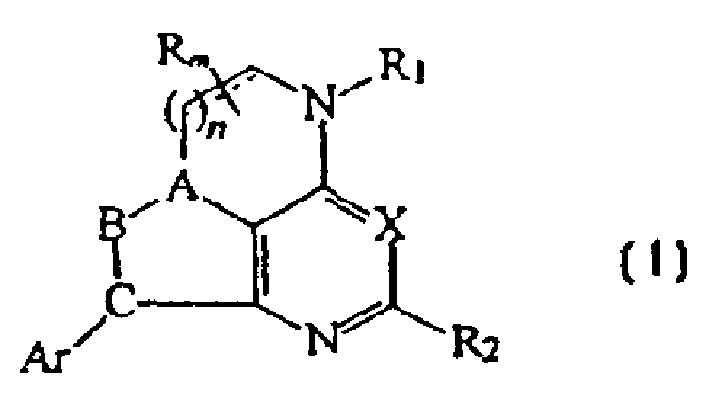
CRF receptor antagonists and methods relating thereto
A technology of stereoisomers and compounds, applied in medical preparations containing active ingredients, organic chemistry, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] Synthesis of Representative Compounds of Structural Formula (I-1)
[0167] Structural formula (I-1a)
[0168]
[0169]
[0170] Compound (2)
[0171] 2,4-Dichloro-6-methyl-3-ethylpyridine (1) (5.0g 21.36mmol), 4-heptylamine (21.36mmol) and triethylamine (2.97ml, 21.36mmol) in ethanol The solution was heated to reflux overnight. Ethanol was distilled off and the residue was dissolved in ethyl acetate, washed with saturated bicarbonate solution and brine. The organic layer was dried over sodium carbonate and concentrated in vacuo. Compound (2) was separated from (1) and (3) by silica gel column eluting with ethyl acetate-hexane.
[0172] Compound (4)
[0173] A solution of compound (2) (20.0 mmol) and hydrazine (25.0 mmol) in ethanol was refluxed overnight. Ethanol was distilled off and the residue was dissolved in ethyl acetate, washed with water, dried over sodium sulfate and concentrated in vacuo to give compound (4)...
Embodiment 2
[0194] Synthesis of Representative Compounds of Structural Formula (I-2)
[0195] Structural formula (I-2a)
[0196]
[0197]
[0198] α-phthalimide-2,4-trichloroacetophenone (1)
[0199] α-2,4-Trichloroacetophenone (15 g, 67 mmol) was added to potassium phthalimide (16 g, 86 mmol) in N, N-dimethylformamide ( 70ml) suspension. After 5 minutes, the resulting solution was allowed to warm to room temperature and then heated at 50°C for 0.5 hours. After heating, the solution was concentrated by pump and the resulting solid was partitioned between ethyl acetate / sodium bicarbonate solution and the resulting organic layers were combined. The organic layer was dried and all solvent was evaporated to obtain a solid. The solid was recrystallized from dichloromethane and diethyl ether to obtain compound (1), 9.6 g.
[0200] Compound (2)
[0201] A solution of α-phthalimide-2,4-trichloroacetophenone (1) (9.6 g) in dimethylformam...
Embodiment 3
[0257] Synthesis of Representative Compounds of Structural Formula (I-3)
[0258]
[0259]
[0260] N-oxides (4)
[0261] Compound (3) (7.31 g, 50 mmol), mCPBA (9.49 g, 55 mmol) prepared by the method disclosed by Clayton and Kenyon ("Chemical Society Journal" (J.Chem.Soc.), 2952-57, 1950) were prepared in The mixture in dichloromethane (200 mL) was stirred at room temperature for 2 hours. The reaction mixture was partitioned between dichloromethane and water. The dichloromethane was dried over sodium sulfate, filtered and concentrated to give the desired product N-oxide (4).
[0262] Compound (5)
[0263] A mixture of N-oxide (4) (4.87 g, 30 mmol) and phosphorus oxychloride (15 mL) was refluxed for 3 h, cooled, poured onto crushed ice, and neutralized with 1N NaOH. The aqueous layer was extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated to give compound (5).
[0264] Compound (6)
[0265] To a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com