CRF receptor antagonists and methods relating thereto
A stereoisomer, CH2OCH3 technology, applied in anti-inflammatory agents, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
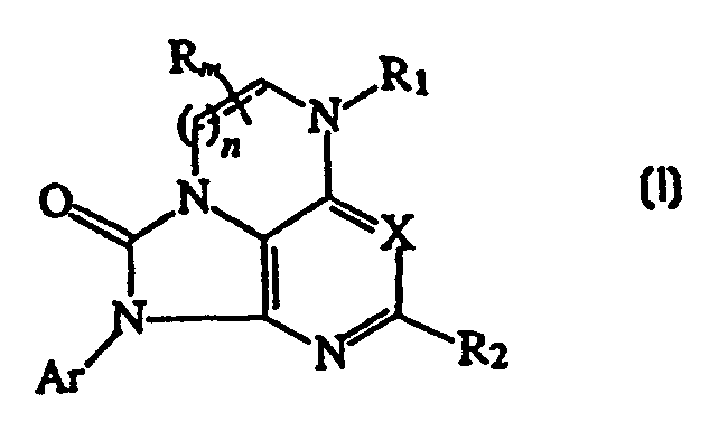
[0099] Synthesis of Representative Compounds of Structural Formula (IA)
[0100]
[0101]
[0102] Compound (4 )
[0103] 4,6-Dichloro-2-methyl-5-nitropyrimidine (3; J.Chem.Soc. 1954, 3836) (2.23 g, 11 mmol) in ethanol (30 mL) A solution of Ethanol was treated with a solution of 1-ethylpropylamine (870 mg, 10 mmol) in ethanol (8 mL) at -30°C and the reaction mixture was stirred at -30°C for 1 hour before warming to room temperature. The volatiles were evaporated and the residue was partitioned between water and ethyl acetate. The organic layer was dried (sodium sulfate), evaporated and purified by flash chromatography (silica gel) to give compound (4).
[0104] Compound (5 )
[0105] A solution of compound (4) (2.07 g, 8 mmol) in acetonitrile (15 mL) was treated with 2,4,6-trimethylaniline (1.35 g, 10 mmol) at room temperature, followed by addition of triethylamine (1.52 g, 15 mmol) . The reaction mixture was stirred at room temperature for 2 hours. T...
Embodiment 2
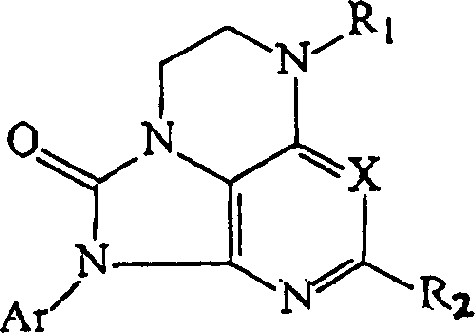
[0115] Synthesis of Compounds of Structural Formula (IB)
[0116] Compounds of formula (IB) can be prepared by the same synthetic route as disclosed in Example 1 above, but substituting the corresponding pyridine compound (1) for pyrimidine. For example, representative compounds of the invention can be prepared by the following reaction schemes:
[0117]
[0118]
Embodiment 3
[0120] Synthesis of Representative Compounds
[0121] Other representative compounds of the invention were prepared by the general reaction schemes disclosed above and / or the methods of Examples 1 and 2, and are shown in the table below.
[0122] surface
[0123] representative compound
[0124]
[0125] Cpd
R
X
R 1
Ar
(I-1)
H
N
-CH(CH 2 CH 2 CH 3 ) 2
2,4,6-Trimethylphenyl
(I-2)
H
CH
-CH(CH 2 CH 2 CH 3 ) 2
2-Chloro-4-methylphenyl
(I-3)
H
CH
-CH(CH 2 CH 2 CH 3 ) 2
2-Bromo-4-isopropylphenyl
[0126]
[0127] Cpd
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com