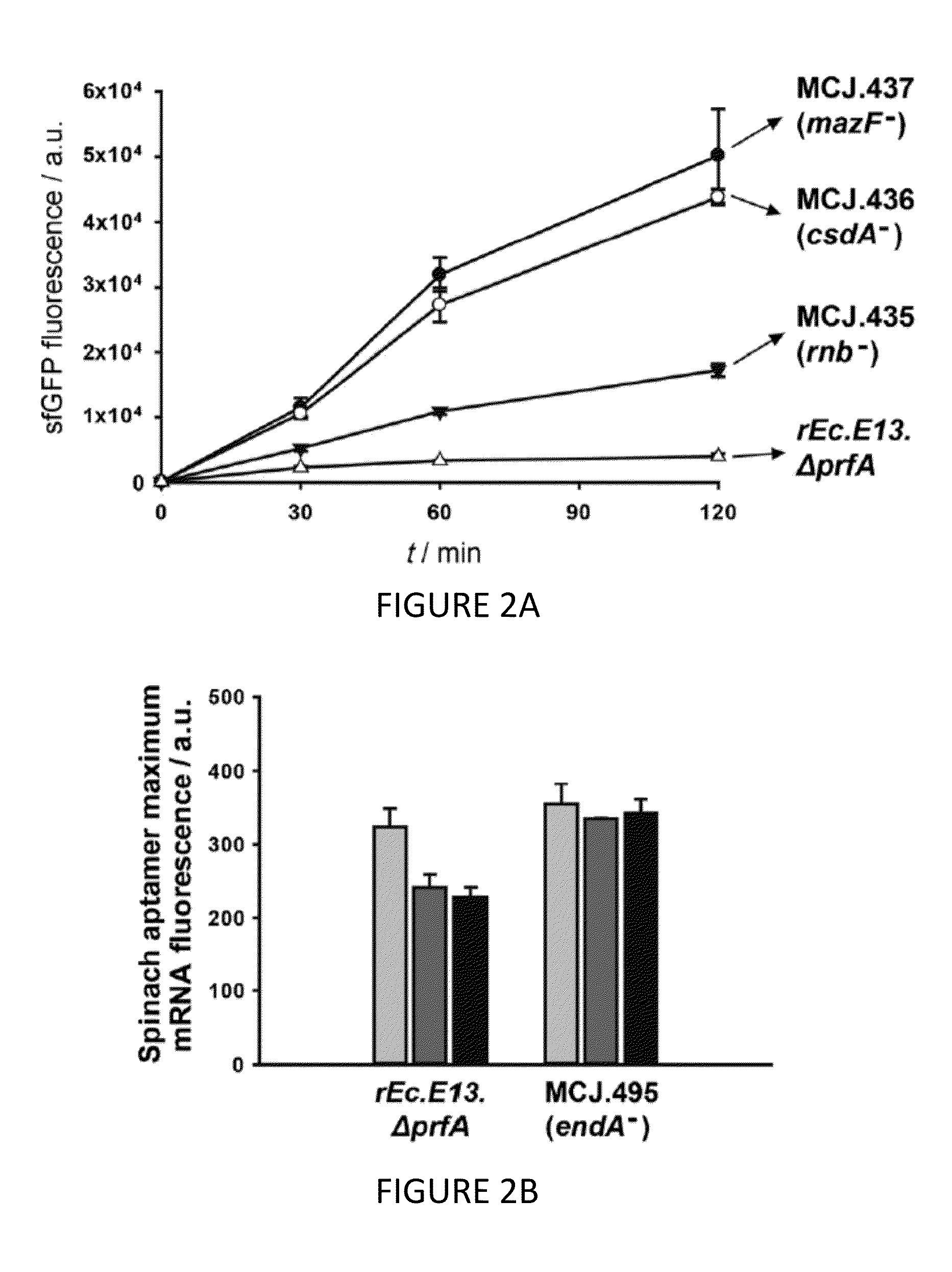
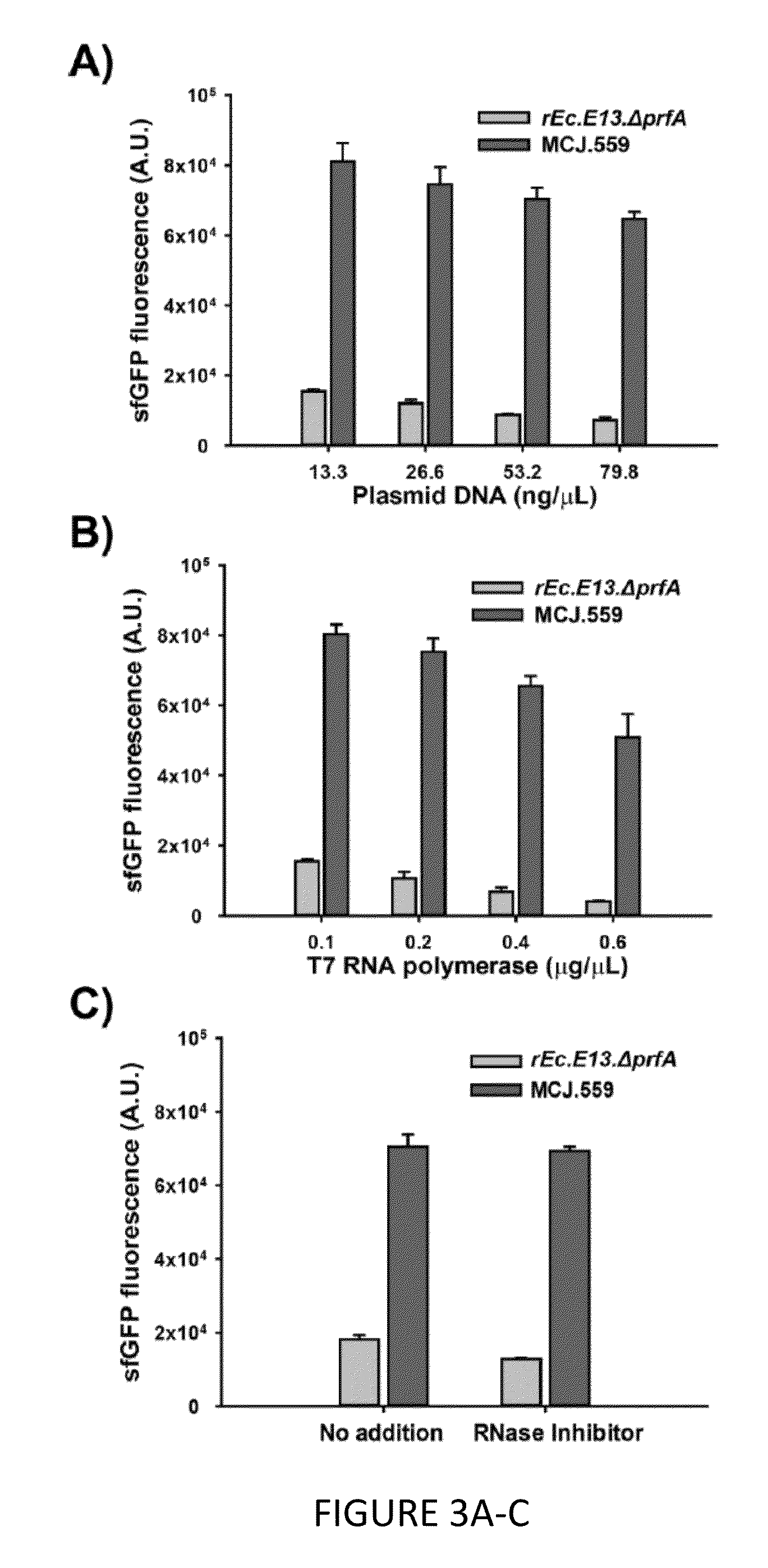
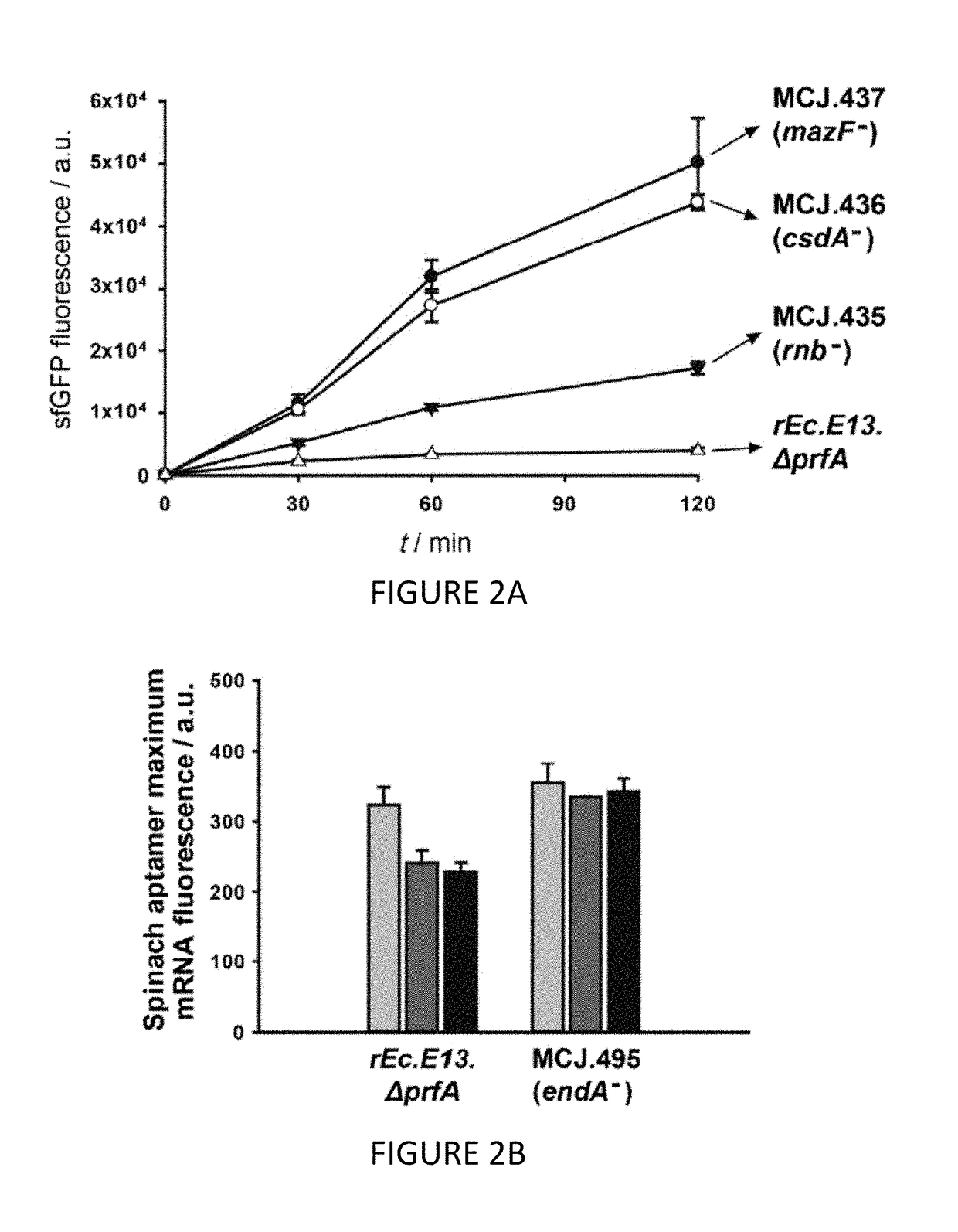
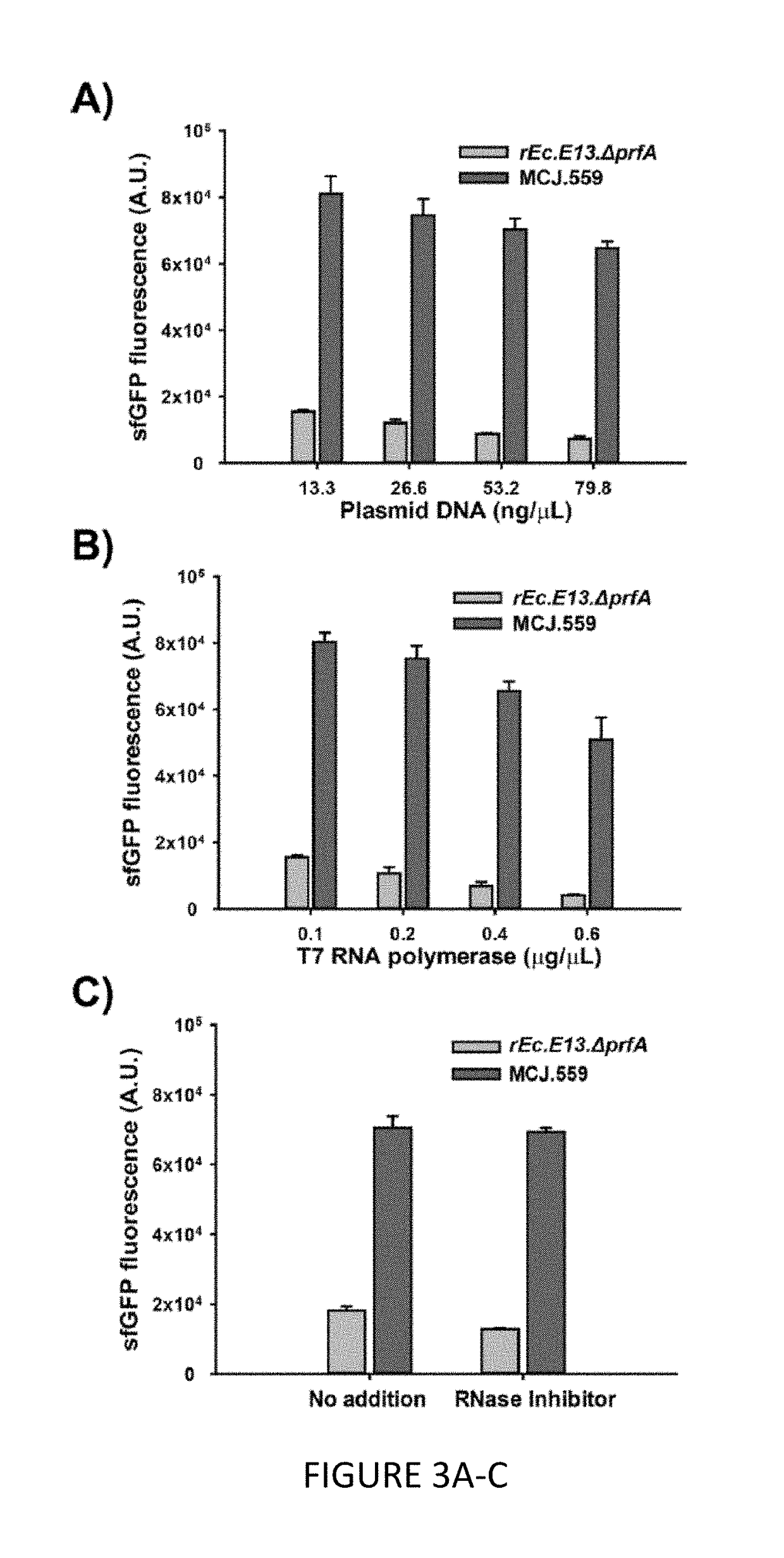
The invention relates to genomically recoded organisms, platforms for preparing sequence defined biopolymers in vitro comprising a cellular extract from a genomically recorded organism, and methods for preparing sequence defined biopolymers in vitro are described. In particular, the invention relates to genomically recoded organisms comprising a strain deficient in release factor 1 (RF-1) or a genetic homolog thereof and at least one of at least one additional genetic knock-out mutation, at least one additional upregulated gene product, or both at least one additional knock-out mutation and at least one additional upregulated gene product.
Owner:NORTHWESTERN UNIV
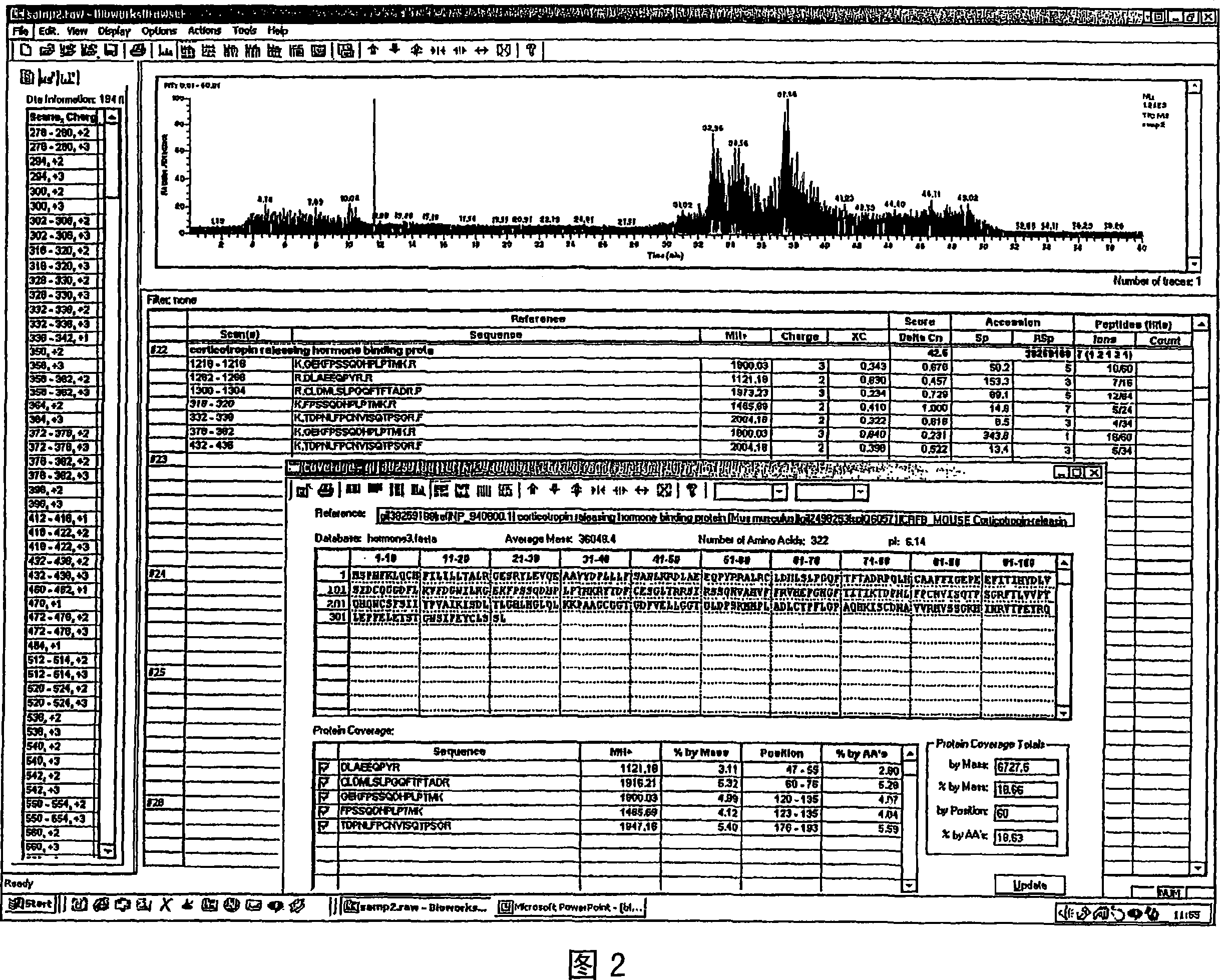
Method for differentially diagnosing ACTH-dependent Cushing's syndrome
This invention provides for an improved method for differentially diagnosing ACTH-dependent Cushing's syndrome. Current practice for differentially diagnosing ectopic ACTH syndrome and Cushing's Disease measures relative ACTH concentrations from the inferior petrosal venous sinus compared to fluid obtained from a periphery venous sample. This is performed before and after administration of exogenous corticotropin releasing factor, or after administration of metyrapone. This invention uses glucocorticoid receptor antagonists to induce release of endogenous CRH which stimulates ACTH to increase in patients with ectopic ACTH syndrome but not in those with Cushing's Disease.
Owner:CORCEPT THERAPEUTICS INC
Compositions for bacterial mediated gene silencing and methods of using the same
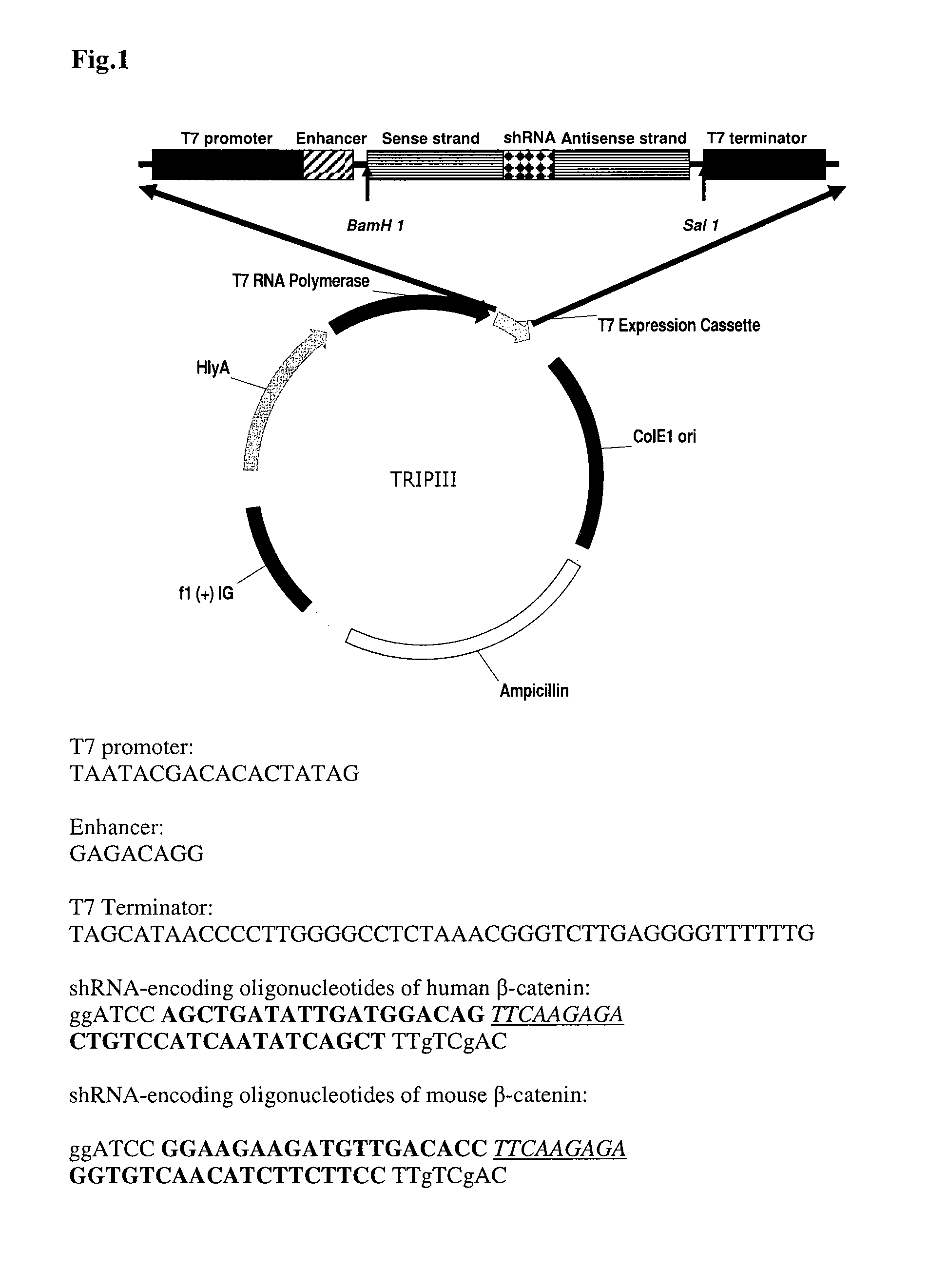
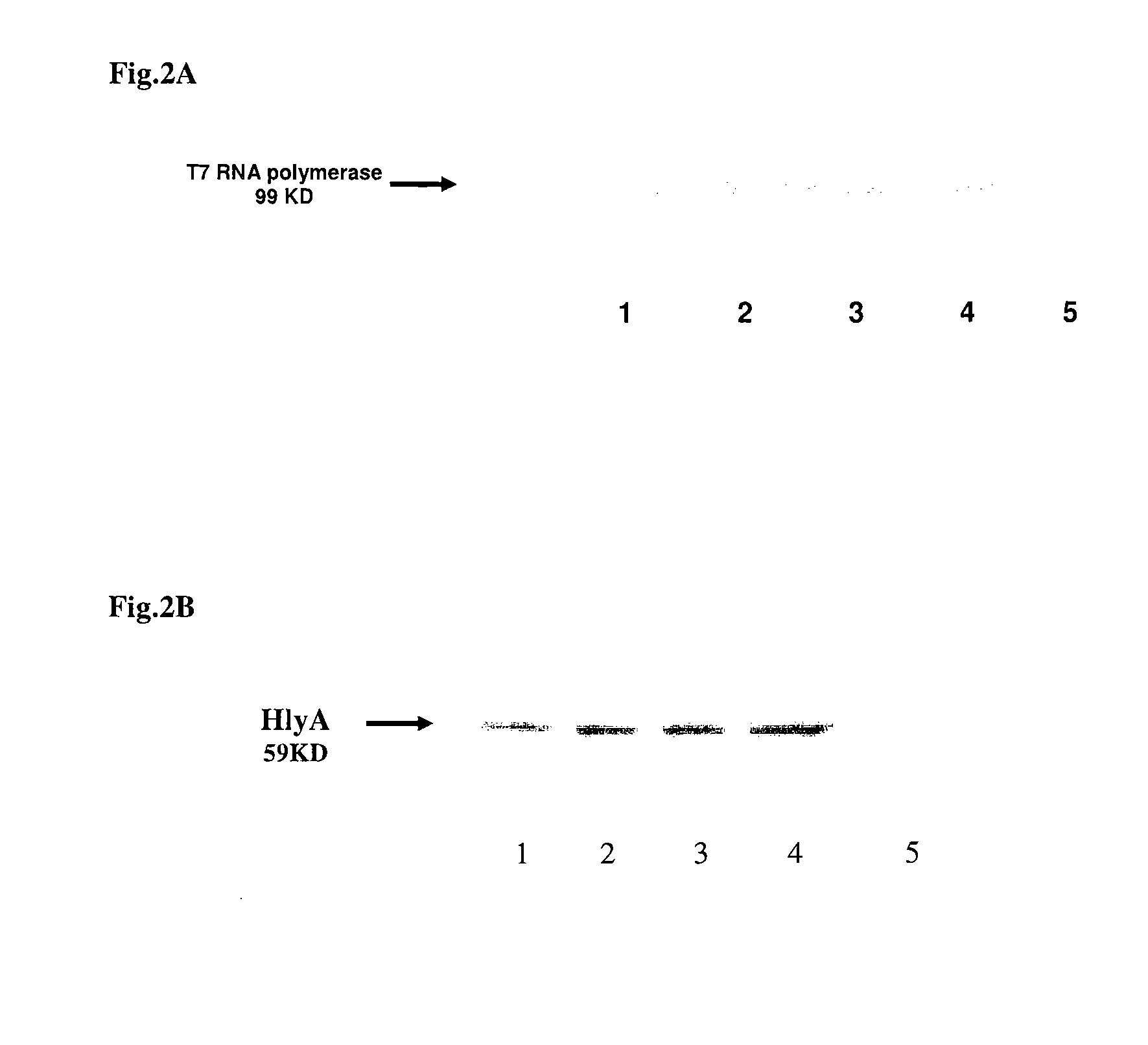
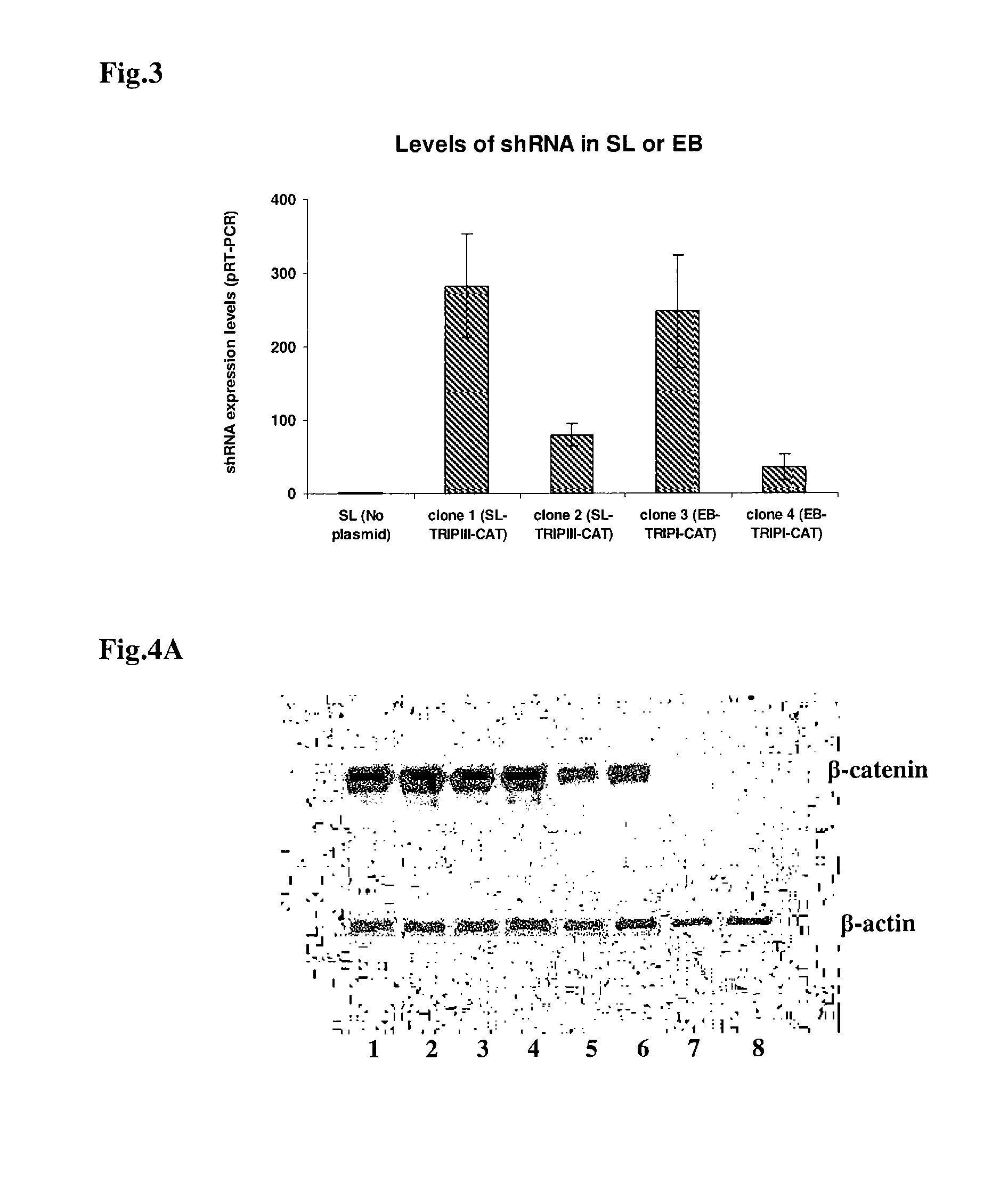
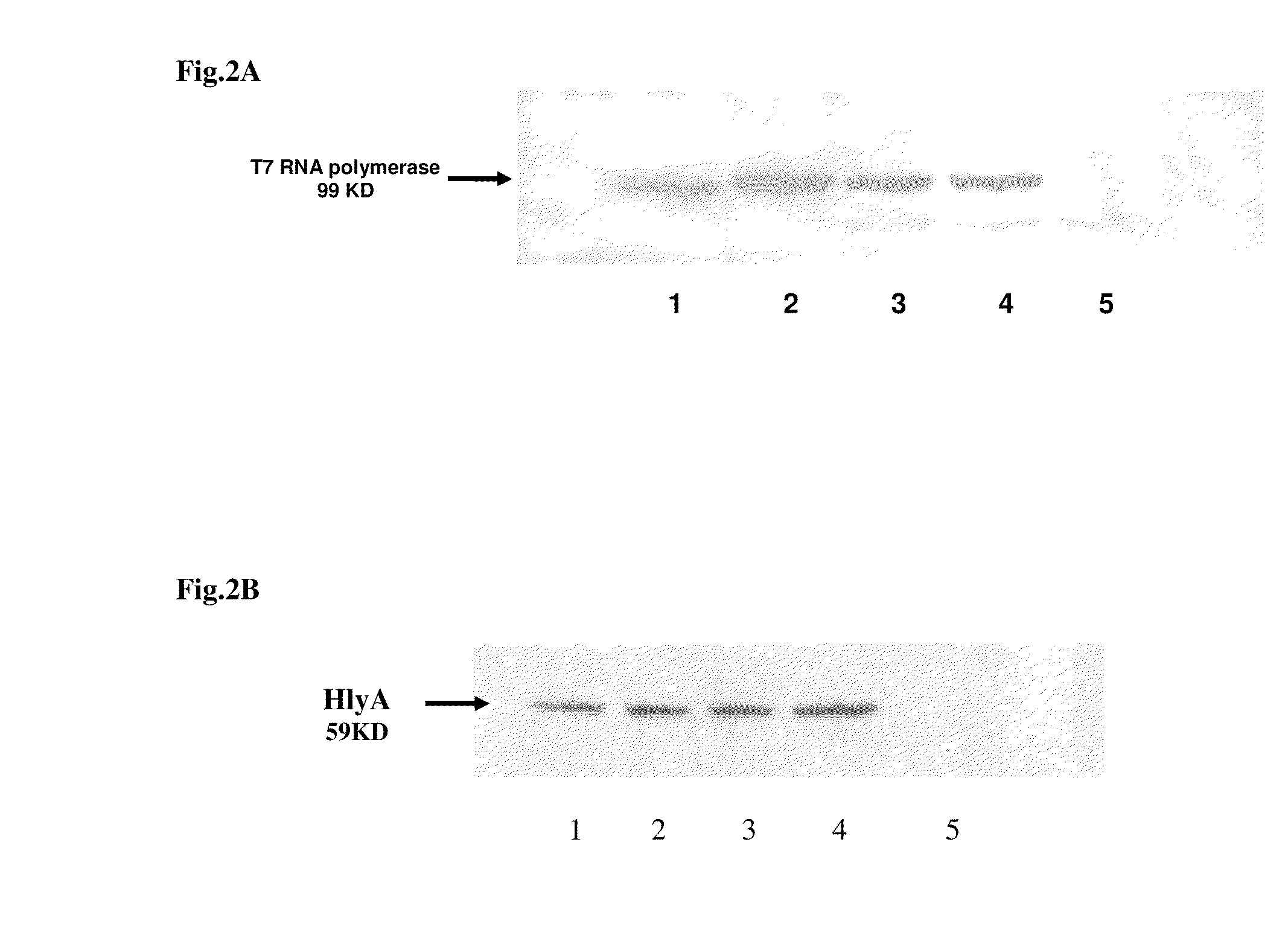
The invention features compositions and methods for delivering small interfering (siRNAs), e.g., shRNAs, to host cells using non-pathogenic strains of Salmonella bacteria containing nucleic acid expression constructs encoding shRNAs. In this process, shRNA expressed by the Salmonella silences or knocks down genes of interest (target genes) inside target cells. The nucleic acid expression constructs of the invention include an RNA polymerase (e.g., a T7 polymerase), an RNA polymerase promoter (e.g., a T7 polymerase promoter), and an RNA polymerase terminator (e.g., a T7 polymerase terminator). The Salmonella bacteria can also include, on the same or different nucleic acid construct, an endosomal release factor (e.g., HlyA).
Owner:GUO HONGNIAN +2
Corticotropin releasing factor 2 receptor agonists
InactiveUS6936585B2Effective treatmentPeptide/protein ingredientsMuscular disorderDiseaseCorticotropins
Isolated corticotropin releasing factor derivatives, and nucleic acids encoding the same, are effective for treating corticotropin releasing factor 2 receptor modulated disorders such as muscular dystrophy.
Owner:APTALIS PHARMA
Platforms for cell-free protein synthesis comprising extracts from genomically recoded E. coli strains having genetic knock-out mutations in release factor 1 (RF-1) and endA
ActiveUS10118950B2Function increaseImprove the level ofPeptide-nucleic acidsTransferasesEscherichia coliBiopolymer
Owner:NORTHWESTERN UNIV
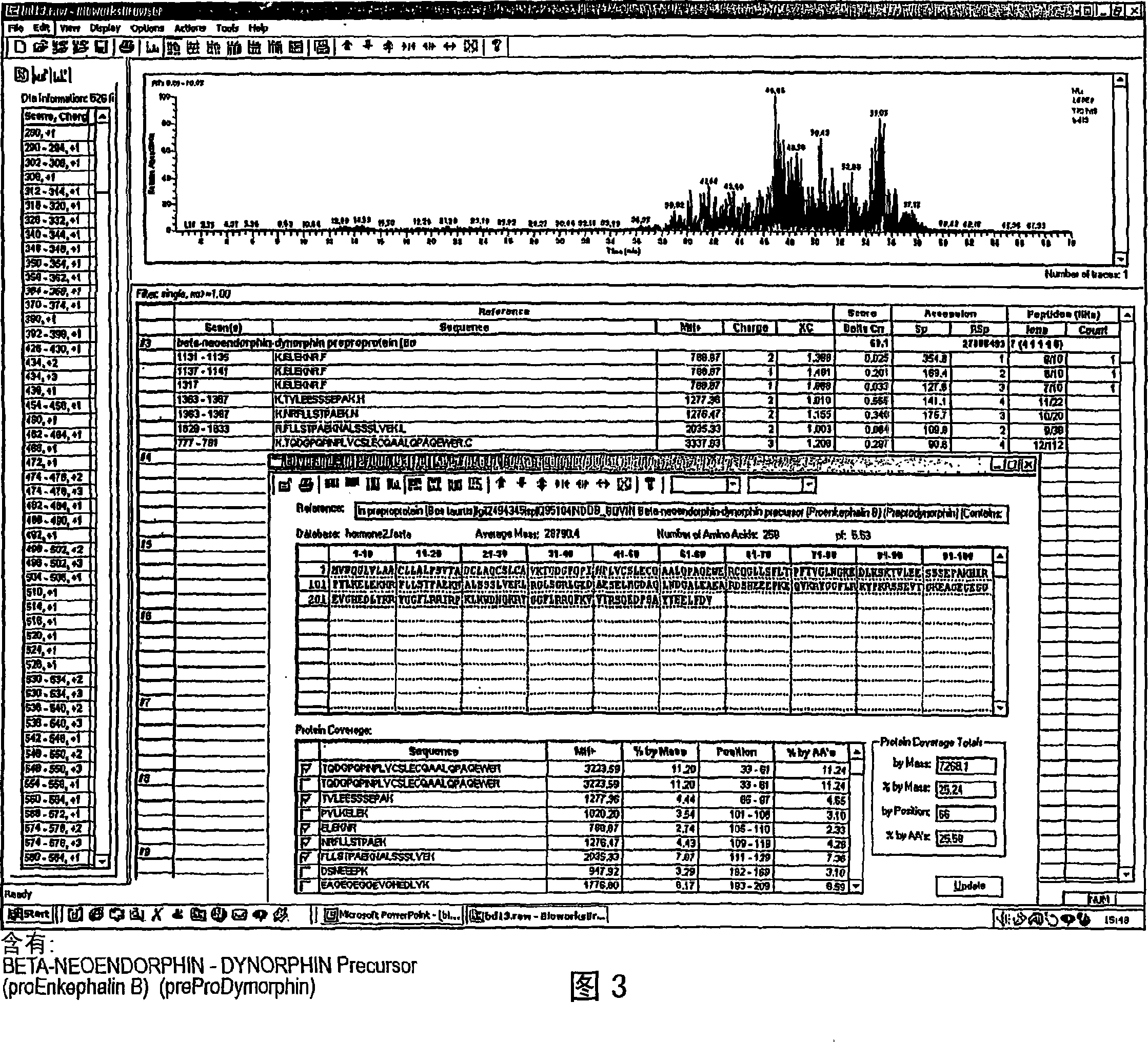
Abstraction of pilose antler releasing somatomedin (DEER GHRF) and preparation method thereof
The present invention relates to process of extracting growth hormone release factor (GHRF) from pilose antler. The process includes the following steps: 1. crushing pilose antler and leaching to obtain leached liquid; 2. ultrafiltering the leached liquid and collecting the pilose antler extract of molecular weight 1-10 kDa; and 3. high efficiency liquid chromatography on the pilose antler extract and collecting component containing GHRF of pilose antler. The present invention also relates to the pilose antler extract with rich GHRF, and its application in medicine, food, cosmetics, feed, etc.
Owner:王利忠
Imidazopyridazine Compounds
The present invention relates to novel substituted imidazo[1,2-b]pyridazine compounds of Formula (I): pharmaceutical compositions thereof, and the use such compounds as corticotropin releasing factor 1 (CRF1) receptor antagonists in the treatment of psychiatric disorders and neurological diseases.
Owner:ELI LILLY & CO
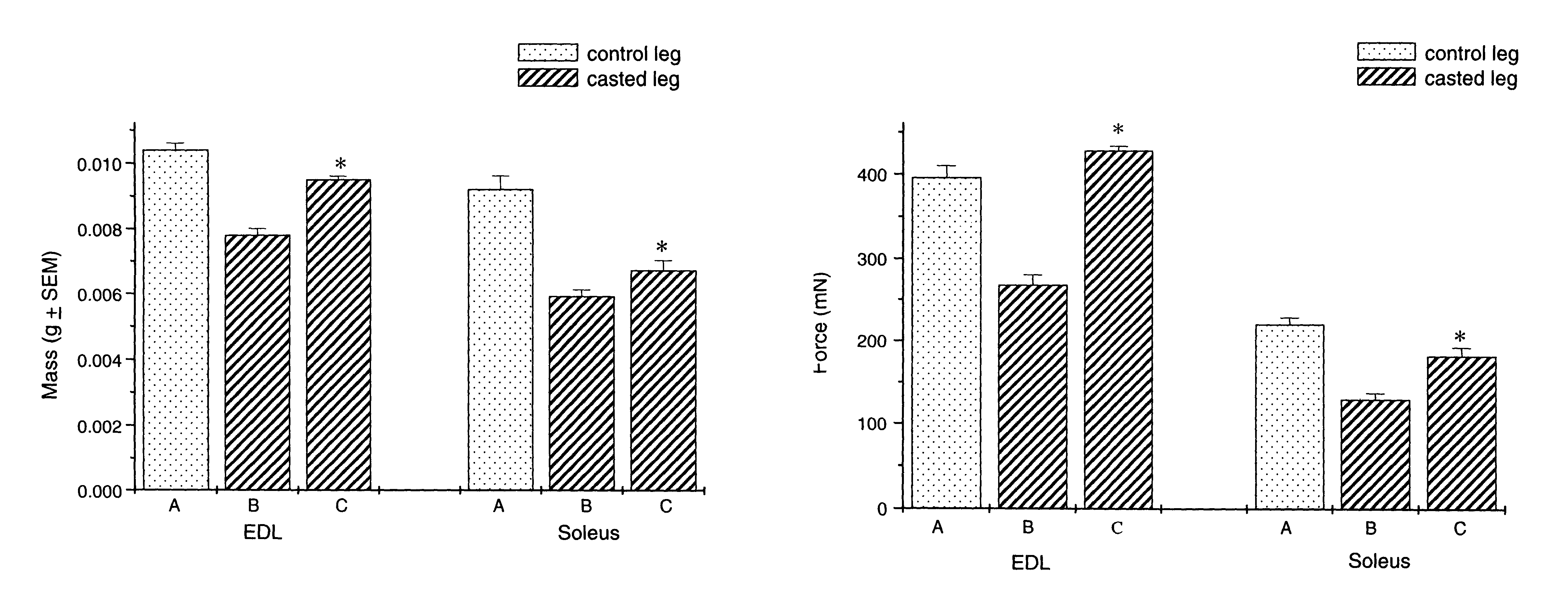
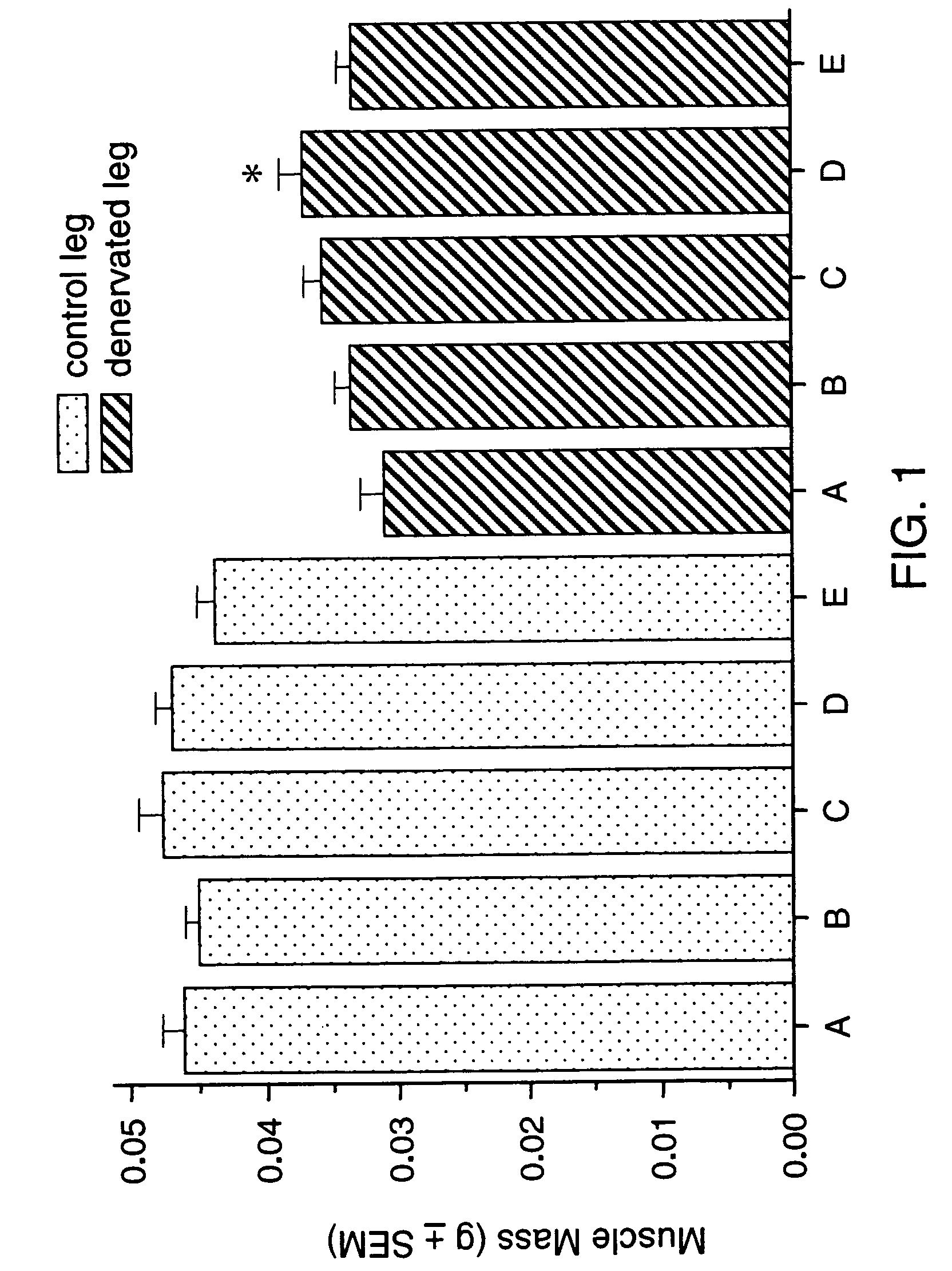
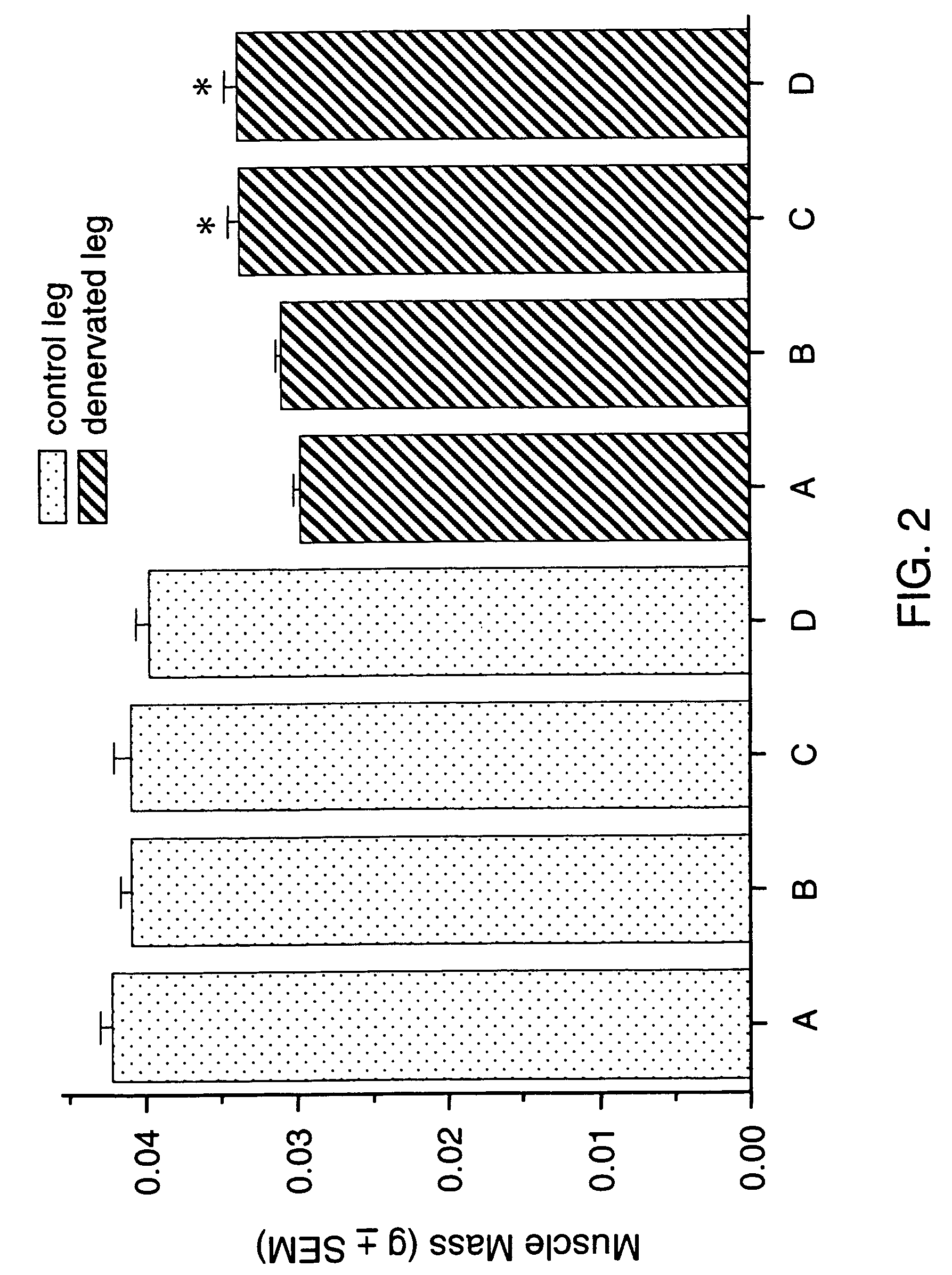
Methods for identifying compounds for regulating muscle mass or function using corticotropin releasing factor receptors
InactiveUS20060198842A1Compounds screening/testingPeptide/protein ingredientsCorticotropinsScreening method
Screening methods for identifying compounds that bind to or activate corticotropin releasing factor2 receptors (CRF2R) and regulate or potentially regulate skeletal muscle mass or function in vivo are disclosed. Also disclosed are screening methods for identifying compounds that prolong or augment the activation of CRF2Rs or of CRF2R signal transduction pathways, increase CRF2R or increase CRF expression are provided. Pharmaceutical compositions comprising CRF2R agonists, antibodies to CRF2R and methods for increasing skeletal muscle mass or function or for the treatment of skeletal muscle atrophy using CRF2R as the target for intervention and methods for treatment of muscular dystrophies are described.
Owner:APTALIS PHARMA
Imidazopyridazine compounds
The present invention relates to novel substituted imidazo[1,2-b]pyridazine compounds of Formula (I) pharmaceutical compositions thereof, and the use such compounds as corticotropin releasing factor 1 (CRF1) receptor antagonists in the treatment of psychiatric disorders and neurological diseases.
Owner:ELI LILLY & CO
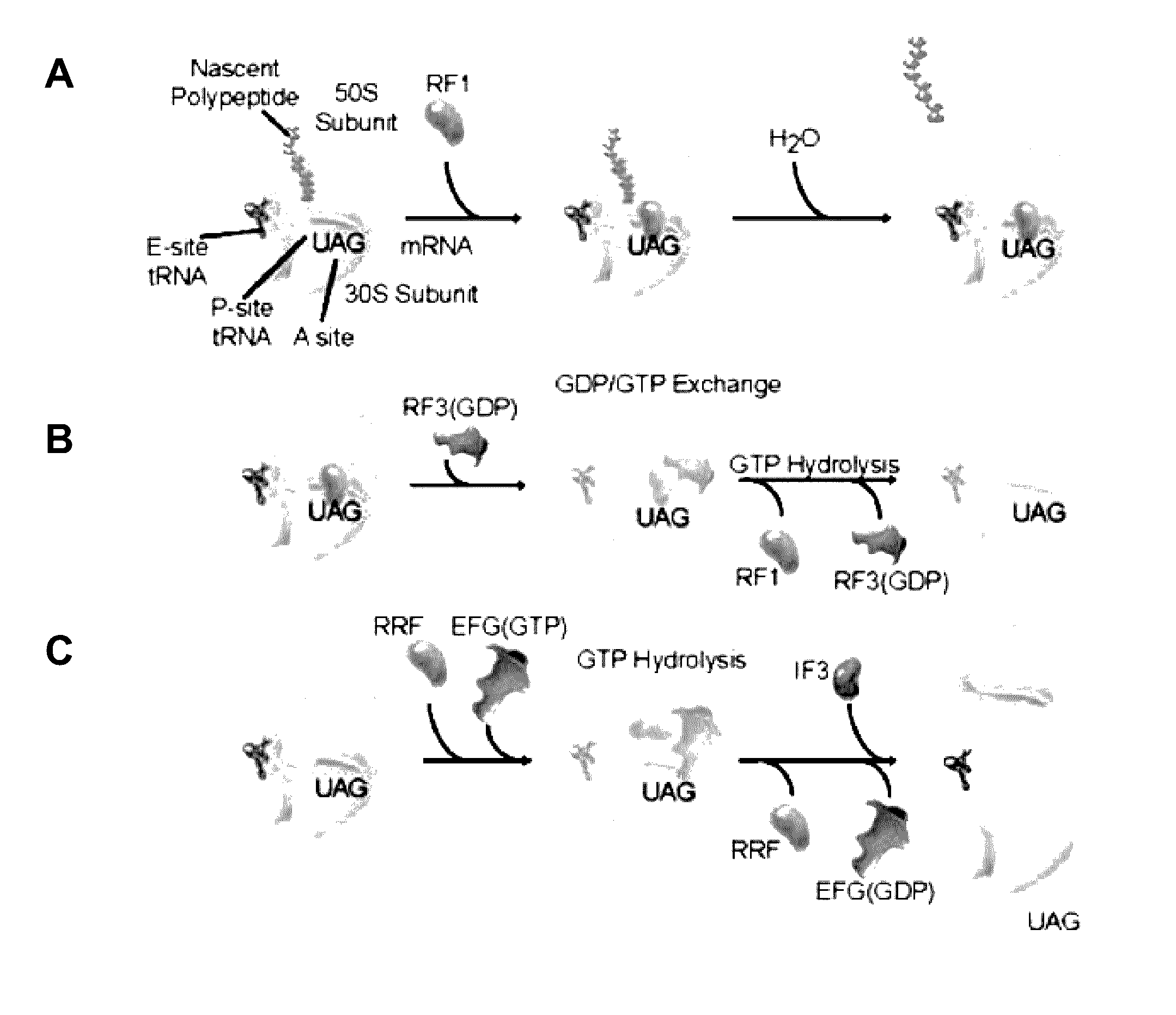
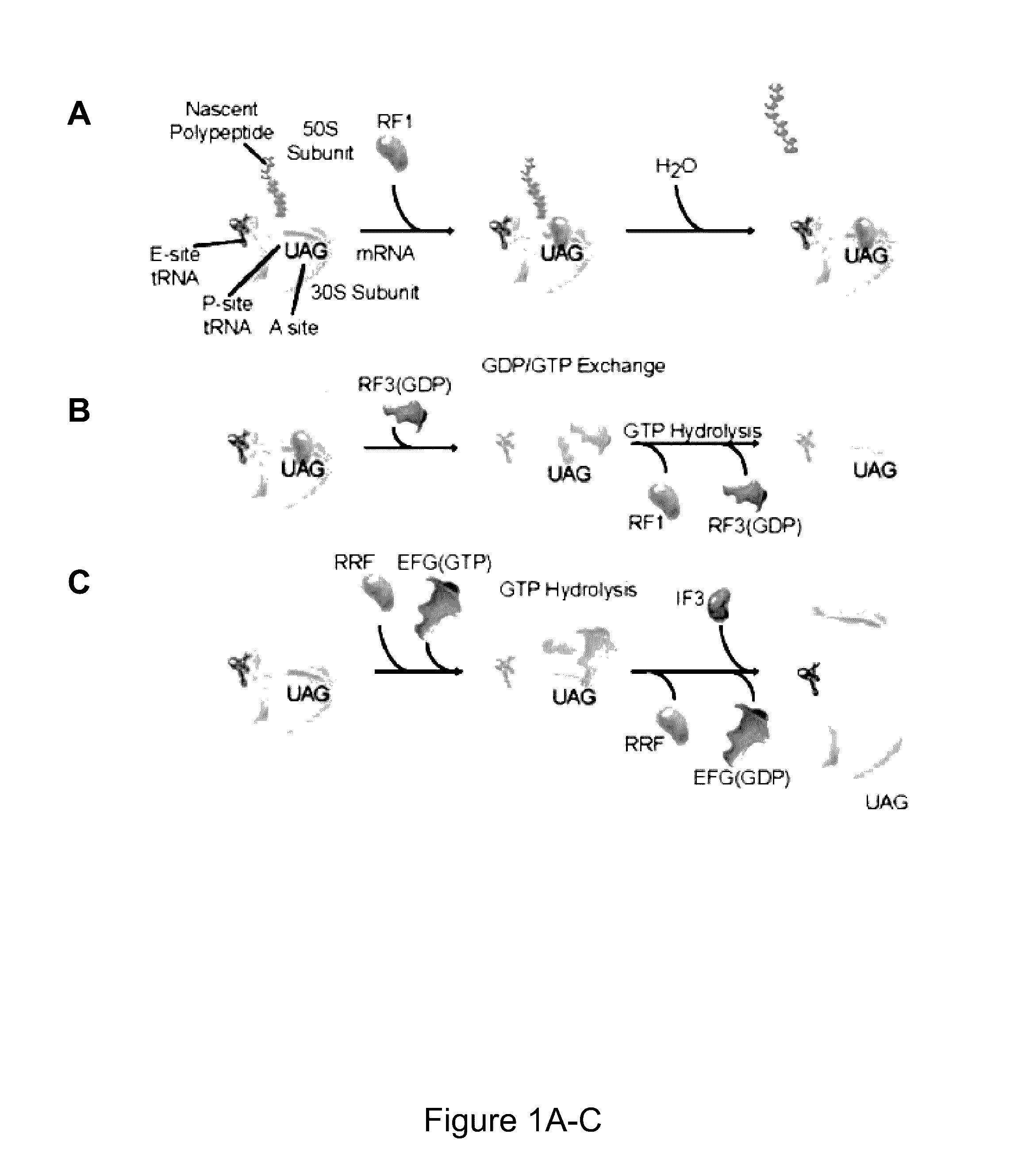
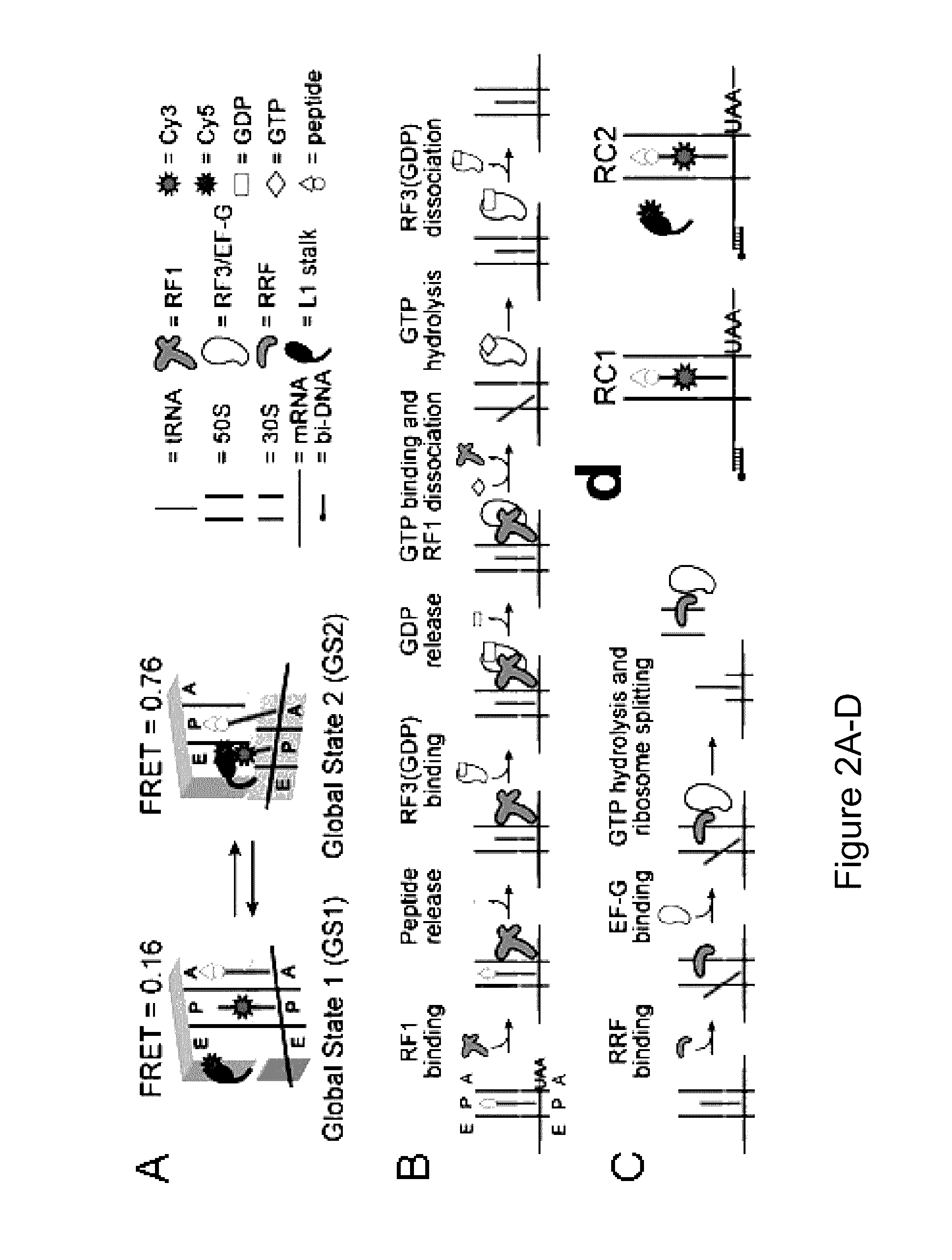
Fluorescence-based approach to monitor release factor-catalyzed termination of protein synthesis
InactiveUS9470680B2Reducing nonsense-mediated decayEfficient transferDepsipeptidesPeptide preparation methodsPremature Stop CodonProtein synthesis termination
Provided are probes comprising a class 1 release factor conjugated to a fluorescent label and methods of making the probes. Also provided are methods for detecting conformational changes in ribosomes and associated molecules, such as class 1 release factors. In addition, methods of identifying a compound for reducing nonsense-mediated decay of mRNA and / or for inhibiting termination of protein synthesis at a premature stop codon, are described. Methods of assaying RF3 activity are also included.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
External use medicine composition and preparing method thereof
InactiveCN104338118ASmooth appearanceDelicate appearancePeptide/protein ingredientsDermatological disorderEffective treatmentAstressin-B
The invention belongs to the technical field of medicine compositions, and discloses an external use medicine composition used for treating alopecia. The external use medicine composition comprises annular adrenocorticotrophic hormone release factor (CRF) antagonism peptide astressin B in effective treatment amount or acceptable salt on the pharmacy. The external use medicine composition is in a gel dosage form preferably and suitable for clinic use and industrialization production. In addition, the invention further discloses a method for preparing the external use medicine composition and application of the astressin B in effective treatment amount or the acceptable salt on the pharmacy to preparing the external use medicine composition used for treating the alopecia. According to the external use medicine composition, the problem of the alopecia for a long time can be effectively treated; and the external use medicine composition is safe, reliable, free of obvious skin irritants and free of sensitization.
Owner:HYBIO PHARMA
Corticotropin releasing factor receptor-2 inhibitors and uses thereof
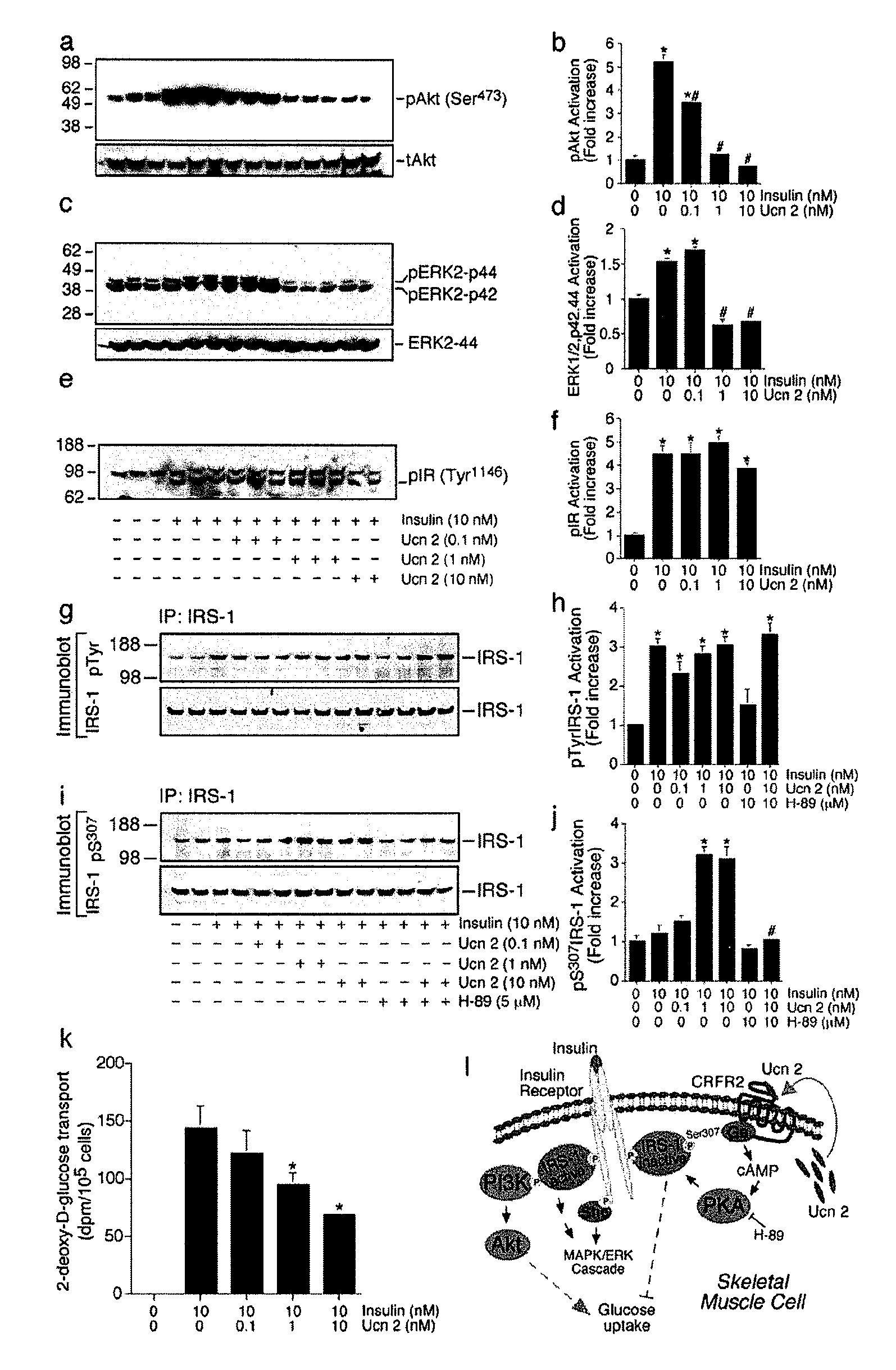
ActiveUS20080161235A1Attenuates glucose induced insulin secretionPeptide/protein ingredientsMetabolism disorderDiseaseCorticotropins
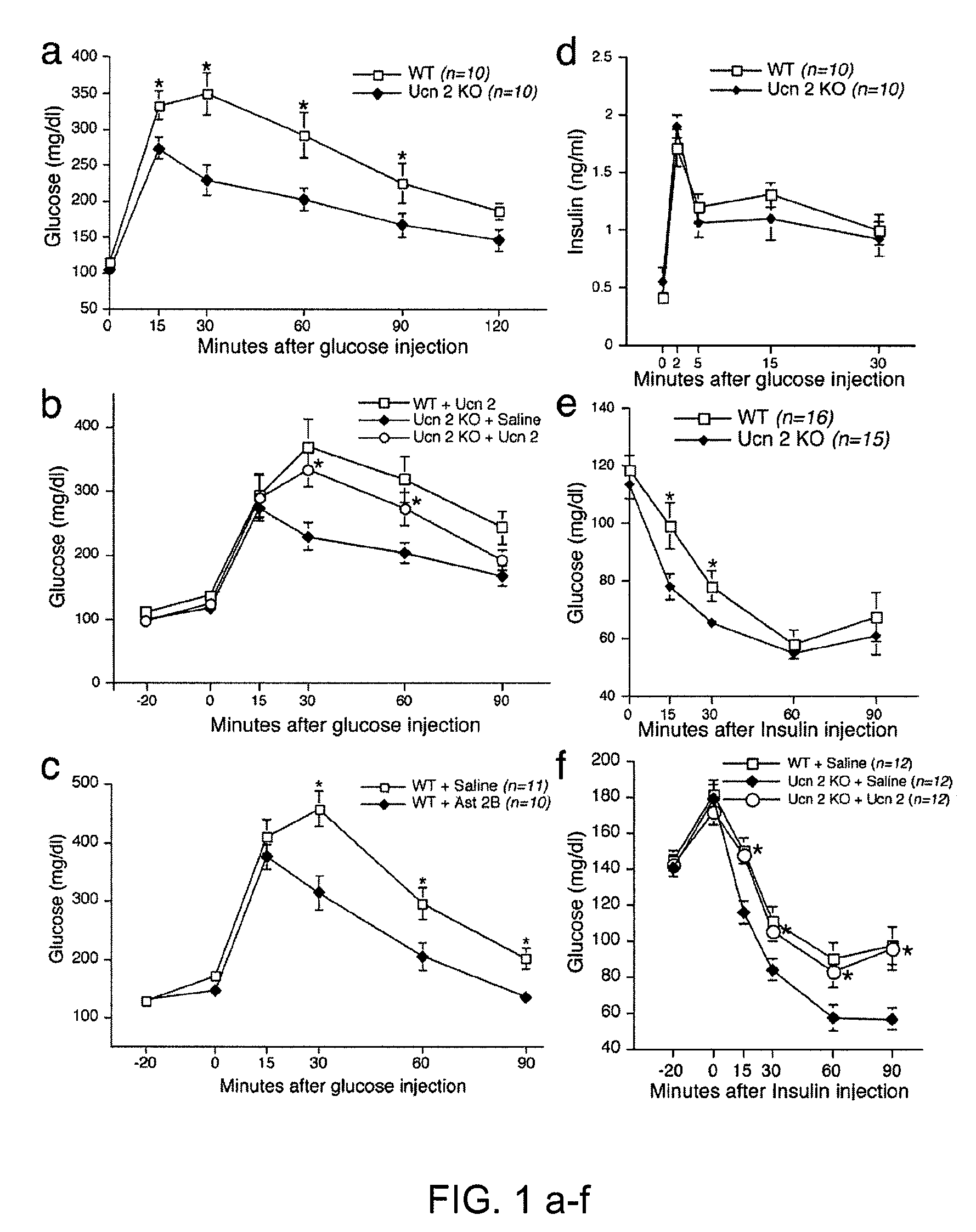
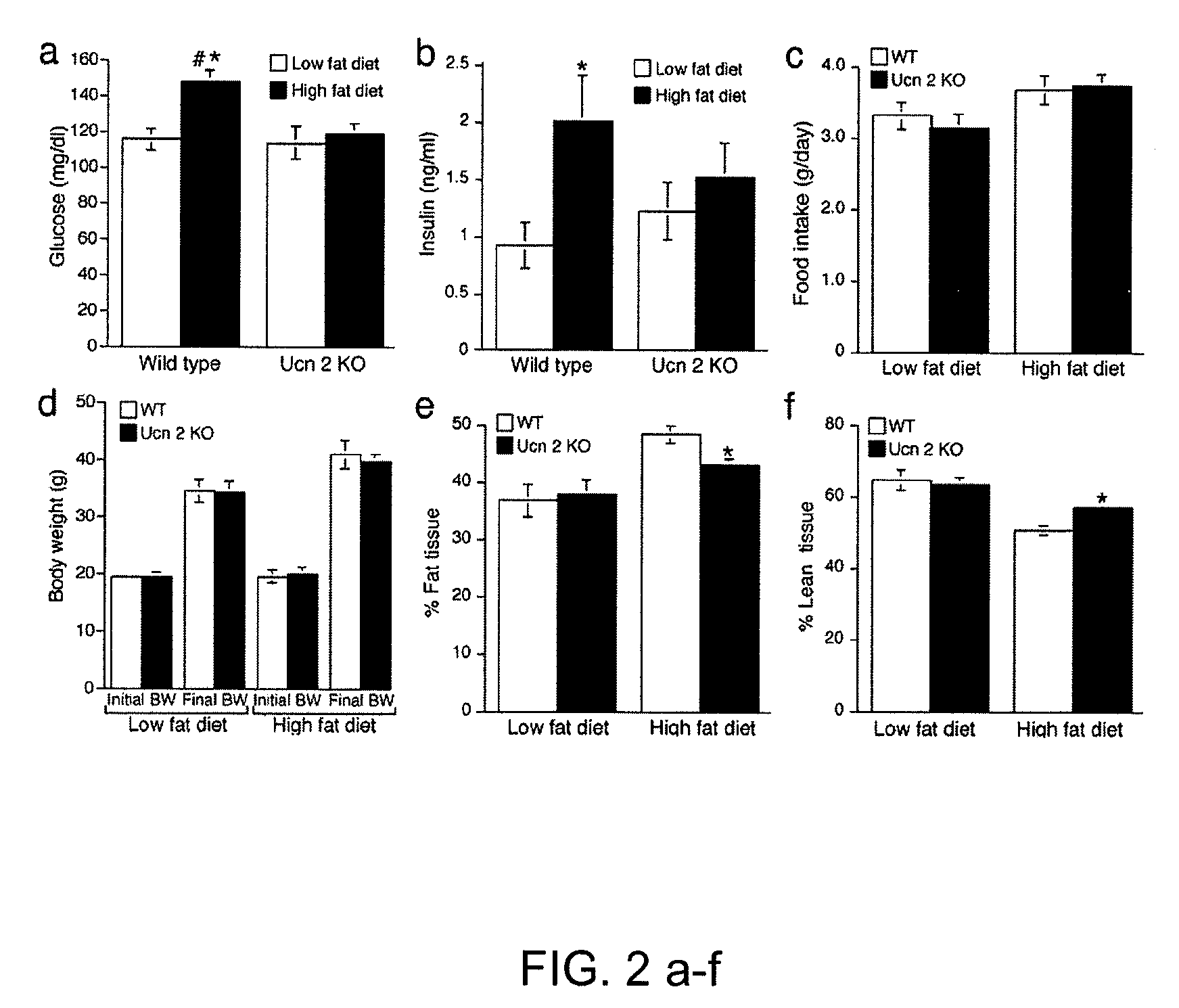
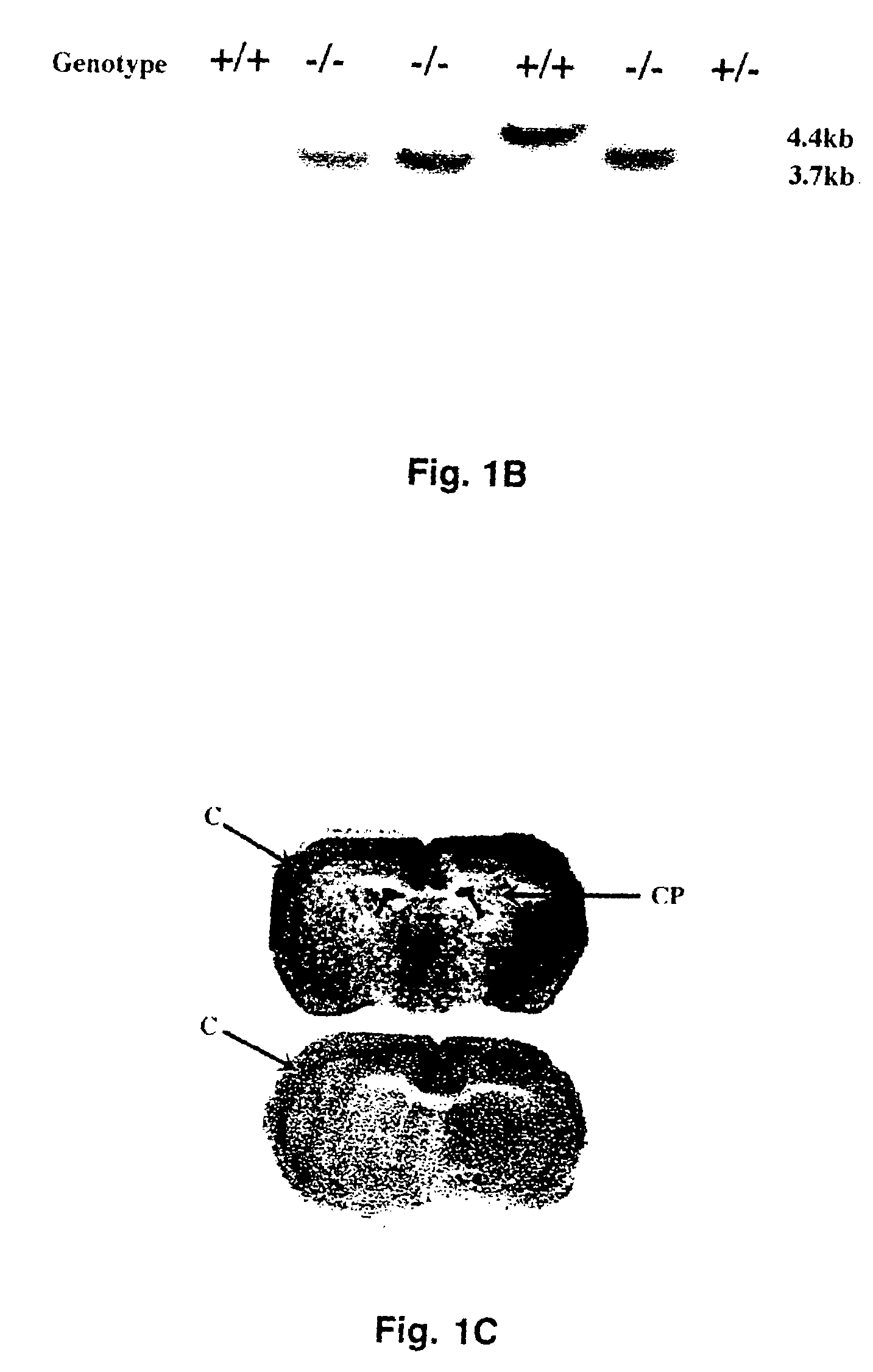
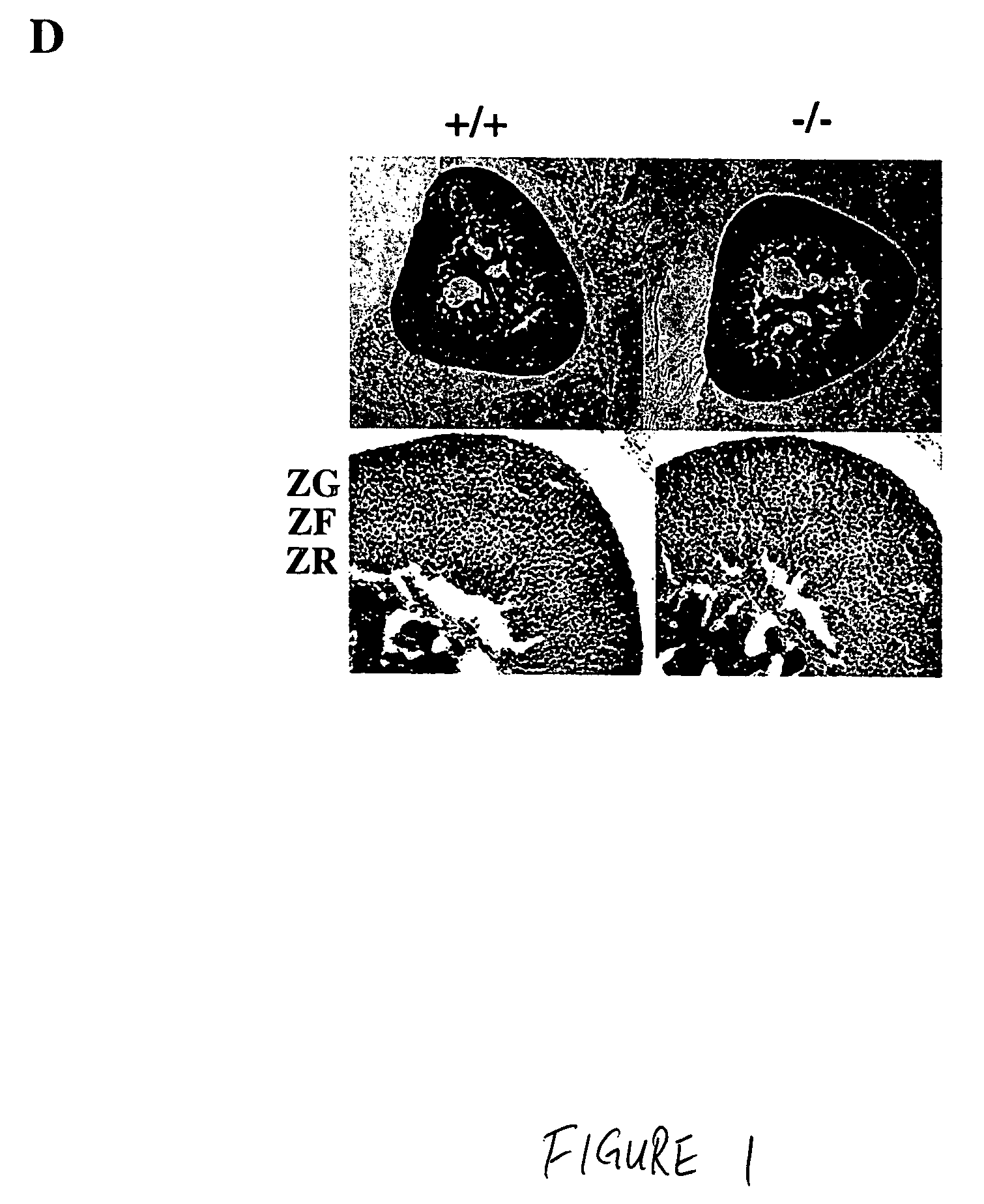
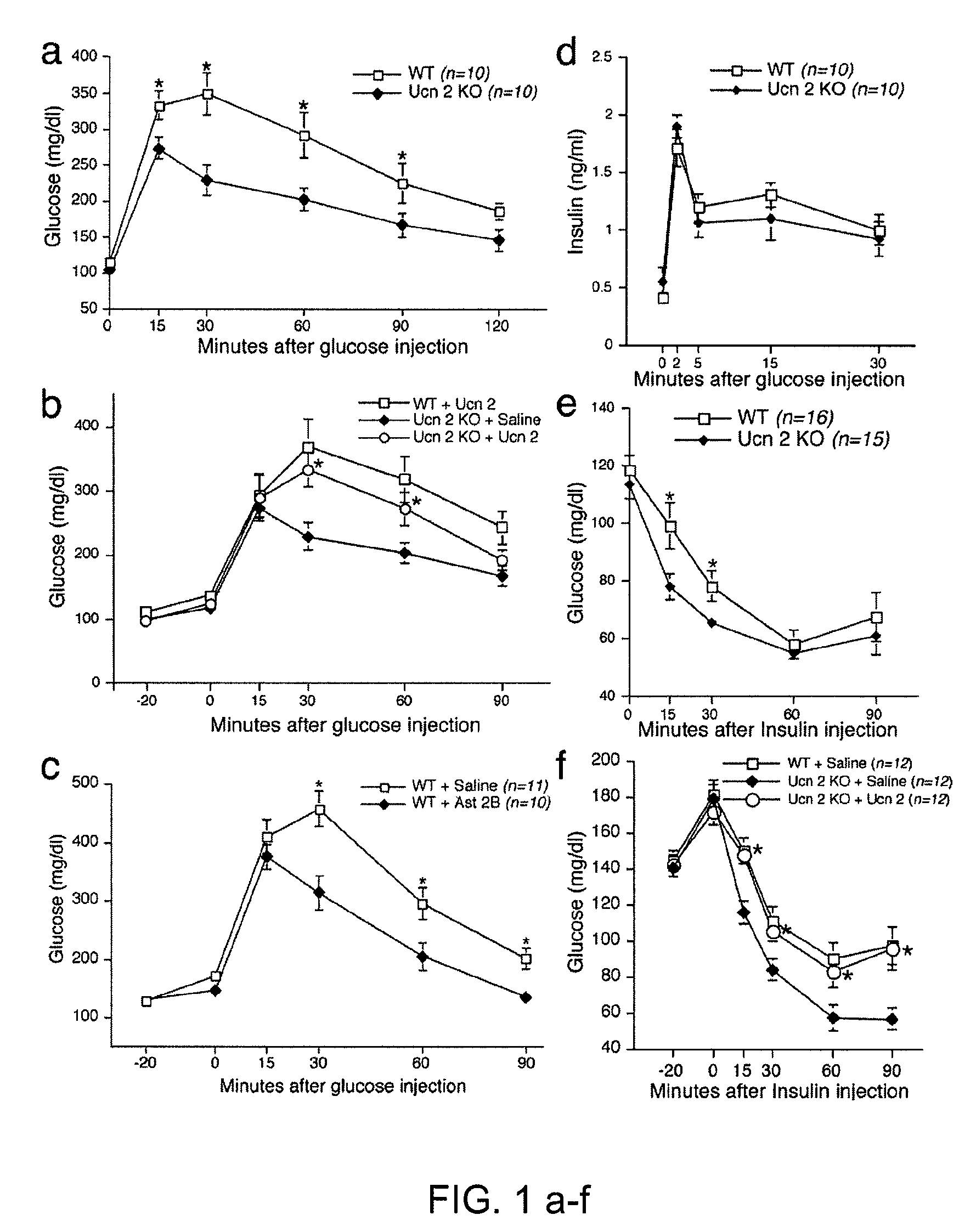
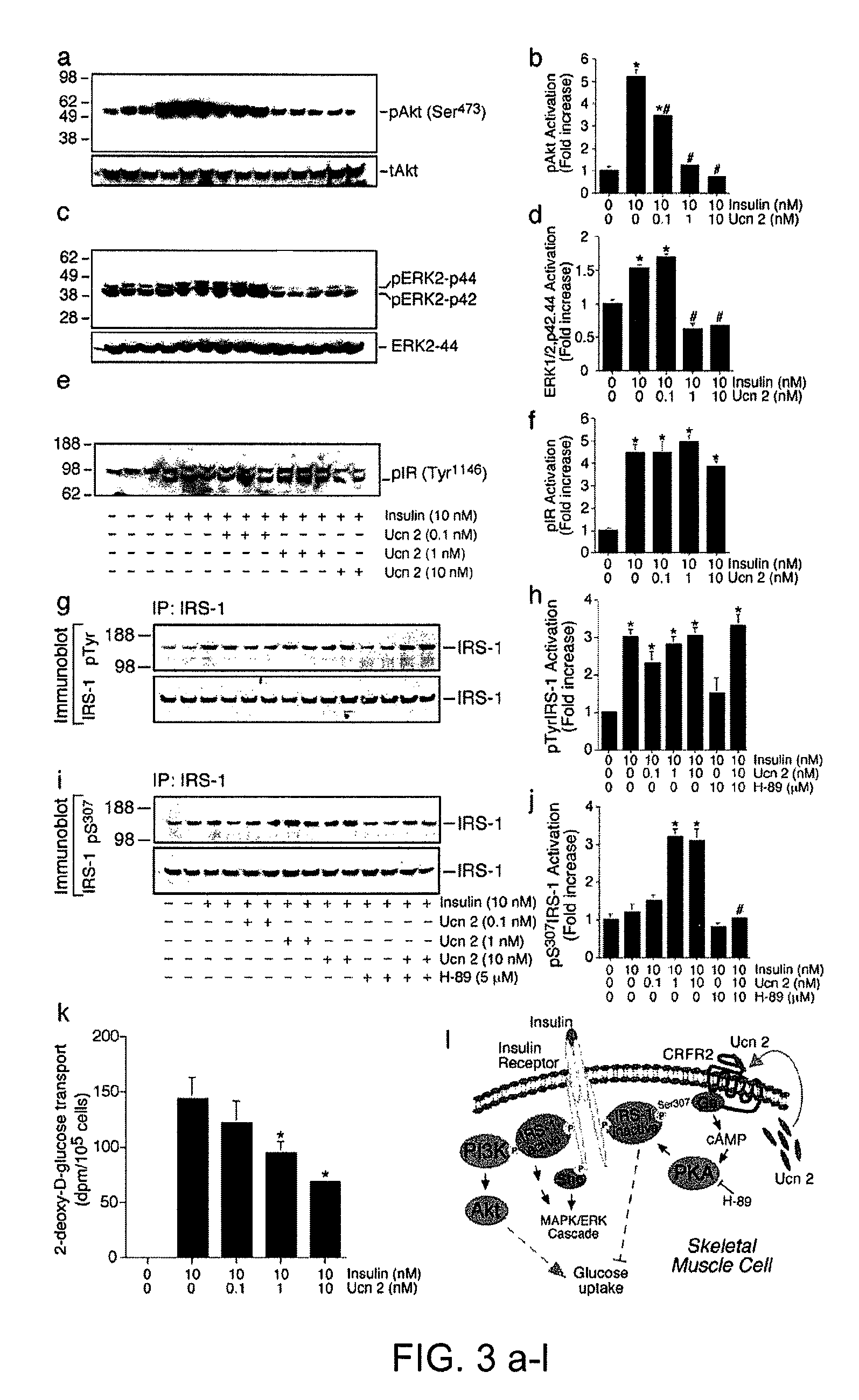
In some aspects, the invention relates to methods for increasing insulin-sensitivity and / or decreasing insulin secretion in an individual by reducing or inhibiting corticotropin releasing factor 2 (CRFR2) signaling. CRFR2 antagonists may block agonism by one or more CRFR2 agonist, for example Ucn 2 or Ucn 3. Methods according to the invention may be use to treat a variety of metabolic diseases such as type 2 diabetes, metabolic syndrome, nonalcoholic fatty liver disease, polycystic ovarian syndrome and obesity.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Recombinant fusion protein with targeted cell for killing tumor
InactiveCN1840546AHigh activityImprove side effectsHybrid peptidesPseudomonas aeruginosa exotoxin AMutant
Owner:BEIJING NORTHLAND BIOTECH
Method of inhibiting angiogenesis by administration of a corticotropin releasing factor receptor 2 agonist
InactiveUS7674463B1Alters anxiety stateReduce appetiteCompounds screening/testingOrganic active ingredientsCorticotropinsTransgene
Owner:RES DEVMENT FOUND
Medicament
InactiveUS20080207500A1Remarkable effectConvenient sourceSenses disorderNervous disorderDiseaseTreatment effect
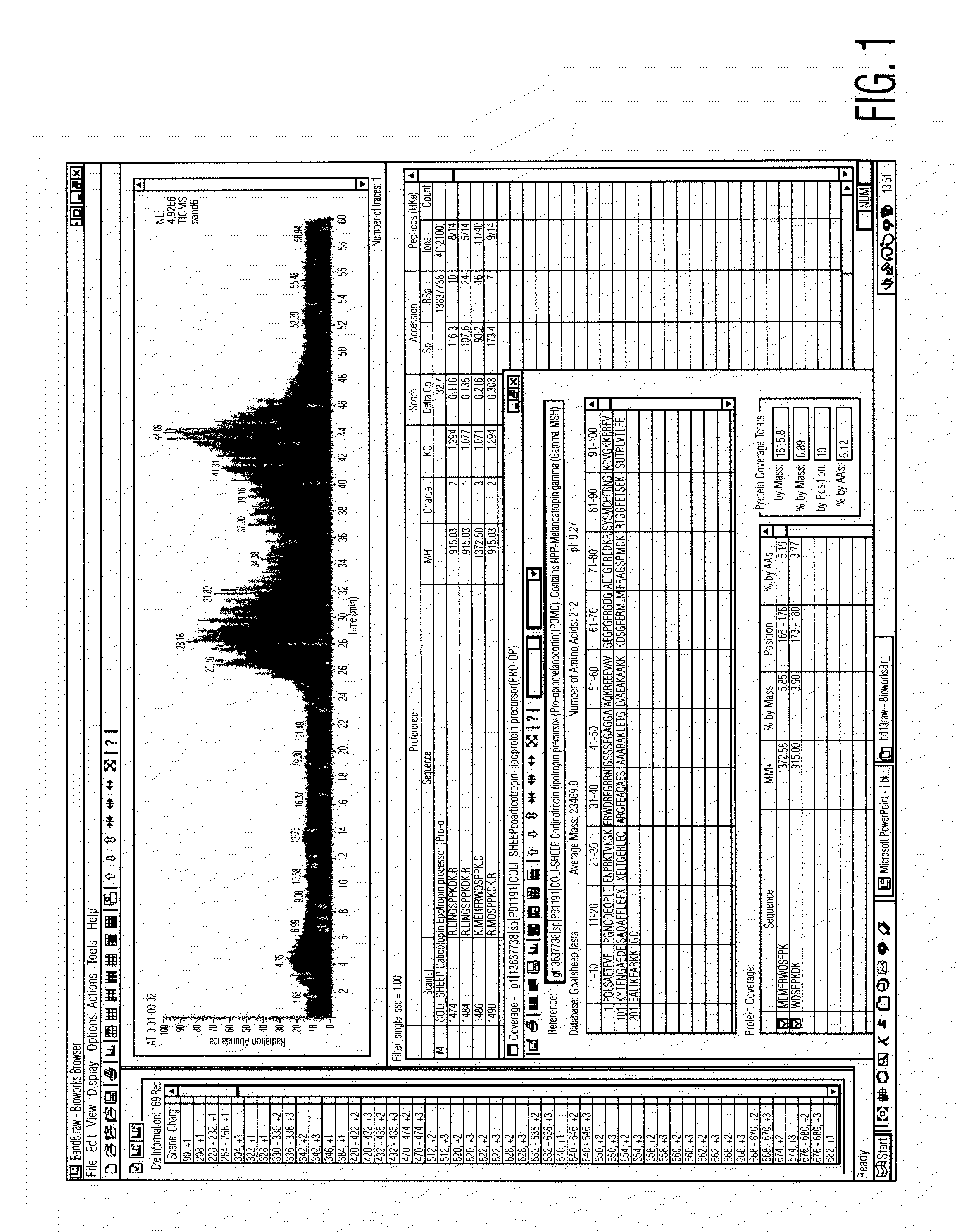
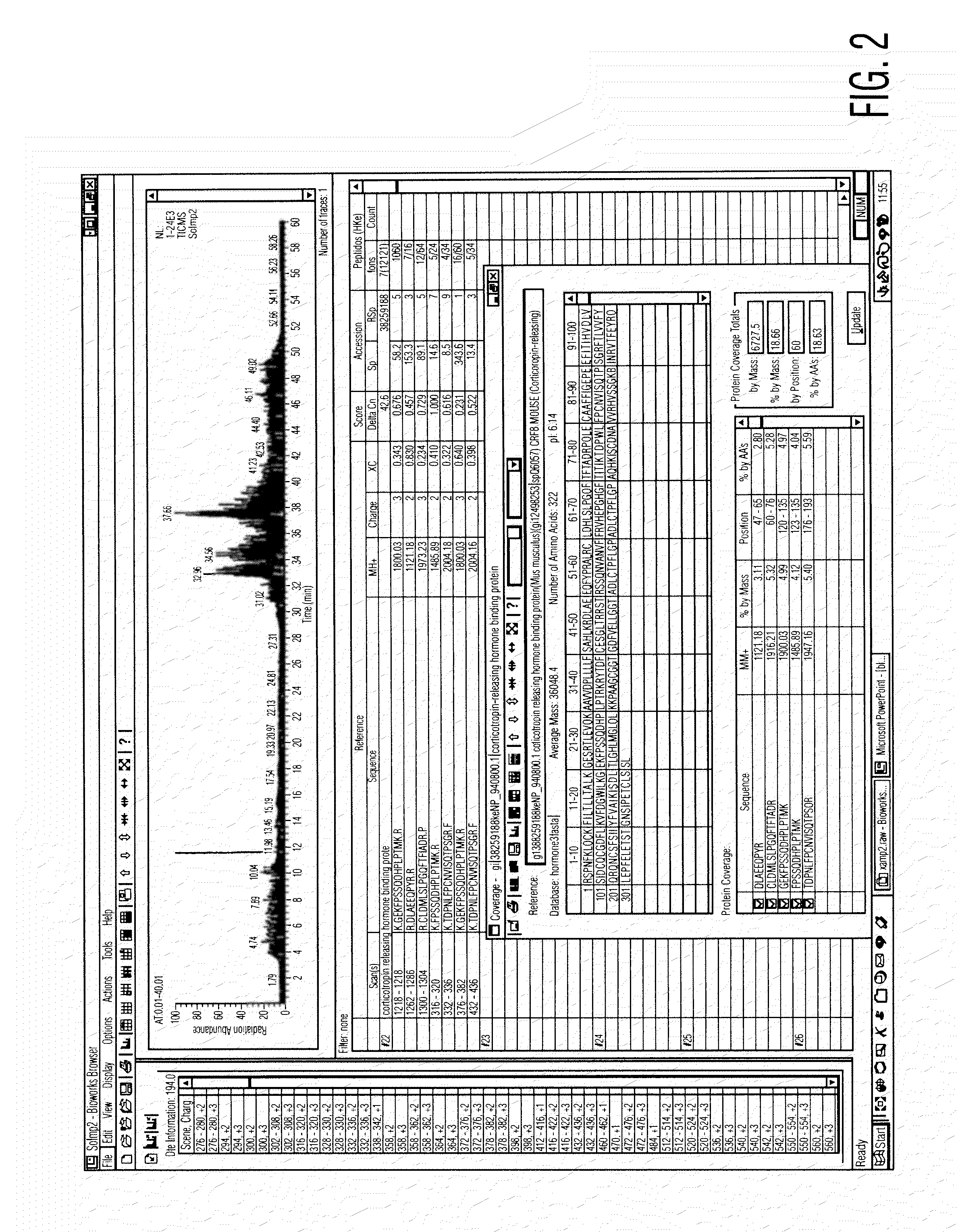
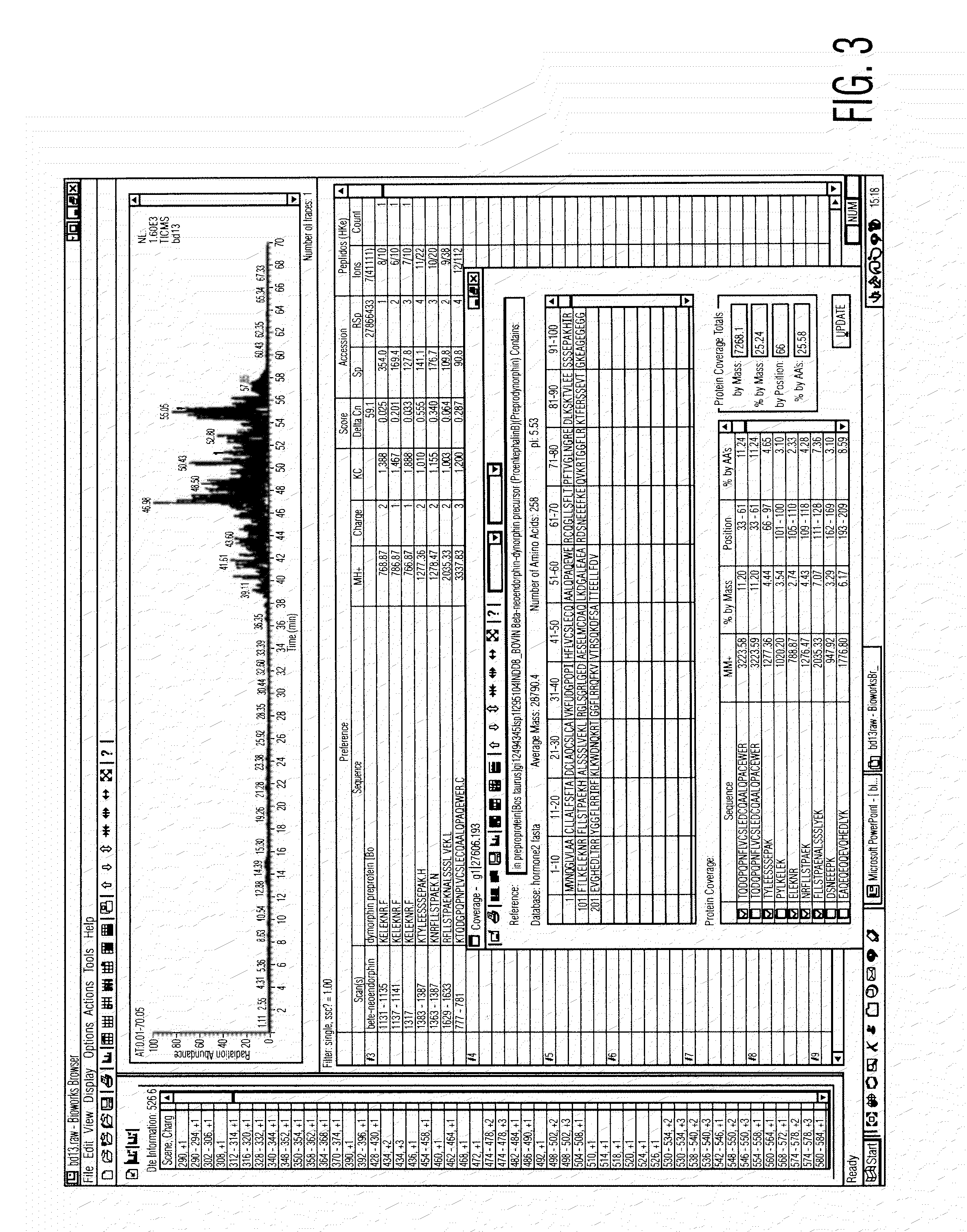
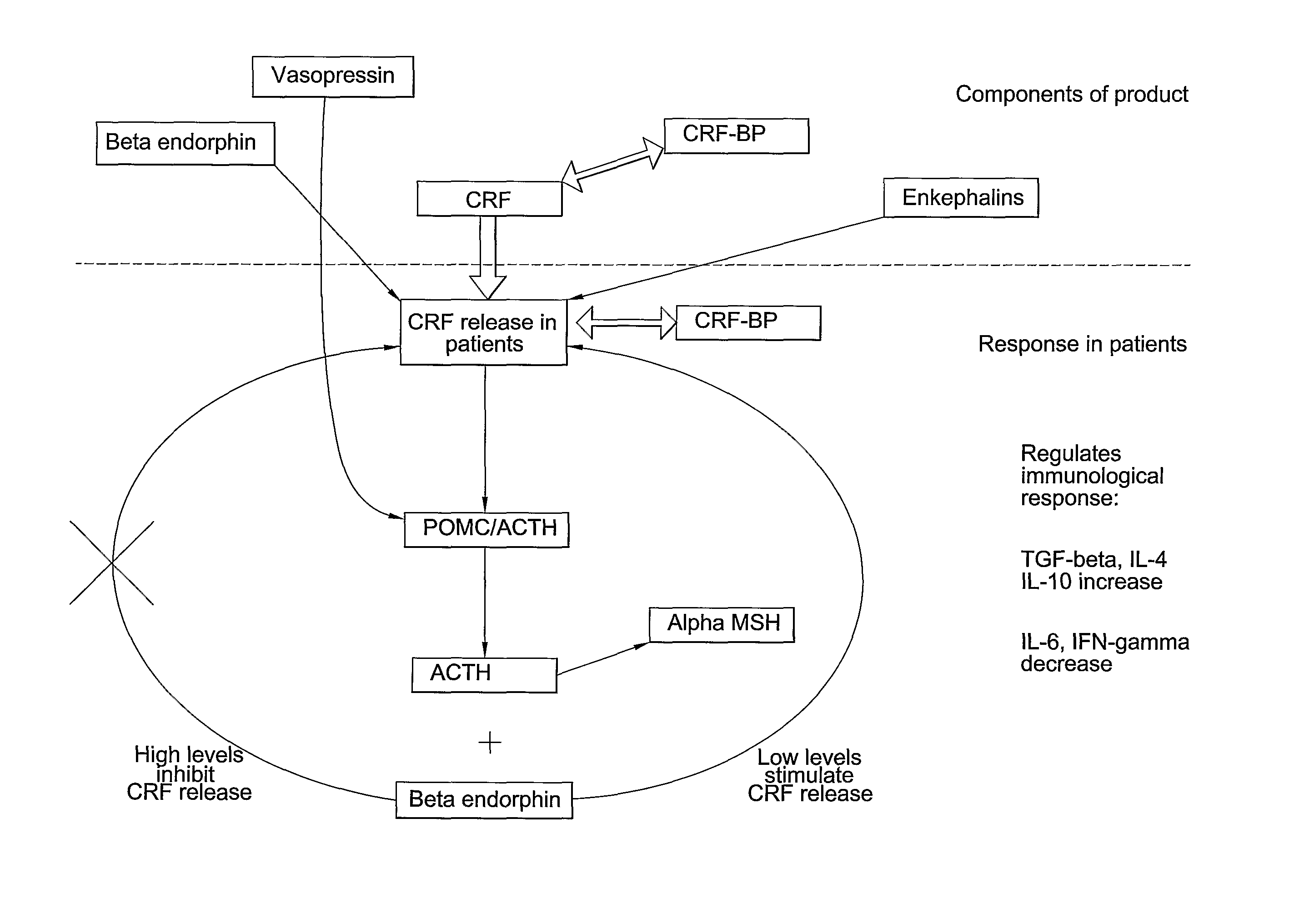
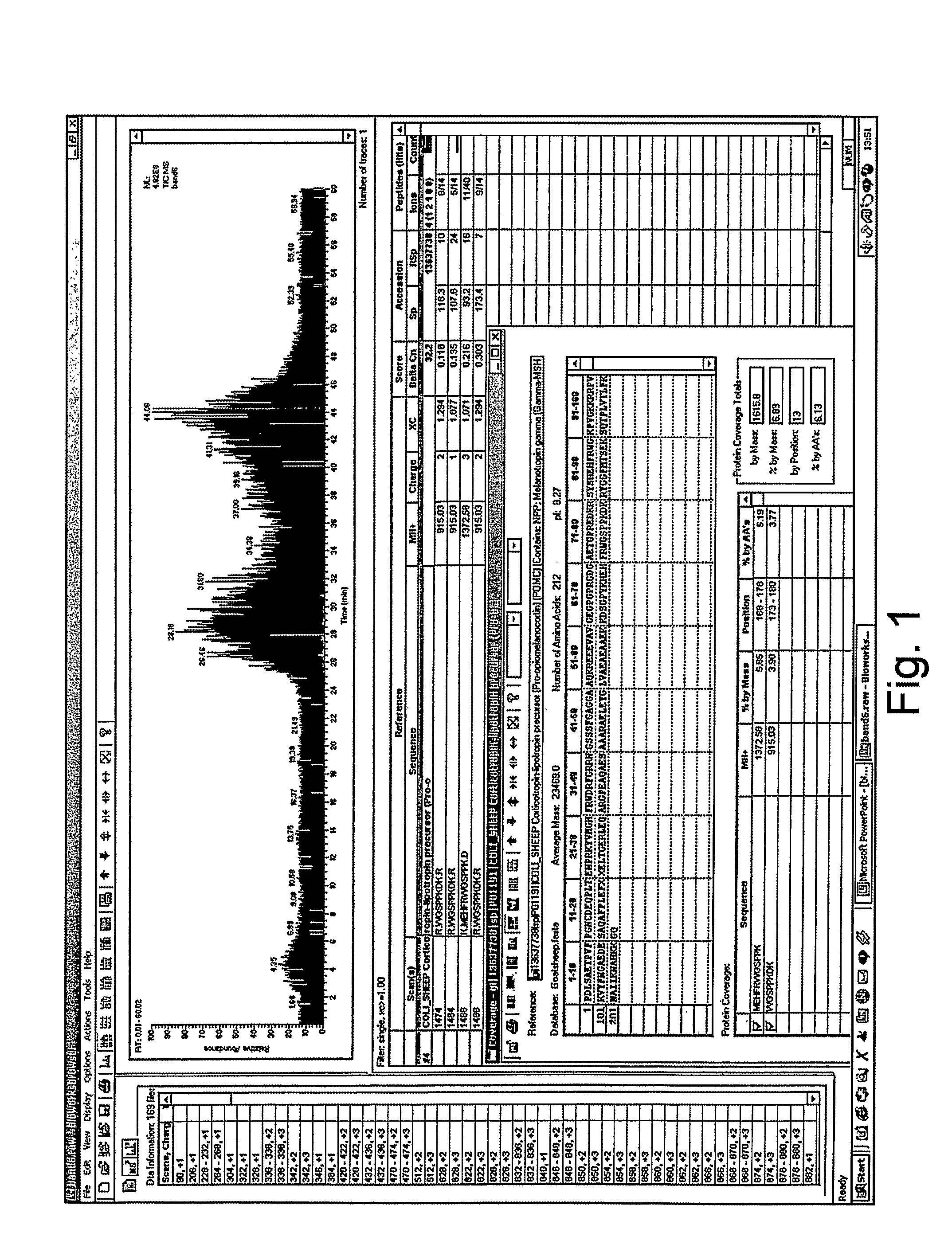
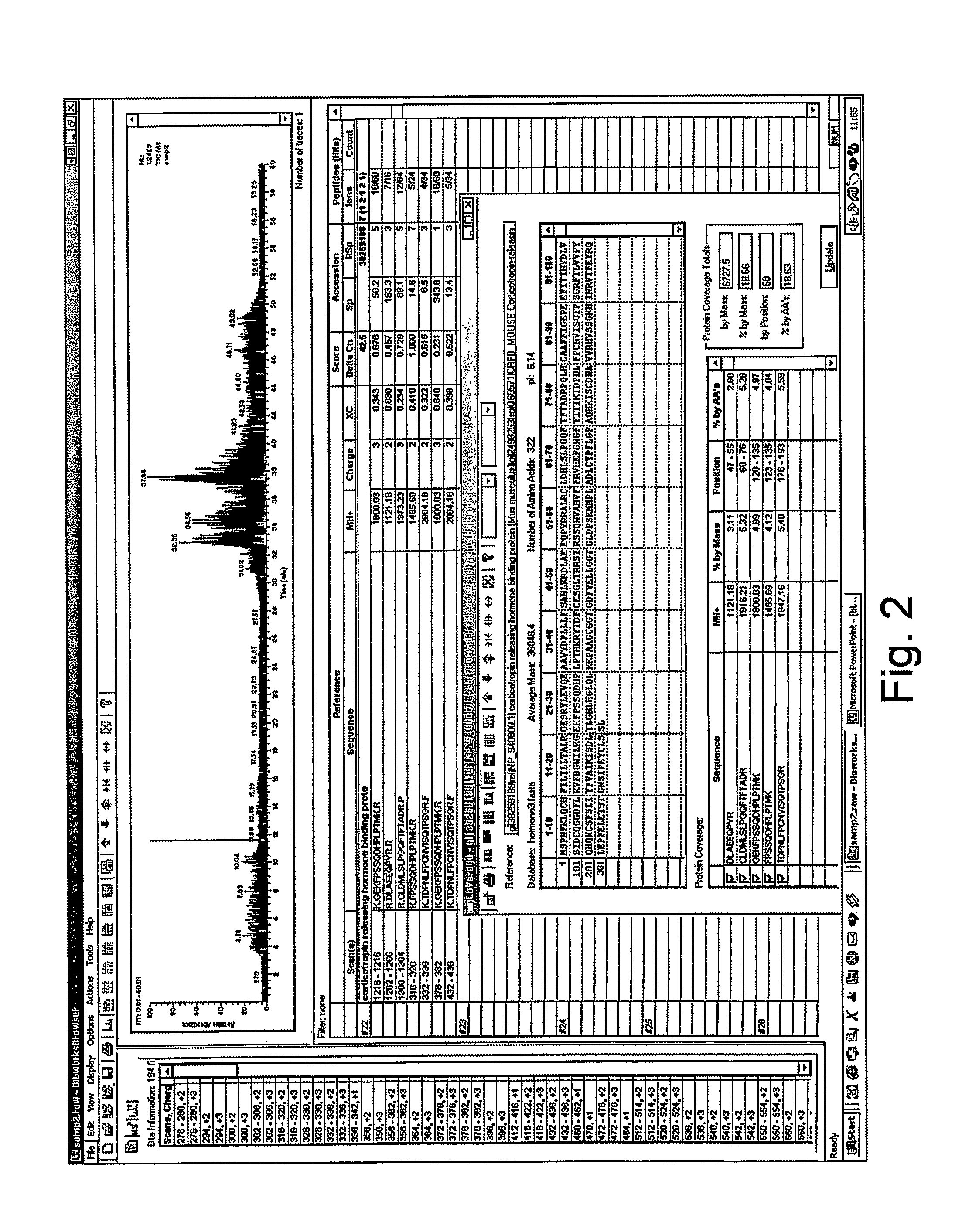
Analysis of a goat serum product with many therapeutic effects is described. The product is identified as containing proopiomelanocortin (POMC) and Corticotropin releasing factor (CRF) peptides, as well as breakdown products of these peptides. We describe methods of treatment of diseases including cancers, multiple sclerosis, and neural disorders using these peptides and their products, as well as medicaments including such peptides and methods of producing the peptides.
Owner:AIMSCO LTD
Methods of increasing insulin sensitivity or decreasing insulin secretion by administering corticotropin releasing factor receptor-2 inhibitors
In some aspects, the invention relates to methods for increasing insulin-sensitivity and / or decreasing insulin secretion in an individual by reducing or inhibiting corticotropin releasing factor 2 (CRFR2) signaling. CRFR2 antagonists may block agonism by one or more CRFR2 agonist, for example Ucn 2 or Ucn 3. Methods according to the invention may be use to treat a variety of metabolic diseases such as type 2 diabetes, metabolic syndrome, nonalcoholic fatty liver disease, polycystic ovarian syndrome and obesity.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Imidazopyridazine Compounds
The present invention relates to novel substituted imidazo[1,2-b]pyridazine compounds of Formula (I) pharmaceutical compositions thereof, and the use such compounds as corticotropin releasing factor 1 (CRF1) receptor antagonists in the treatment of psychiatric disorders and neurological diseases.
Owner:ELI LILLY & CO
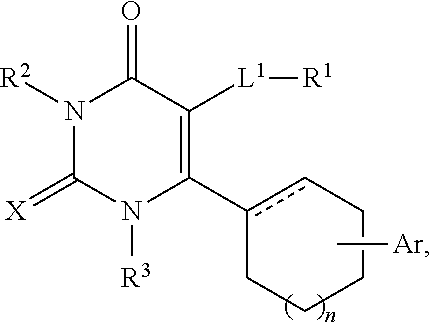
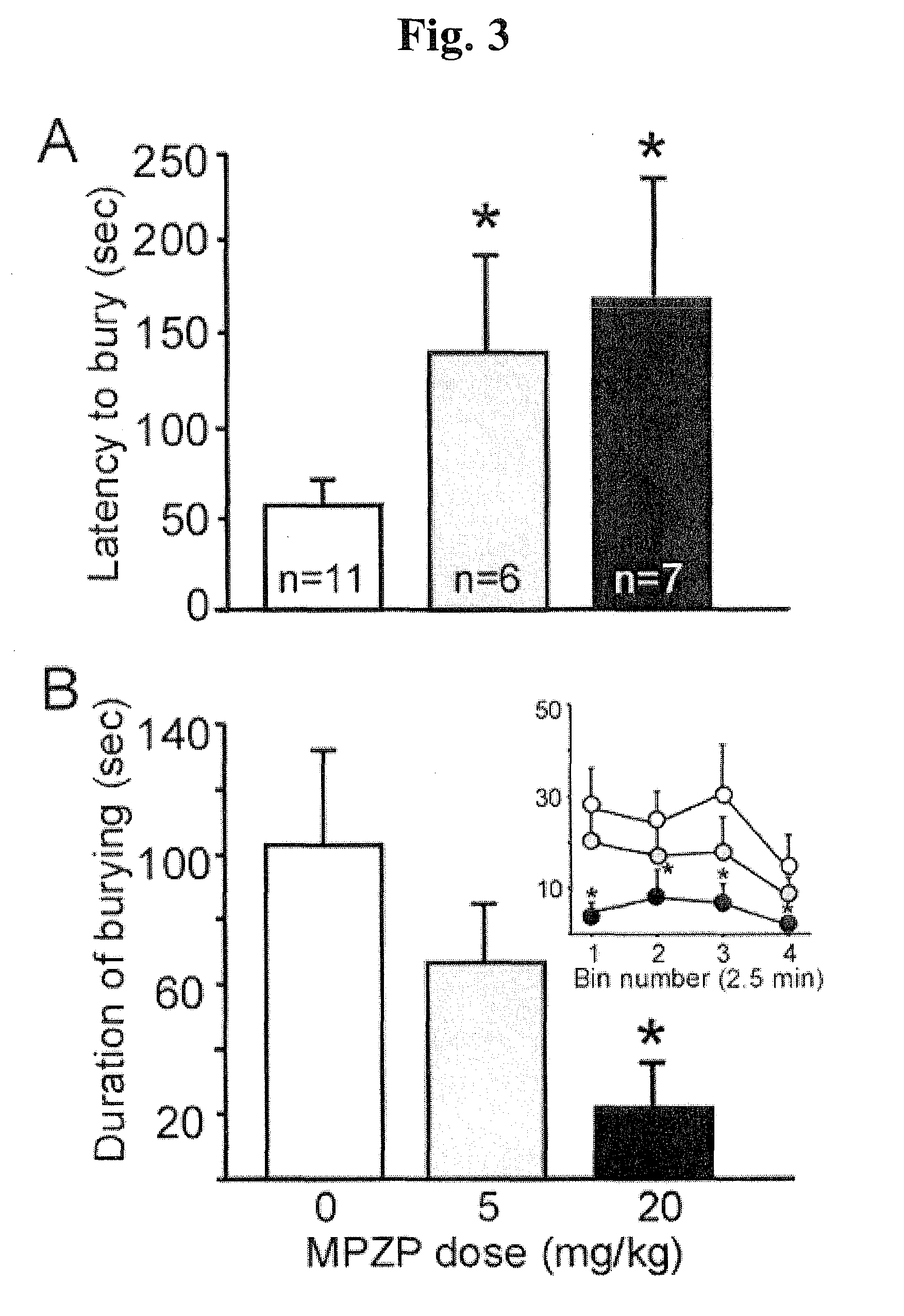
Mpzp: a small molecule corticotropin-releasing factor type 1 receptor (CRF1) antogonist
A method for treating or preventing a host mammal that exhibits aversive signs and symptoms present during protracted abstinence or extended discontinuation syndromes as seen after cessation of compulsive activity, behaviors, or substance use is disclosed. That method comprises administering to a host mammal in need a pharmaceutical composition containing an aversive sign and symptom lessening amount a compound of Formula I or a pharmaceutically acceptable salt thereof dissolved or dispersed in a physiologically acceptable diluent, and repeating the administration as needed,wherein W, X, Y and Z, R1 and Ar are defined within. Data are provided in rats as host mammals using behavioral models dependent on the CRF1 system: defensive burying, alcohol dependence, cocaine dependence and nicotine dependence. A contemplated method also is useful for inhibiting relapse of such a behavior. A contemplated method also is useful for treating substance-related or substance-induced psychiatric disorders that include aversive signs and symptoms.
Owner:THE SCRIPPS RES INST
Process for improving calf beef production property by using growth hormone releasing factor gene expression plasmid
The invention discloses a calf beef producing property improving method, which comprises the following steps: 1) injecting expressive plasmid of growth hormone releasing factor gene in the calf musculus semitendinosus positing, 2) setting the fitful agent quantity at 5-7mg per calf, 3) adopting the density of injection dilution liquid of expressive plasmid at 0.25% bupivacaine PSS, 4) improving beef producing property without influencing quality and safety.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Methods for identifying compounds for regulating muscle mass or function using corticotropin releasing factor receptors
Screening methods for identifying compounds that bind to or activate corticotropin releasing factor2 receptors (CRF2R) and regulate or potentially regulate skeletal muscle mass or function in vivo are disclosed. Also disclosed are screening methods for identifying compounds that prolong or augment the activation of CRF2Rs or of CRF2R signal transduction pathways, increase CRF2R or increase CRF expression are provided. Pharmaceutical compositions comprising CRF2R agonists, antibodies to CRF2R and methods for increasing skeletal muscle mass or function or for the treatment of skeletal muscle atrophy using CRF2R as the target for intervention and methods for treatment of muscular dystrophies are described.
Owner:APTALIS PHARMA
Preparation method of protein containing non-natural amino acid
ActiveCN113699124APrecise verification of orthogonalityIncrease productionNucleic acid vectorLigasesForeign proteinHistone protein
The invention relates to a method for expressing recombinant protein containing unnatural amino acid in animal cells, which comprises the following steps: on the basis of four groups of aminoacyl-tRNA synthetases and corresponding tRNA, constructing aminoacyl-tRNA for identifying termination codons, and screening to obtain three gene codon expansion systems. The three gene codon expansion systems are high in termination codon reading efficiency and high in mutual compatibility, so that three non-natural amino acids can be inserted into one foreign protein, or different non-natural amino acids can be inserted into three foreign proteins of the same cell respectively. In order to further improve the readthrough rate, the release factor eRF1 is mutated to weaken the interaction between the release factor eRF1 and the termination codon.
Owner:PEKING UNIV
Medicament
Analysis of a goat serum product with many therapeutic effects is described. The product is identified as containing proopiomelanocortin (POMC) and Corticotropin releasing factor (CRF) peptides, as well as breakdown products of these peptides. We describe methods of treatment of diseases including cancers, multiple sclerosis, and neural disorders using these peptides and their products, as well as medicaments including such peptides and methods of producing the peptides.
Owner:AIMSCO LTD
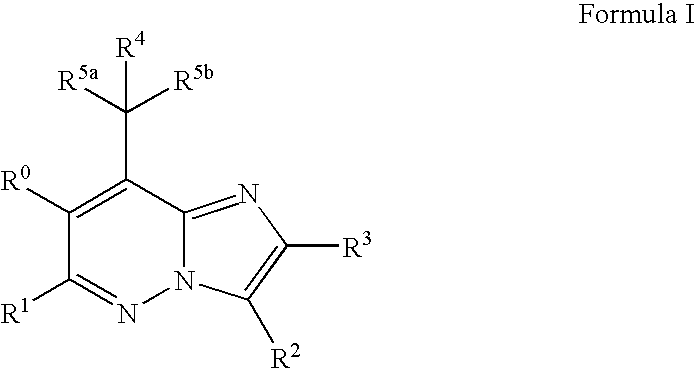
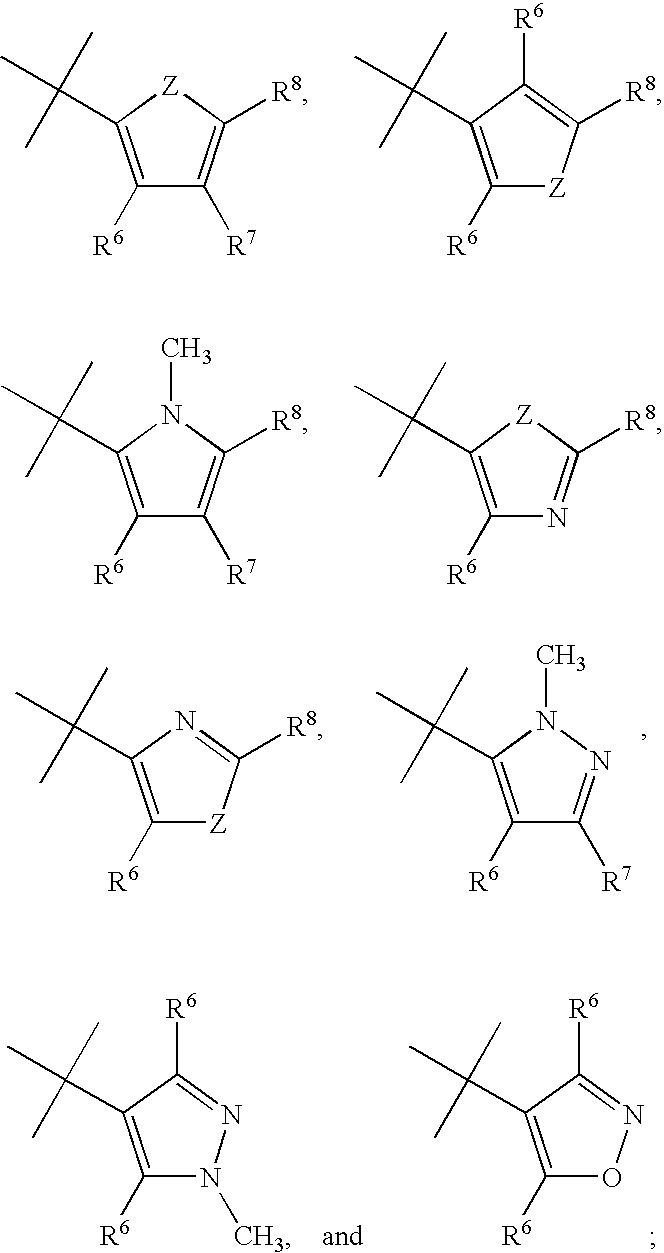
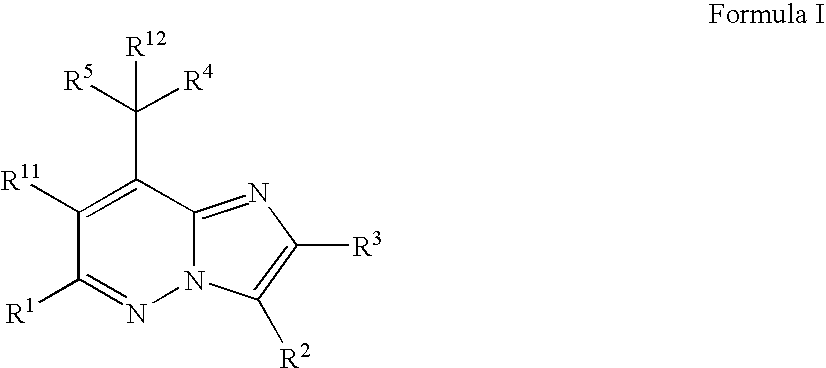
Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists
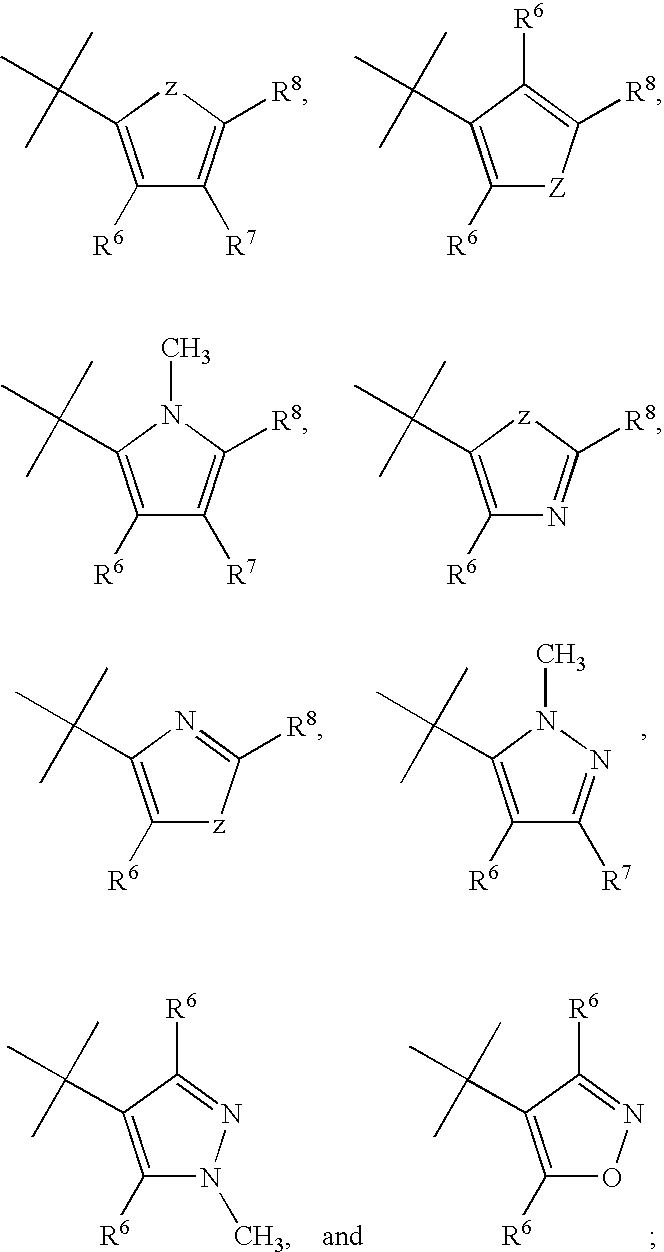
CRF receptor antagonists are disclosed which may have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in mammals. The CRF receptor antagonists of this invention have the following structure: (I);and pharmaceutically acceptable salts, esters, solvates, stereoisomers and prodrugs thereof, wherein R1, R2a, R2b, Y, Het, n, o, R6, Ar and R7 are as defined herein. Compositions containing a CRF receptor antagonist in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Owner:NEUROCRINE BIOSCI INC
Medicament
InactiveUS20130203669A1Effect in patientImprove the level ofSenses disorderNervous disorderDiseaseSerum ige
Owner:AIMSCO LTD
Compositions for bacterial mediated gene silencing and methods of using the same
The invention features compositions and methods for delivering small interfering (siRNAs), e.g., shRNAs, to host cells using non-pathogenic strains of Salmonella bacteria containing nucleic acid expression constructs encoding shRNAs. In this process, shRNA expressed by the Salmonella silences or knocks down genes of interest (target genes) inside target cells. The nucleic acid expression constructs of the invention include an RNA polymerase (e.g., a T7 polymerase), an RNA polymerase promoter (e.g., a T7 polymerase promoter), and an RNA polymerase terminator (e.g., a T7 polymerase terminator). The Salmonella bacteria can also include, on the same or different nucleic acid construct, an endosomal release factor (e.g., HlyA).
Owner:GUO HONGNIAN +2
Corticotropin releasing factor 2 receptor agonists
InactiveCN1869068AEffective treatmentHormone peptidesPeptide/protein ingredientsCorticotropinsMuscular dystrophy
Isolated corticotropin releasing factor derivatives, and nucleic acids encoding the same, are effective for treating corticotropin releasing factor 2 receptor modulated disorders such as muscular dystrophy.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com















![Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8acdc4f6-2c35-4e2b-8051-7f951315a125/US07879862-20110201-D00001.png)
![Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8acdc4f6-2c35-4e2b-8051-7f951315a125/US07879862-20110201-D00002.png)
![Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8acdc4f6-2c35-4e2b-8051-7f951315a125/US07879862-20110201-D00003.png)