Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "CRF Receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclic compounds
There is provided a CRF receptor antagonist comprising a compound of the formula (I):A-W—Ar (I)wherein, A is a group represented by the formula (A1) or (A2): (wherein, ring Aa is a 5- or 6-membered ring which may be further substituted; ring Ab is a 5- or 6-membered ring which may be further substituted; ring Ac is a 5- or 6-membered ring which may be substituted; R1 is optionally substituted alkyl, substituted amino, substituted hydroxy, etc.; X is carbonyl, —O—, —S—, etc.; Y1, Y2 and Q are independently optionally substituted carbon or nitrogen; is a single or double bond); W is a bond, optionally substituted methylene, optionally substituted imino, —O—, —S—, etc.; Ar is optionally substituted aryl or optionally substituted heteroaryl; or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Cyclic compounds
There is provided a CRF receptor antagonist comprising a compound of the formula (I):A-W—Ar (I)wherein, A is a group represented by the formula (A1) or (A2):(wherein, ring Aa is a 5- or 6-membered ring which may be further substituted; ring Ab is a 5- or 6-membered ring which may be further substituted; ring Ac is a 5- or 6-membered ring which may be substituted; R1 is optionally substituted alkyl, substituted amino, substituted hydroxy, etc.; X is carbonyl, —O—, —S—, etc.; Y1, Y2 and Q are independently optionally substituted carbon or nitrogen; is a single or double bond);W is a bond, optionally substituted methylene, optionally substituted imino, —O—, —S—, etc.; Ar is optionally substituted aryl or optionally substituted heteroaryl; or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Cloning and recombinant production of CRF receptor(s)
InactiveUS20050186593A1Peptide/protein ingredientsAntibody mimetics/scaffoldsBinding siteSerine Kinase
In accordance with the present invention, there are provided novel receptor proteins characterized by having the following domains, reading from the N-terminal end of said protein: an extracellular, ligand-binding domain, a hydrophobic, trans-membrane domain, and an intracellular, receptor domain having serine kinase-like activity. The invention receptors optionally further comprise a second hydrophobic domain at the amino terminus thereof. The invention receptor proteins are further characterized by having sufficient binding affinity for at least one member of the activin / TGF-β superfamily of polypeptide growth factors such that concentrations of ≦10 nM of said polypeptide growth factor occupy ≦50% of the binding sites of said receptor protein. A presently preferred member of the invention superfamily of receptors binds specifically to activins, in preference to inhibins, transforming growth factor-β, and other non-activin-like proteins. DNA sequences encoding such receptors, assays employing same, as well as antibodies derived therefrom, are also disclosed.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Crf Receptor Antagonists and Methods
CRF receptor antagonists are disclosed which have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in a warm-blooded animals, such as stroke. The CRF receptor antagonists of this invention have the following structure: including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein R1, R2, R3, Y, Ar, and Het are as defined herein. Compositions containing a CRF receptor antagonist in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Owner:NEUROCRINE BIOSCI INC +1
Crf Receptor Antagonists And Methods Relating Thereto
CRF receptor antagonists are disclosed which may have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in mammals, such as stroke. The CRF receptor antagonists of this invention have the following structure: and pharmaceutically acceptable salts, esters, solvates, stereoisomers and prodrugs thereof, wherein R1, R2, n, R5, Ar, and Het are as defined herein. Compositions containing a CRF receptor antagonists in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Owner:NEUROCRINE BIOSCI INC +1
Pyrazolothiazole compound
InactiveUS20110086882A1Exhibit anti-depressantAmeliorating depression, depressive symptoms, anxietyBiocideNervous disorderArylCompound a
A compound represented by the formula (I) or pharmacologically acceptable salt thereof exhibits an excellent CRF receptor antagonismwherein X is a nitrogen atom or CH; R1 is -A11-A12; A11 is a single bond or a C1-6 alkylene group; A12 is a hydrogen atom, a C1-6 alkyl group or a C3-6 cycloalkyl group, etc.; R2 is -A21-A22; A21 is a single bond or a C1-6 alkylene group; A22 is a hydrogen atom, a C1-6 alkyl group, a C3-6 cycloalkyl group, a non-aromatic heterocyclic group, or a heteroaryl group, etc.; R3 is a C1-6 alkyl group, a C3-6 cycloalkyl group, a C1-6 alkoxy group, a C3-6 cycloalkoxy C1-6 alkyl group, di-C1-6 alkyl amino group, a halogen atom, a cyano group, a formyl group, or a carboxyl group, etc; R4 is a hydrogen atom or a C1-6 alkoxy group; R5 is a halogen atom, a C1-6 alkyl group, or a C1-6 alkoxy group; R6 is a hydrogen atom, a C1-6 alkyl group, a C1-6 alkoxy group, a C1-6 alkylthio group, or a C1-6 alkyl sulfinyl group etc.; and R7 is a C1-6 alkyl group, a C1-6 alkoxy group, or a C1-6 alkylthio group.
Owner:EISIA R&D MANAGEMENT CO LTD
CRF receptor antagonists and methods relating thereto
CRF receptor antagonists are disclosed which have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in a warm-blooded animals, such as stroke.
Owner:BRISTOL MYERS SQUIBB PHARMA CO
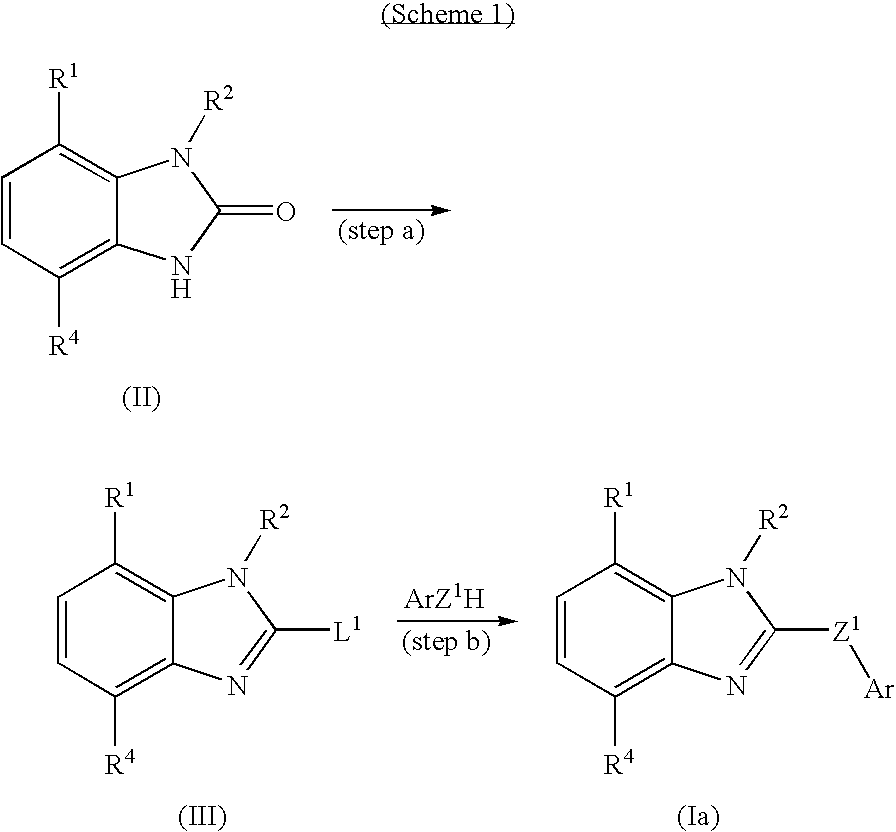
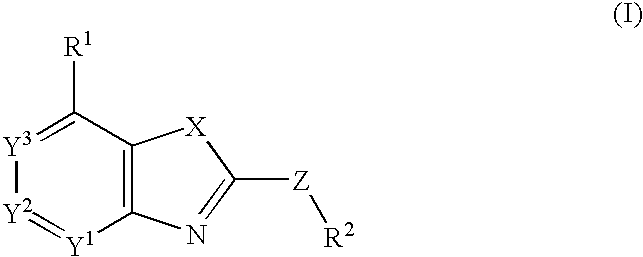
Fused heterocyclic compounds
InactiveCN101166729AExcellent CRF antagonistic activityPrevent emotional disordersOrganic chemistryHydrogenSulfur
There is provided a CRF receptor antagonist comprising a compound of the formula (I) : wherein R1 is an optionally substituted hydrocarbyl, an optionally substituted C-linked heterocyclic group, an optionally substituted N-linked heteroaryl group, a cyano or an acyl; R2 is an optionally substituted cyclic hydrocarbyl or an optionally substituted heterocyclic group; X is oxygen, sulfur or -NR3- (wherein R3 is a hydrogen, an optionally substituted hydrocarbyl or an acyl) ; Y1, Y2 and Y3 are each an optionally substituted carbon or a nitrogen, provided that one or less of Y1, Y2 and Y3 is nitrogen; and Z is a bond, -CO-, oxygen, sulfur, -SO-, -SO2-, -NR4-, -NR4-alk-, -CONR4- or -NR4CO- (wherein alk is an optionally substituted C3.-4 alkylene and R4 is a hydrogen, an optionally substituted hydrocarbyl or an acyl) ; or a salt thereof or a prodrug thereof .
Owner:TAKEDA PHARMA CO LTD
Crf receptor antagonists, their preparations, their pharmaceutical composition, and their uses
CRF receptor antagonists are disclosed which have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in warm-blooded animals. CRF receptor antagonists which are labeled with a positron emitting isotope for use in PET are also disclosed.
Owner:PHARMCO PUERTO RICO INC +1
3-PHENYLPYRAZOLO[5,1-b]THIAZOLE COMPOUNDS
ActiveUS20090259049A1Sufficient pharmacokinetic propertySufficient pharmacological activityBiocideOrganic active ingredientsCompound aThiazole
A compound represented by the following formula (I), or salt thereof exhibits excellent CRF receptor antagonism, and sufficient pharmacological activity, safety and pharmacokinetic properties as a drug.wherein R1 represents the formula -A11-A12; R2 represents tetrahydrofurylmethyl, tetrahydropyranylmethyl or tetrahydropyranyl; A11 represents a single bond, methylene or 1,2-ethylene; A12 represents C1-6 alkyl, C3-6 cycloalkyl or C3-6 cycloalkyl having methyl; R3 represents methoxy, cyano, cyclobutyloxymethyl, methoxymethyl or ethoxymethyl; and R4 represents methoxy or chlorine.
Owner:EISIA R&D MANAGEMENT CO LTD
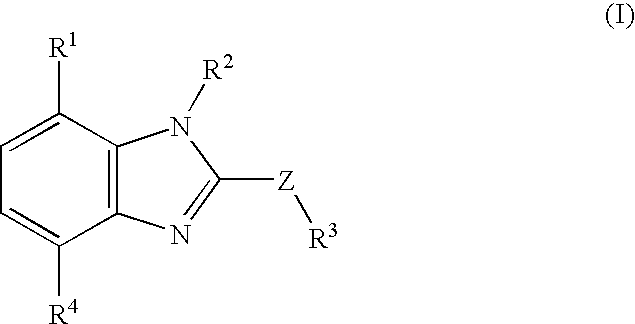
Benzimidazole compounds
InactiveUS20100056515A1Improve responseExcellent corticotropin releasing factor antagonistic activityBiocideNervous disorderHalogenHydrogen
There is provided a compound of the formula (I):wherein R1 is an optionally substituted C1-10 alkyl; R2 is H, or a C1-6 alkyl which may be substituted with 1 to 3 substituents; R3 is a 5- or 6-membered aromatic group which may be substituted with 1 to 5 substituents, wherein the 5- or 6-membered aromatic group may be fused with a 5- or 6-membered ring which may be substituted with 1 to 3 C1-6 alkyls; R4 is a hydrogen, a halogen, a hydroxy, a cyano, a C1-6 alkyl or a C1-6 alkoxy; Z is —O—, —S—, —SO—, —SO2—, or —NR5— wherein R5 is a hydrogen or a C1-6 alkyl; or a salt thereof or a prodrug thereof, which have CRF receptor antagonist activity and use thereof.
Owner:TAKEDA PHARMA CO LTD
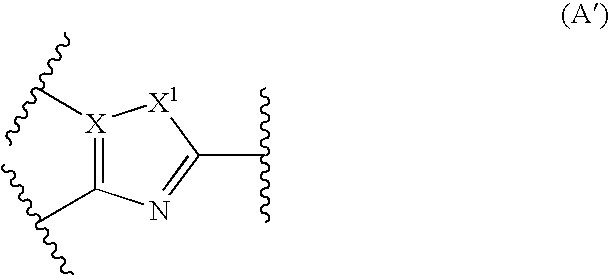
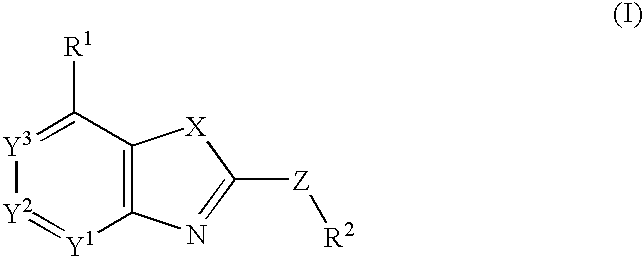
Nitrogen-containing fused heterocyclic compounds
There is provided a CRF receptor antagonist comprising a compound of the formula (I): wherein, ring A is a 5-membered ring represented by the formula (A′): wherein X is a carbon and X1 is an oxygen, a sulfur or —NR5—, or formula (A″): wherein X is a nitrogen and R6 is an optionally substituted hydrocarbyl, R1 is an amino substituted by two optionally substituted hydrocarbyl groups, R2 is an phenyl, Y1 is CR3a or a nitrogen, y2 is CR3b or a nitrogen and Y3 is CR3c or a nitrogen, provided that one or less of Y1, Y2, and Y3 is nitrogen, W is a bond, —(CH2)n-, and Z is a bond, —NR4—, etc.; or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMACEUTICALS CO LTD
Fused heterocyclic compounds
InactiveUS8039500B2Excellent corticotropin releasing factor antagonistic activityImproved profileOrganic active ingredientsBiocideHydrogenSulfur
There is provided a compound of the formula:wherein R1 is an optionally substituted hydrocarbyl, a substituted amino, etc.; R2 is an aromatic group substituted with one or two substituents at the positions adjacent to the position bonded to Z, and said aromatic group may have additional substituent(s); X is —NR3— wherein R3 is a hydrogen, an optionally substituted hydrocarbyl or an acyl, or sulfur; Y1, Y2 and Y3 are an optionally substituted methine or a nitrogen, etc.; and Z is an optionally substituted methylene, provided that carbonyl is excluded; or a salt thereof or a prodrug thereof, which have CRF receptor antagonist activity and use thereof.
Owner:TAKEDA PHARMA CO LTD
Nitrogen-containing fused heterocyclic compounds
There is provided a CRF receptor antagonist comprising a compound of the formula (I):wherein, ring A is a 5-membered ring represented by the formula (A′):wherein X is a carbon and X1 is an oxygen, a sulfur or —NR5—, or formula (A″):wherein X is a nitrogen and R6 is an optionally substituted hydrocarbyl, R1 is an amino substituted by two optionally substituted hydrocarbyl groups, R2 is an phenyl, Y1 is CR3a or a nitrogen, Y2 is CR3b or a nitrogen and Y3 is CR3c or a nitrogen, provided that one or less of Y1, Y2, and Y3 is nitrogen, W is a bond, —(CH2)n-, and Z is a bond, —NR4—, etc.; or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Fused heterocyclic compounds
InactiveUS20100048658A1Excellent corticotropin releasing factor antagonistic activityImproved profileOrganic active ingredientsBiocideHydrogenSulfur
There is provided a compound of the formula:wherein R1 is an optionally substituted hydrocarbyl, a substituted amino, etc.; R2 is an aromatic group substituted with one or two substituents at the positions adjacent to the position bonded to Z, and said aromatic group may have additional substituent(s); X is —NR3— wherein R3 is a hydrogen, an optionally substituted hydrocarbyl or an acyl, or sulfur; Y1, Y2 and Y3 are an optionally substituted methine or a nitrogen, etc.; and Z is an optionally substituted methylene, provided that carbonyl is excluded; or a salt thereof or a prodrug thereof, which have CRF receptor antagonist activity and use thereof.
Owner:TAKEDA PHARMA CO LTD
Tri-and tetraaza-acenaphthylen derivatives as CRF receptor antagonists
Owner:GLAXO GROUP LTD +2
Fused Heterocyclic Compounds
There is provided a CRF receptor antagonist comprising a compound of the formula (I):wherein R1 is an optionally substituted hydrocarbyl, an optionally substituted C-linked heterocyclic group, an optionally substituted N-linked heteroaryl group, a cyano or an acyl; R2 is an optionally substituted cyclic hydrocarbyl or an optionally substituted heterocyclic group; X is oxygen, sulfur or —NR3— (wherein R3 is a hydrogen, an optionally substituted hydrocarbyl or an acyl); Y1, Y2 and Y3 are each an optionally substituted carbon or a nitrogen, provided that one or less of Y1, Y2 and Y3 is nitrogen; and Z is a bond, —CO—, oxygen, sulfur, —SO—, —SO2—, —NR4—, —NR4-alk-, —CONR4— or —NR4CO— (wherein alk is an optionally substituted C1-4 alkylene and R4 is a hydrogen, an optionally substituted hydrocarbyl or an acyl); or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Heteroaryl fused pyridines, pyrazines and pyrimidines as CRF1 receptor ligands
Substituted heteroaryl fused pyridine, pyrazine, and pyrimidine compounds that act as selective modulators of CRF 1 receptors are provided. These compounds are useful in the treatment of a number of CNS and periphereal disorders, particularly stress, anxiety, depression, cardiovascular disorders, and eating disorders. Methods of treatment of such disorders and well as packaged pharmaceutical compositions are also provided. Compounds of the invention are also useful as probes for the localization of CRF receptors and as standards in assays for CRF receptor binding. Methods of using the compounds in receptor localization studies are given.
Owner:NEUROGEN +1
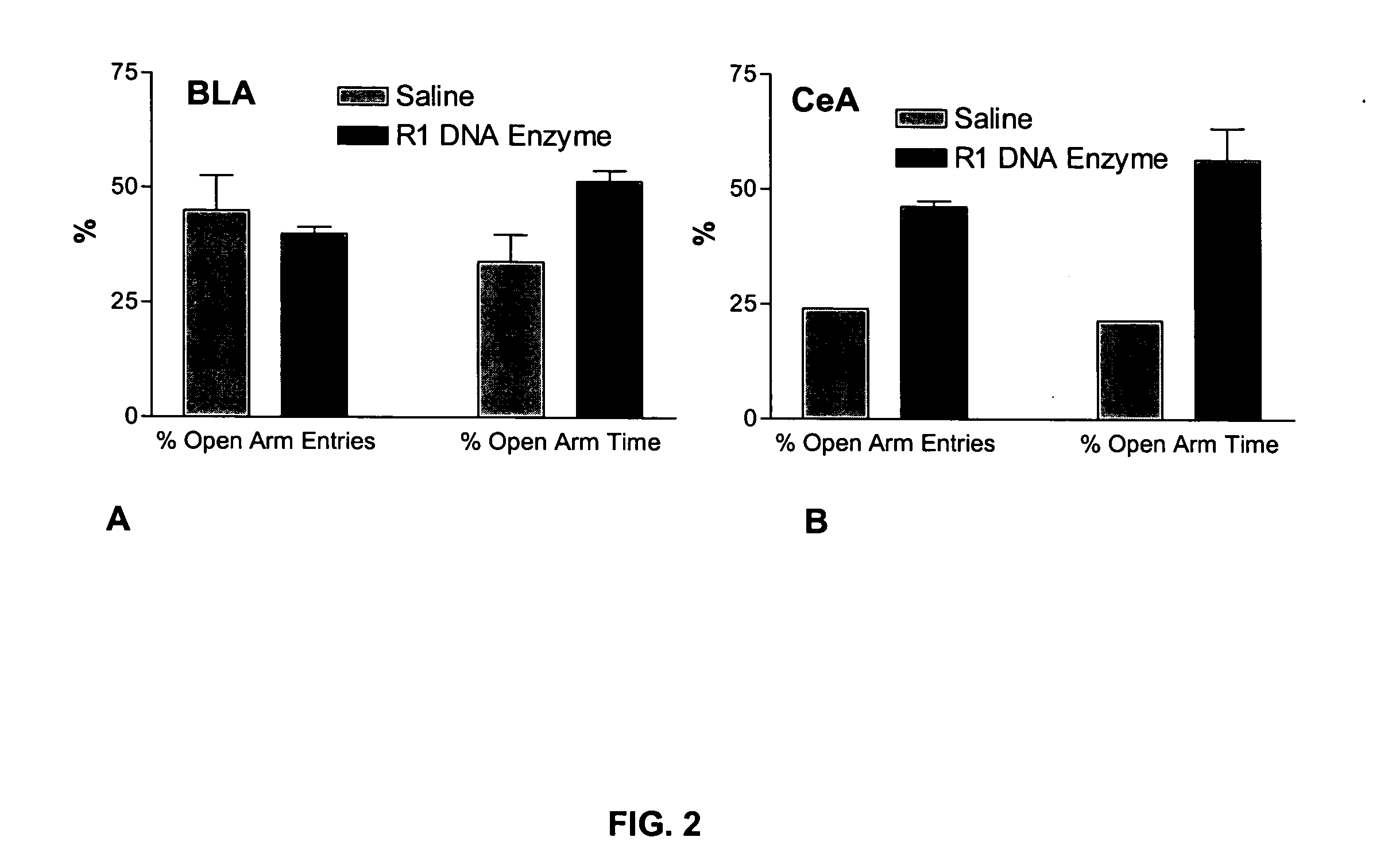
Method of reducing CRF receptor mRNA
InactiveUS20050042212A1Nervous disorderPeptide/protein ingredientsCorticotropinsCorticotropin Releasing-Factor Receptors
A method of treating a patient is disclosed. In one embodiment, the method comprises the step of reducing the amount of mRNA encoding corticotropin-releasing factor receptor, wherein a portion of the mRNA encoding corticotropin-releasing factor receptor is destroyed and wherein the amount of corticotrophin releasing factor receptor is reduced.
Owner:WISCONSIN ALUMNI RES FOUND
CRF receptor antagonists and methods relating thereto
CRF receptor antagonists are disclosed which may have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in mammals, such as stroke. The CRF receptor antagonists of this invention have the following structure:and pharmaceutically acceptable salts, esters, solvates, stereoisomers and prodrugs thereof, wherein R1, R2, n, R5, Ar, and Het are as defined herein. Compositions containing a CRF receptor antagonists in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Owner:NEUROCRINE BIOSCI INC +1
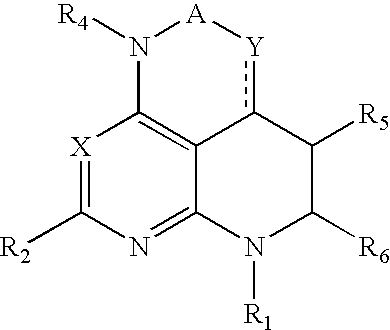
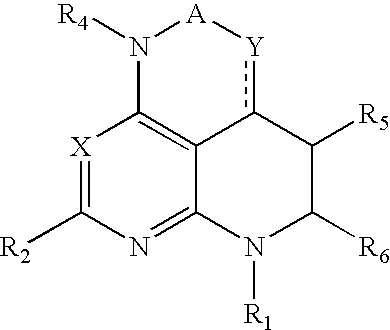
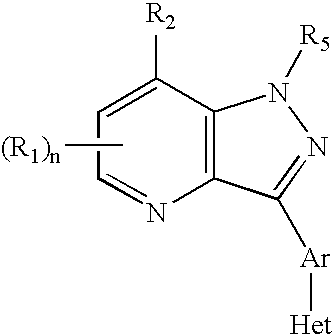
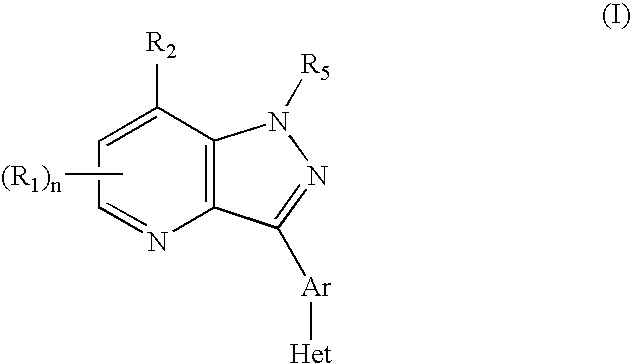
Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists
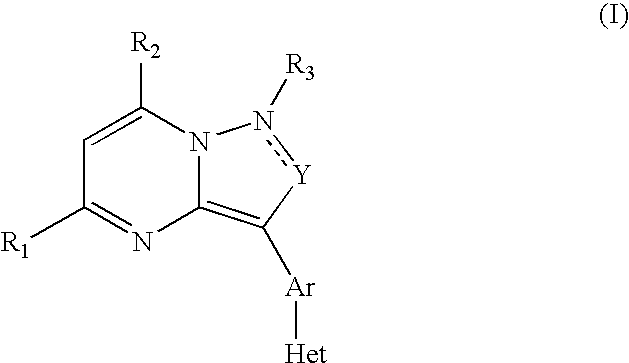
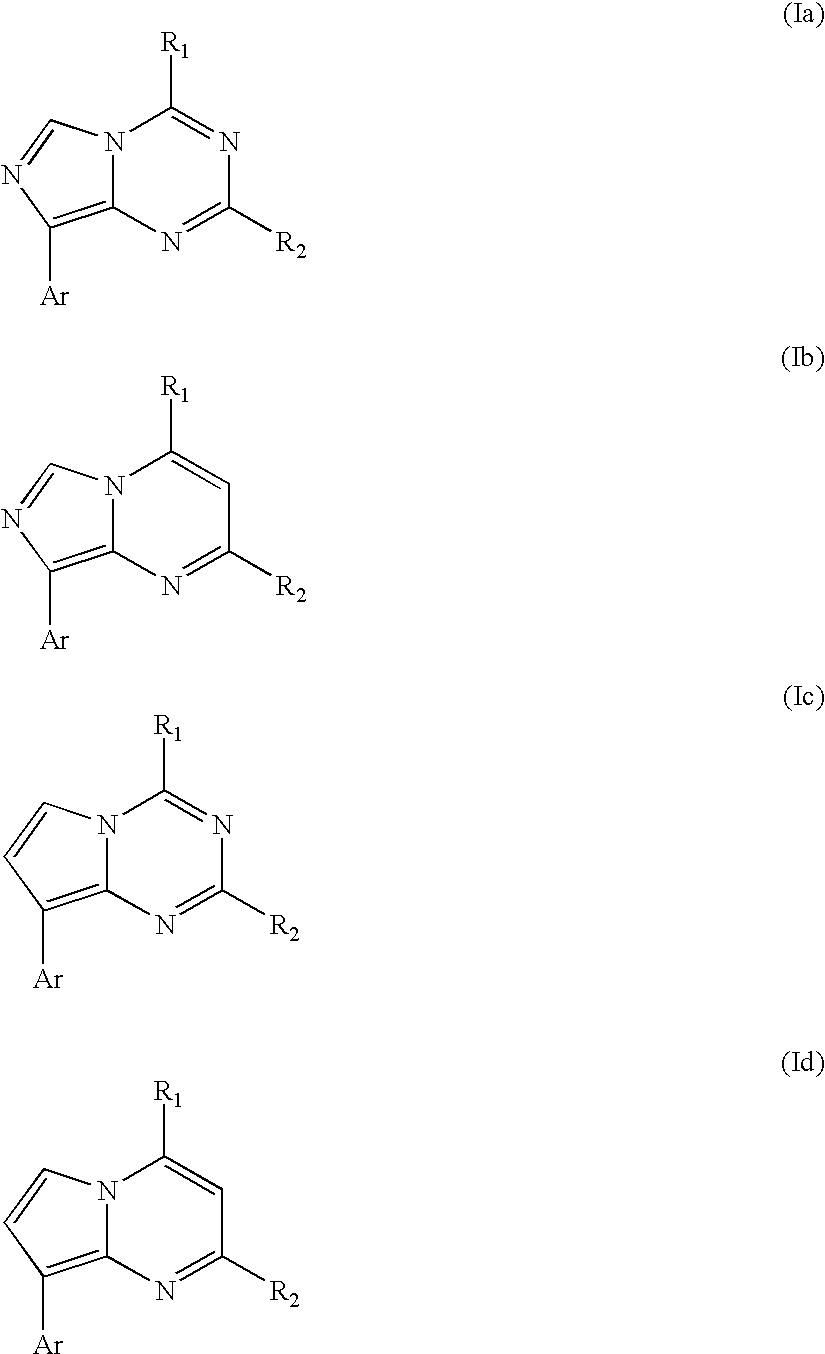
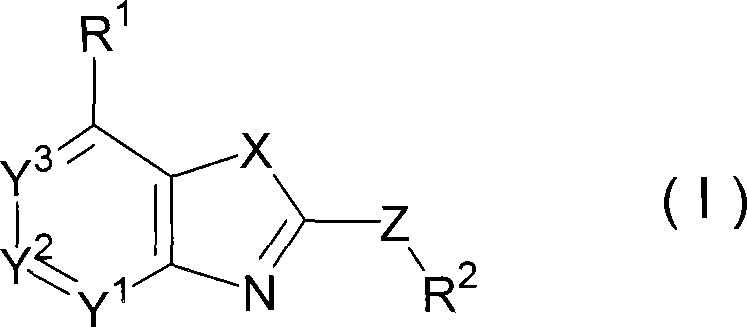
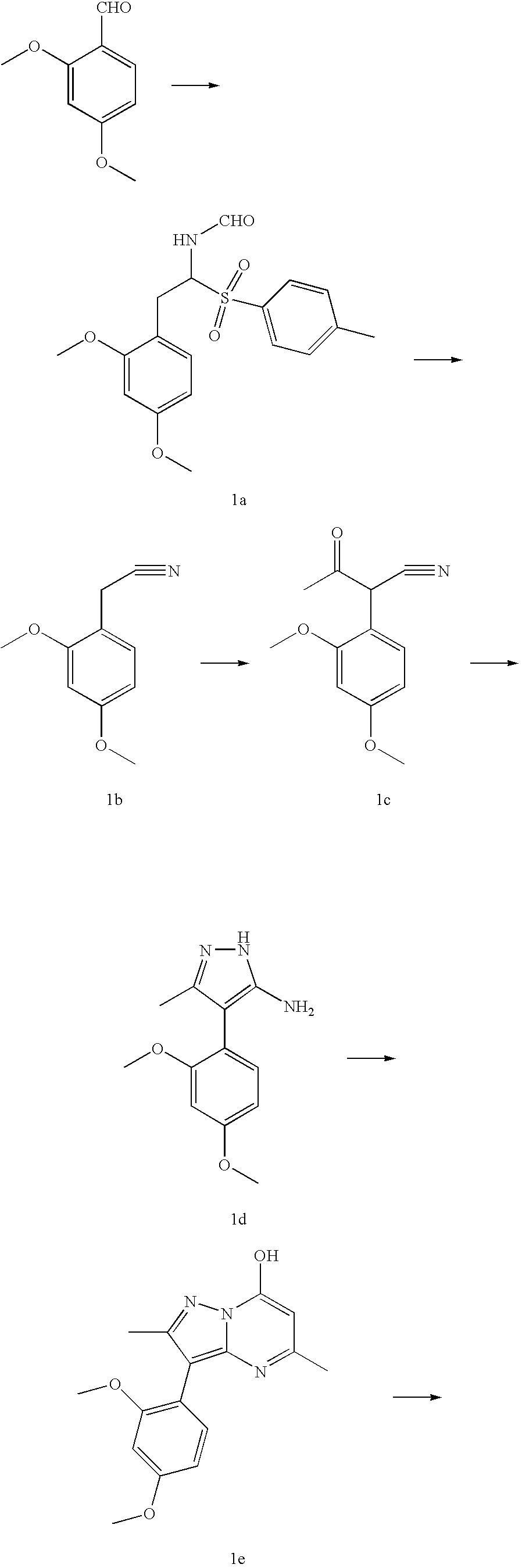
CRF receptor antagonists are disclosed which may have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in mammals. The CRF receptor antagonists of this invention have the following structure: (I);and pharmaceutically acceptable salts, esters, solvates, stereoisomers and prodrugs thereof, wherein R1, R2a, R2b, Y, Het, n, o, R6, Ar and R7 are as defined herein. Compositions containing a CRF receptor antagonist in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Owner:NEUROCRINE BIOSCI INC
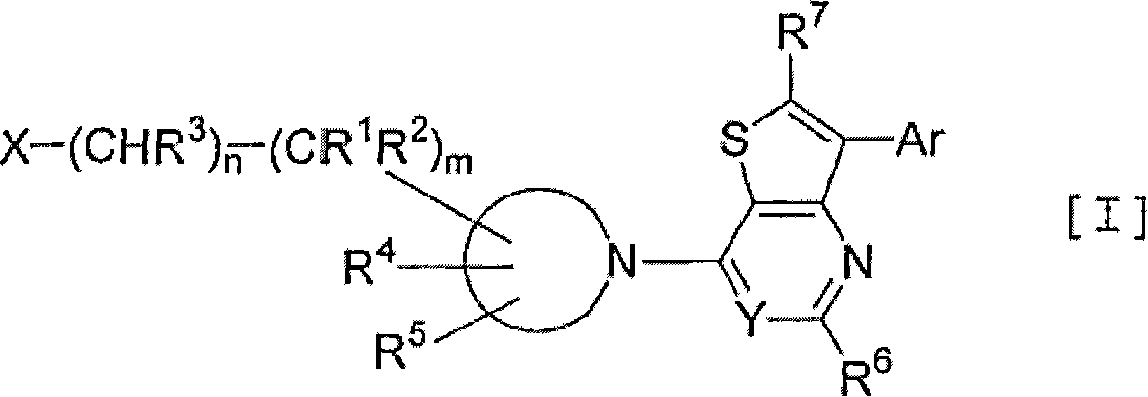
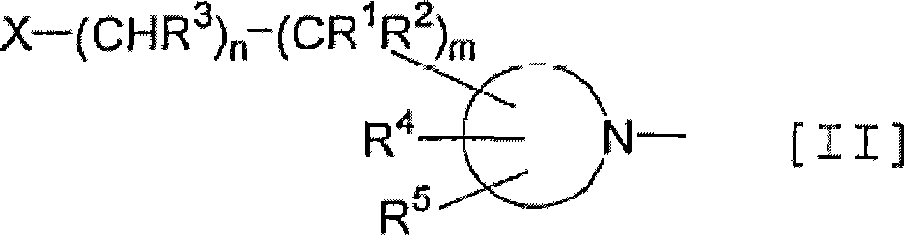
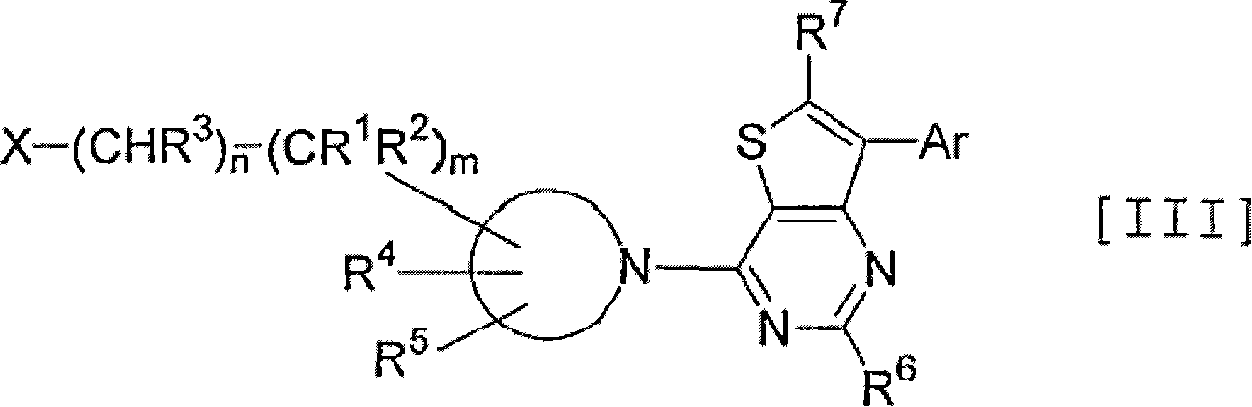
Thienopyrimidine and thienopyridine derivatives substituted with cyclic amino group
An object of the present invention is to provide an antagonist against CRF receptors which is effective as a therapeutic or prophylactic agent for diseases in which CRF is considered to be involved, such as depression, anxiety, Alzheimer's disease, Parkinson's disease, Huntington's chorea, eating disorder, hypertension, gastral diseases, drug dependence, epilepsy, cerebral infarction, cerebral ischemia, cerebral edema, cephalic external wound, inflammation, immunity-related diseases, alpecia, irritable bowel syndrome, sleep disorders, dermatitides, schizophrenia, pain, etc. A thienopyrimidine or thienopyridine derivative substituted with a cyclic amino group represented by the following formula [I]: has a high affinity for CRF receptors and is effective against diseases in which CRF is considered to be involved.
Owner:TAISHO PHARMACEUTICAL CO LTD
Crf receptor antagonists and methods relating thereto
CRF receptor antagonists are disclosed which have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in a warm-blooded animals, such as stroke. The CRF receptor antagonists of this invention have the following structure (I), including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein R1, R2, R3, Y, Ar, and Het are as defined herein. Compositions containing a CRF receptor antagonist in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Owner:SMITHKLINE BEECHAM (CORK) LTD +1
Crf Receptor Antagonists and Methods Relating Thereto
CRF receptor antagonists are disclosed which have utility in the treatment of a variety of disorders in mammals, including the treatment of disorders, such as stroke, manifesting hypersecretion of CRF. The CRF receptor antagonists of this invention have the following structure: including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein R1, R2, R5, Ar, and Het are as defined herein. Compositions containing a CRF receptor antagonist in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Owner:SMITHKLINE BEECHAM (CORK) LTD +1
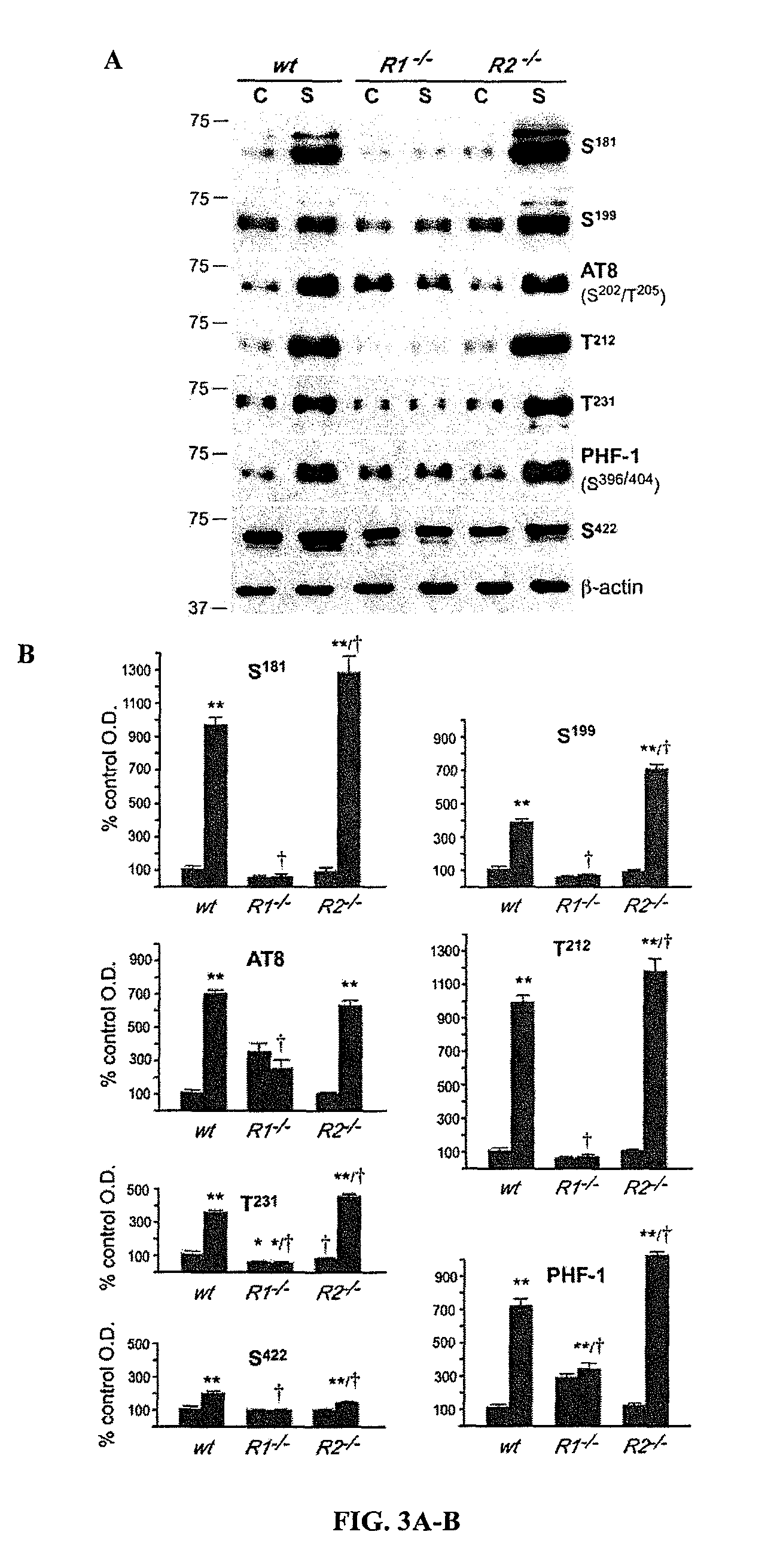
Methods for treatment and prevention of tauopathies and amyloid beta amyloidosis by modulating CRF receptor signaling
Methods for treating or preventing tauopathies and / or Aβ amyloidosis by modulating CRF receptor signaling. Accumulation of hyperphosphorlyated tau protein in the CNS may be reduced by administration of CRF-R1 selective antagonists and / or CRF-R2 selective agonists. For example, in some aspects, methods for preventing the onset of Alzheimer's disease by administration of CRF-R1 selective antagonist are provided.
Owner:RES DEVMENT FOUND
CRF receptor antagonists and methods relating thereto
CRF receptor antagonists are disclosed which have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in a warm-blooded animals, such as stroke. The CRF receptor antagonists of this invention have the following structure:including stereoisomers and pharmaceutically acceptable salts thereof, wherein n, m, A, B, C, R, R1, R2 and Ar are as defined herein. Compositions containing a CRF receptor antagonist in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same
Owner:NEUROCRINE BIOSCI INC
CRF receptor antagonists and methods relating thereto
CRF receptor antagonists are disclosed which have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in a warm-blooded animals, such as stroke. The CRF receptor antagonists of this invention have structure (I) including stereoisomers and pharmaceutically acceptable salts thereof, wherein n, m, A, B, C, R, R1, R2 and Ar are as defined herein. Compositions containing a CRF receptor antagonist in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Owner:NEUROCRINE BIOSCIENCES INC
CRF receptor antagonists and methods relating thereto
Owner:NEUROCRINE BIOSCIENCES INC
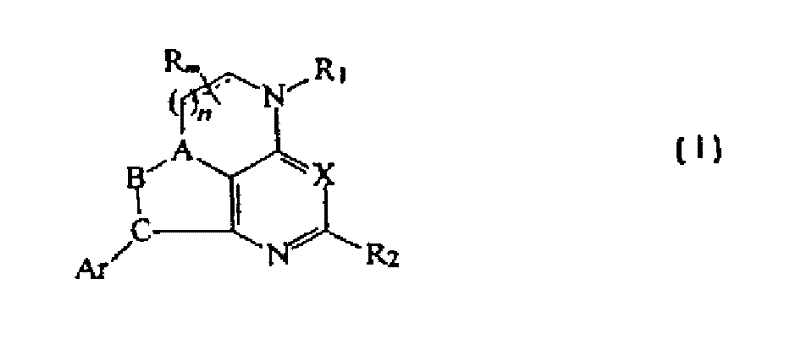
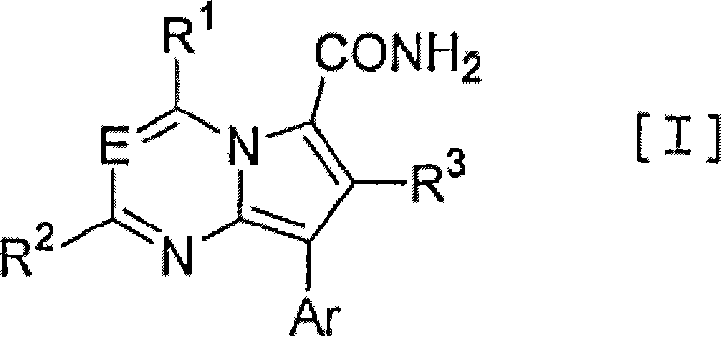
Pyrrolopyrimidine and pyrrolotriazine derivatives as CRF receptor antagon
An object of the present invention is to provide an antagonist against CRF receptors which is effective as a therapeutic or prophylactic agent for diseases in which CRF is considered to be involved, such as depression, anxiety, Alzheimer's disease, Parkinson's disease, Huntington's chorea, eating disorder, hypertension, gastral diseases, drug dependence, epilepsy, cerebral infarction, cerebral ischemia, cerebral edema, cephalic external wound, inflammation, immunity-related diseases, alpecia, irritable bowel syndrome, sleep disorders, dermatitides, schizophrenia, pain, etc. A pyrrolopyrimidine or pyrrolotriazine derivative substituted with a carbamoyl group represented by the following formula [I]: has a high affinity for CRF receptors and is effective against diseases in which CRF is considered to be involved.
Owner:TAISHO PHARMACEUTICAL CO LTD
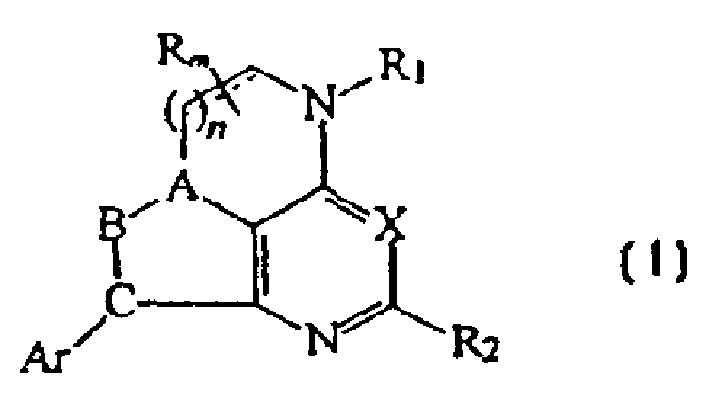
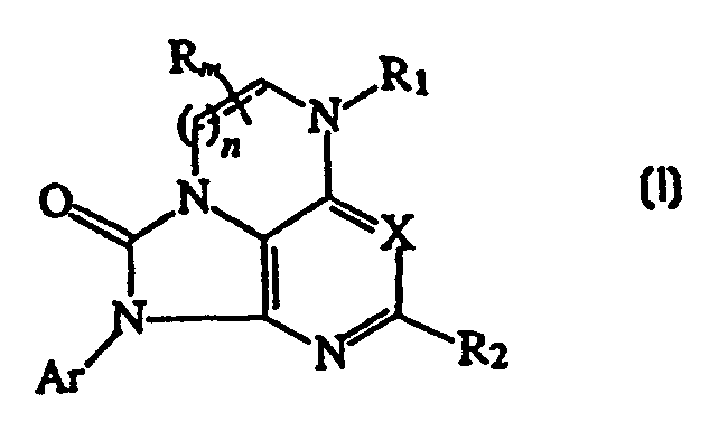
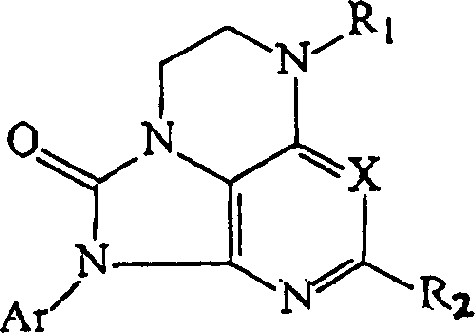
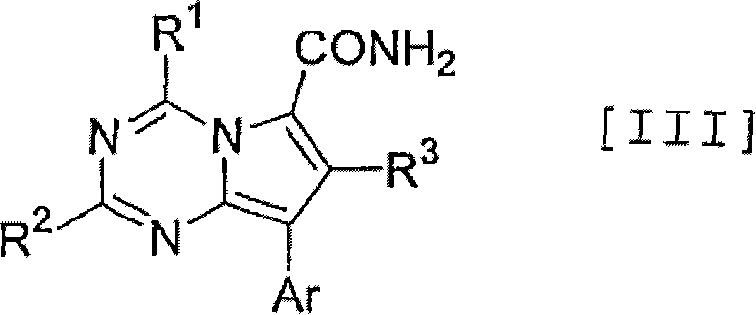
3-phenylpyrazolo[5,1-b]thiazole compounds
ActiveUS8431603B2Excellent CRF receptor antagonismUseful in therapyOrganic active ingredientsBiocideAntagonismPhenyl group
A compound represented by the following formula (I), or salt thereof exhibits excellent CRF receptor antagonism, and sufficient pharmacological activity, safety and pharmacokinetic properties as a drug.wherein R1 represents the formula -A11-A12; R2 represents tetrahydrofurylmethyl, tetrahydropyranylmethyl or tetrahydropyranyl; A11 represents a single bond, methylene or 1,2-ethylene; A12 represents C1-6 alkyl, C3-6 cycloalkyl or C3-6 cycloalkyl having methyl; R3 represents methoxy, cyano, cyclobutyloxymethyl, methoxymethyl or ethoxymethyl; and R4 represents methoxy or chlorine.
Owner:EISIA R&D MANAGEMENT CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com























![3-PHENYLPYRAZOLO[5,1-b]THIAZOLE COMPOUNDS 3-PHENYLPYRAZOLO[5,1-b]THIAZOLE COMPOUNDS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0d1bf306-31b9-4165-9f17-472829411d66/US20090259049A1-20091015-C00001.png)
![3-PHENYLPYRAZOLO[5,1-b]THIAZOLE COMPOUNDS 3-PHENYLPYRAZOLO[5,1-b]THIAZOLE COMPOUNDS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0d1bf306-31b9-4165-9f17-472829411d66/US20090259049A1-20091015-C00002.png)
![3-PHENYLPYRAZOLO[5,1-b]THIAZOLE COMPOUNDS 3-PHENYLPYRAZOLO[5,1-b]THIAZOLE COMPOUNDS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0d1bf306-31b9-4165-9f17-472829411d66/US20090259049A1-20091015-C00003.png)











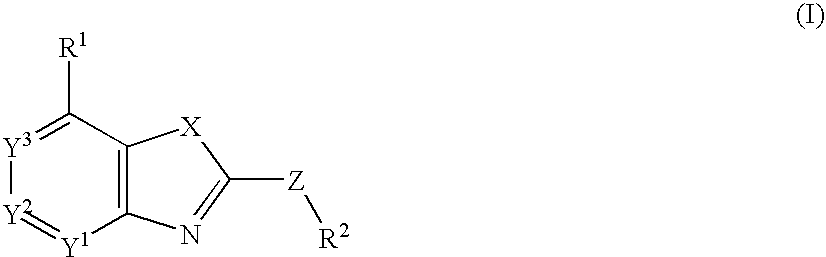








![Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8acdc4f6-2c35-4e2b-8051-7f951315a125/US07879862-20110201-D00001.png)
![Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8acdc4f6-2c35-4e2b-8051-7f951315a125/US07879862-20110201-D00002.png)
![Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists Pyrazolo[1,5-alpha]pyrimidinyl derivatives useful as corticotropin-releasing factor (CRF) receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8acdc4f6-2c35-4e2b-8051-7f951315a125/US07879862-20110201-D00003.png)












![3-phenylpyrazolo[5,1-<i>b</i>]thiazole compounds 3-phenylpyrazolo[5,1-<i>b</i>]thiazole compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d254f4d-c47f-4937-9052-c44b24bbdf0f/US08431603-20130430-C00001.png)
![3-phenylpyrazolo[5,1-<i>b</i>]thiazole compounds 3-phenylpyrazolo[5,1-<i>b</i>]thiazole compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d254f4d-c47f-4937-9052-c44b24bbdf0f/US08431603-20130430-C00002.png)
![3-phenylpyrazolo[5,1-<i>b</i>]thiazole compounds 3-phenylpyrazolo[5,1-<i>b</i>]thiazole compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d254f4d-c47f-4937-9052-c44b24bbdf0f/US08431603-20130430-C00003.png)