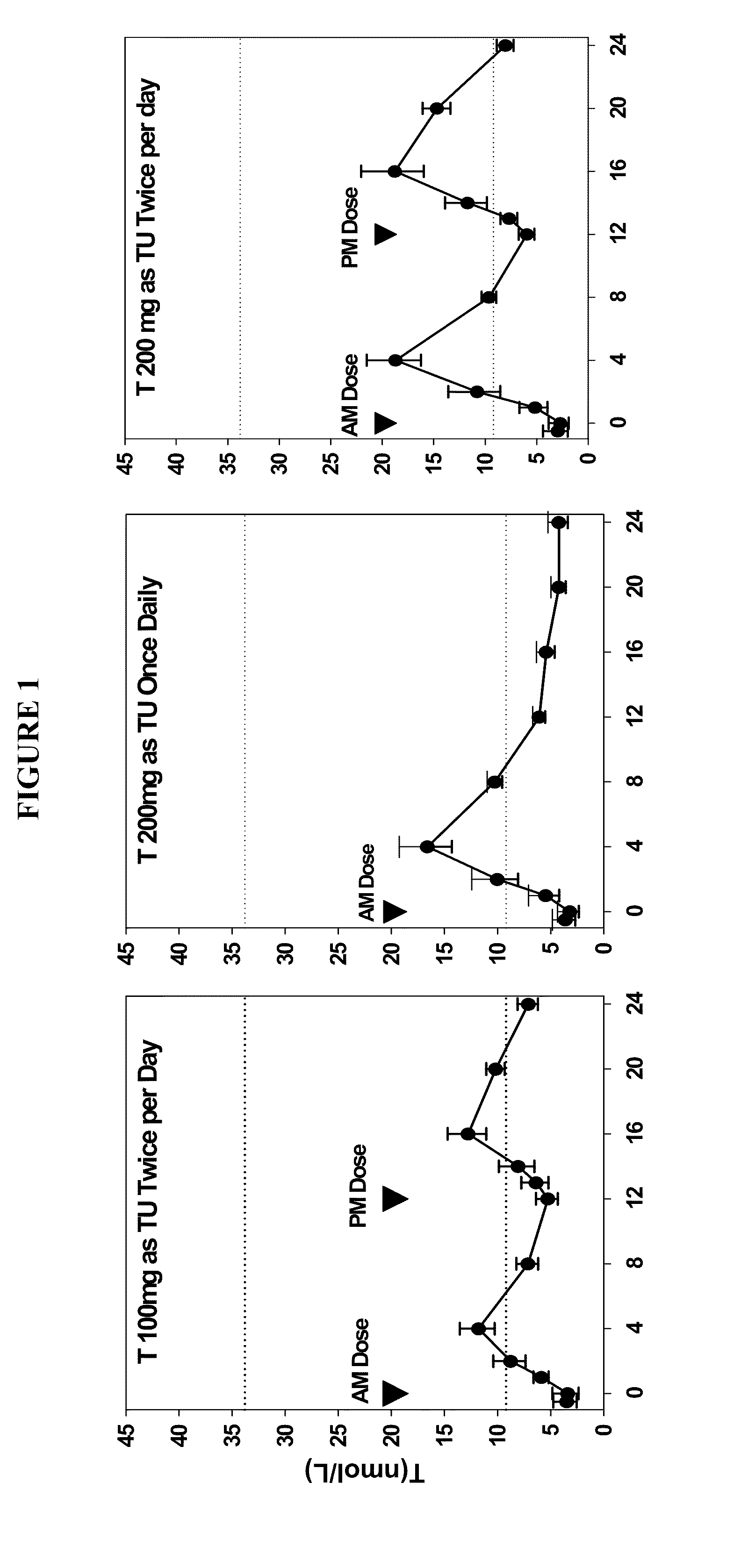
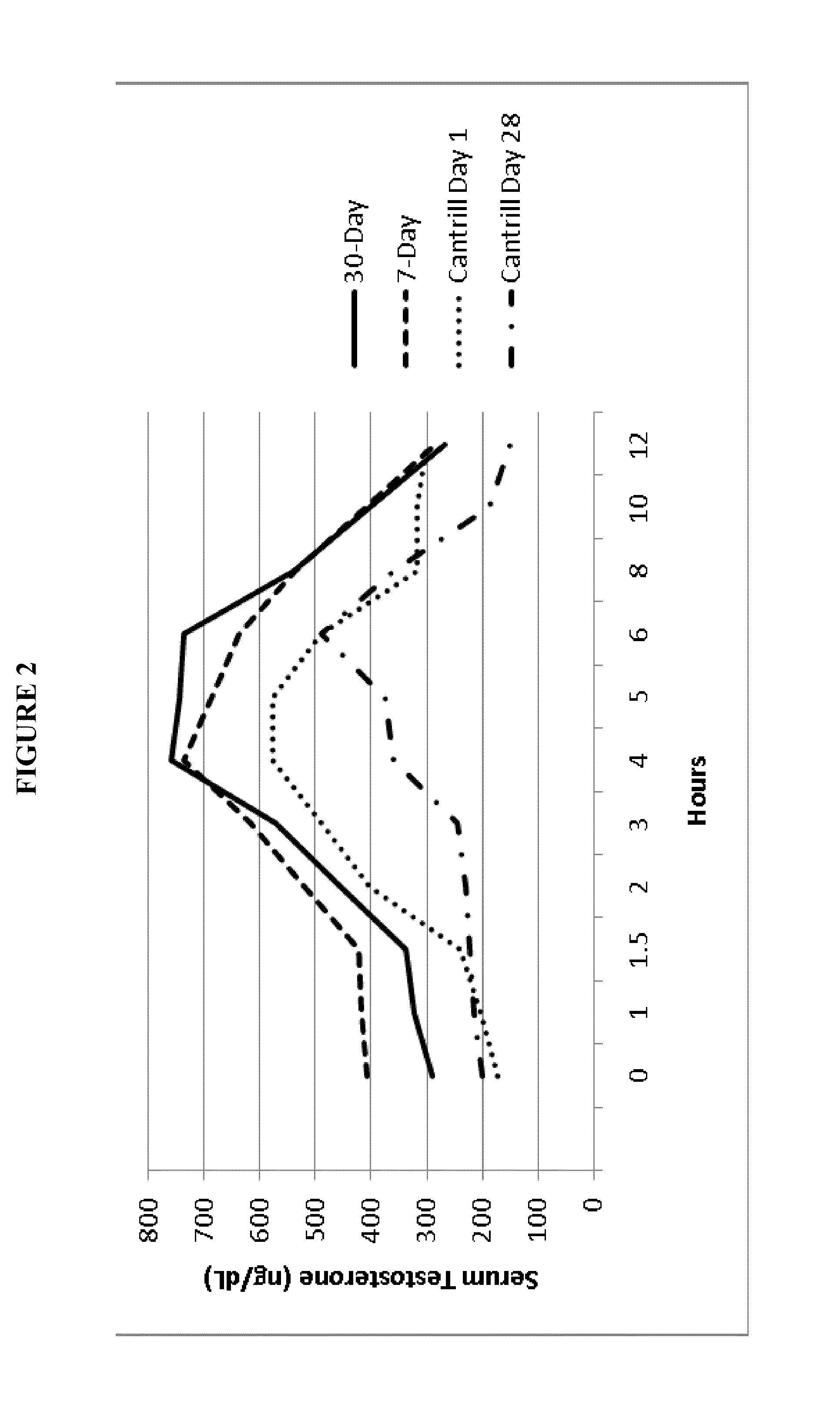
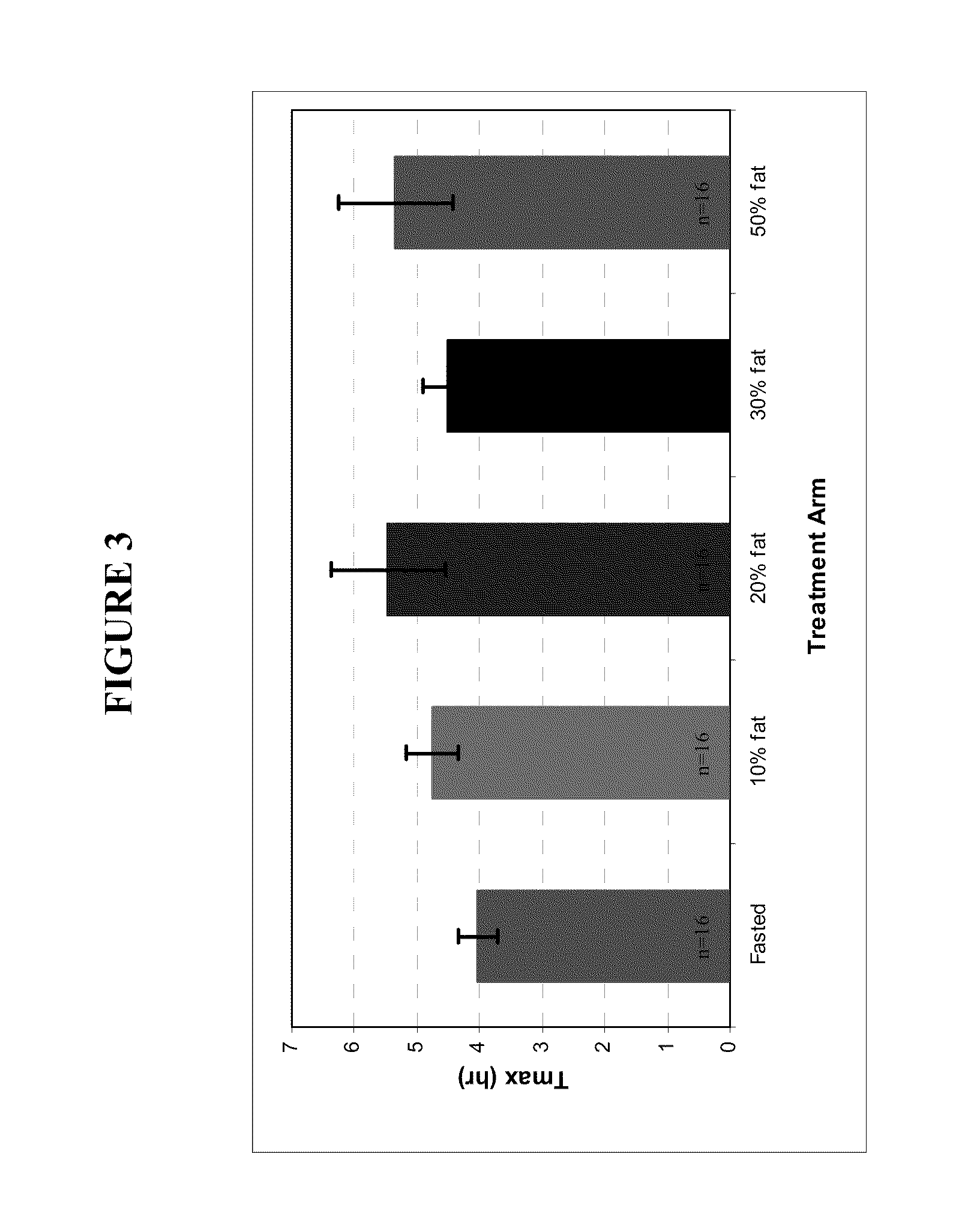
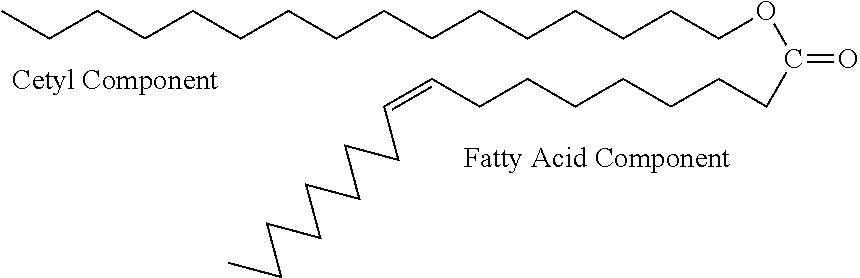
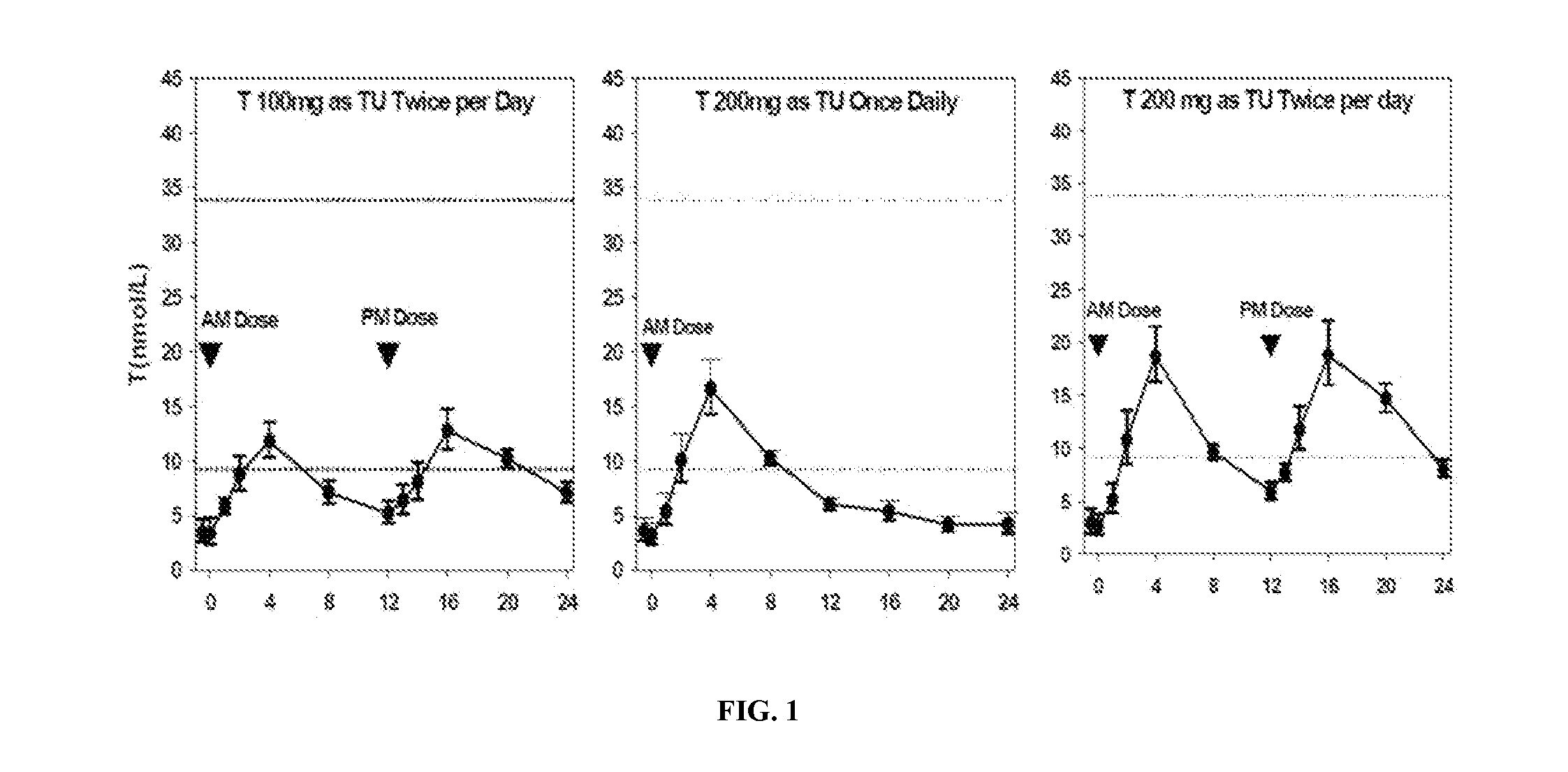
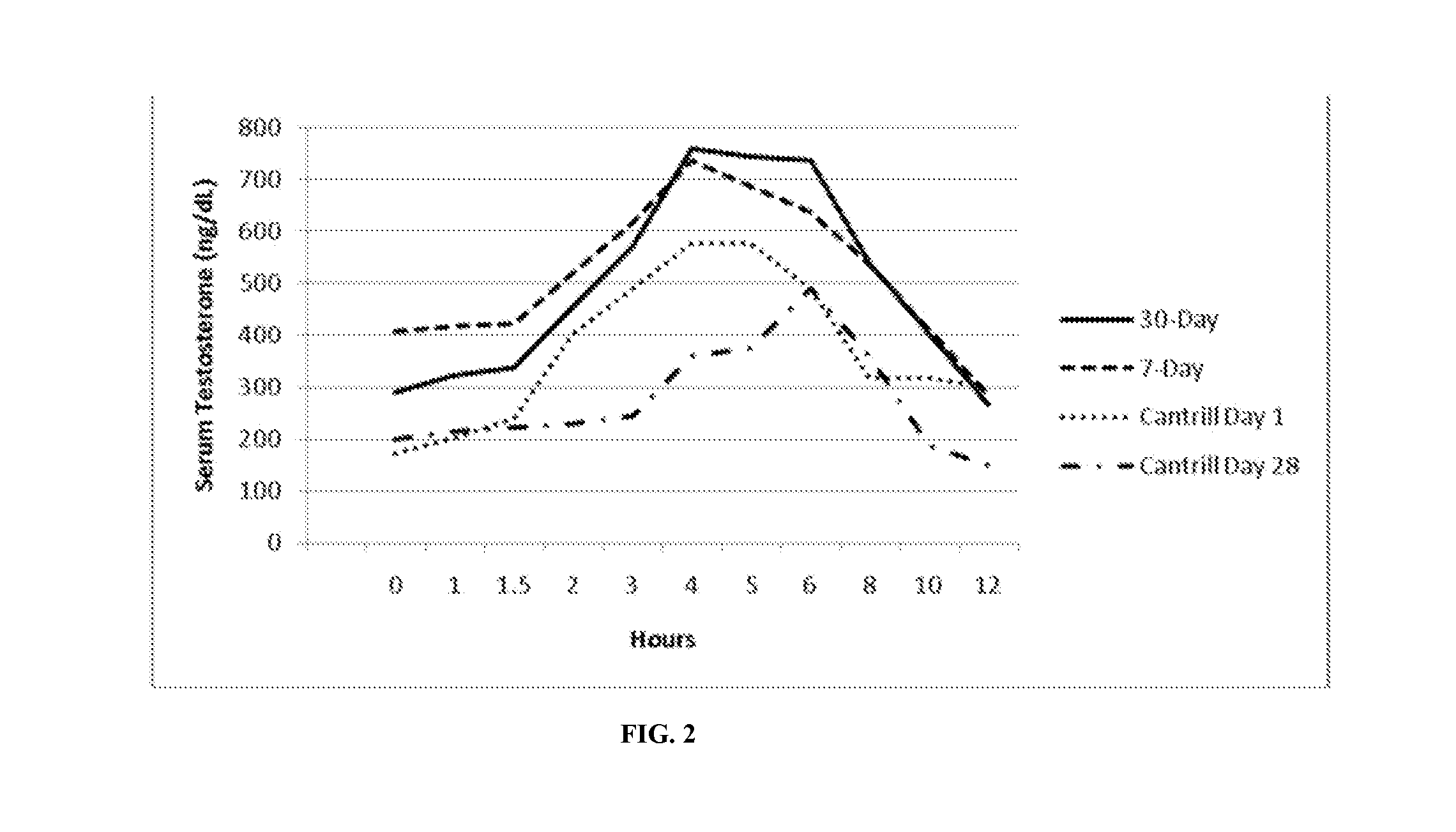
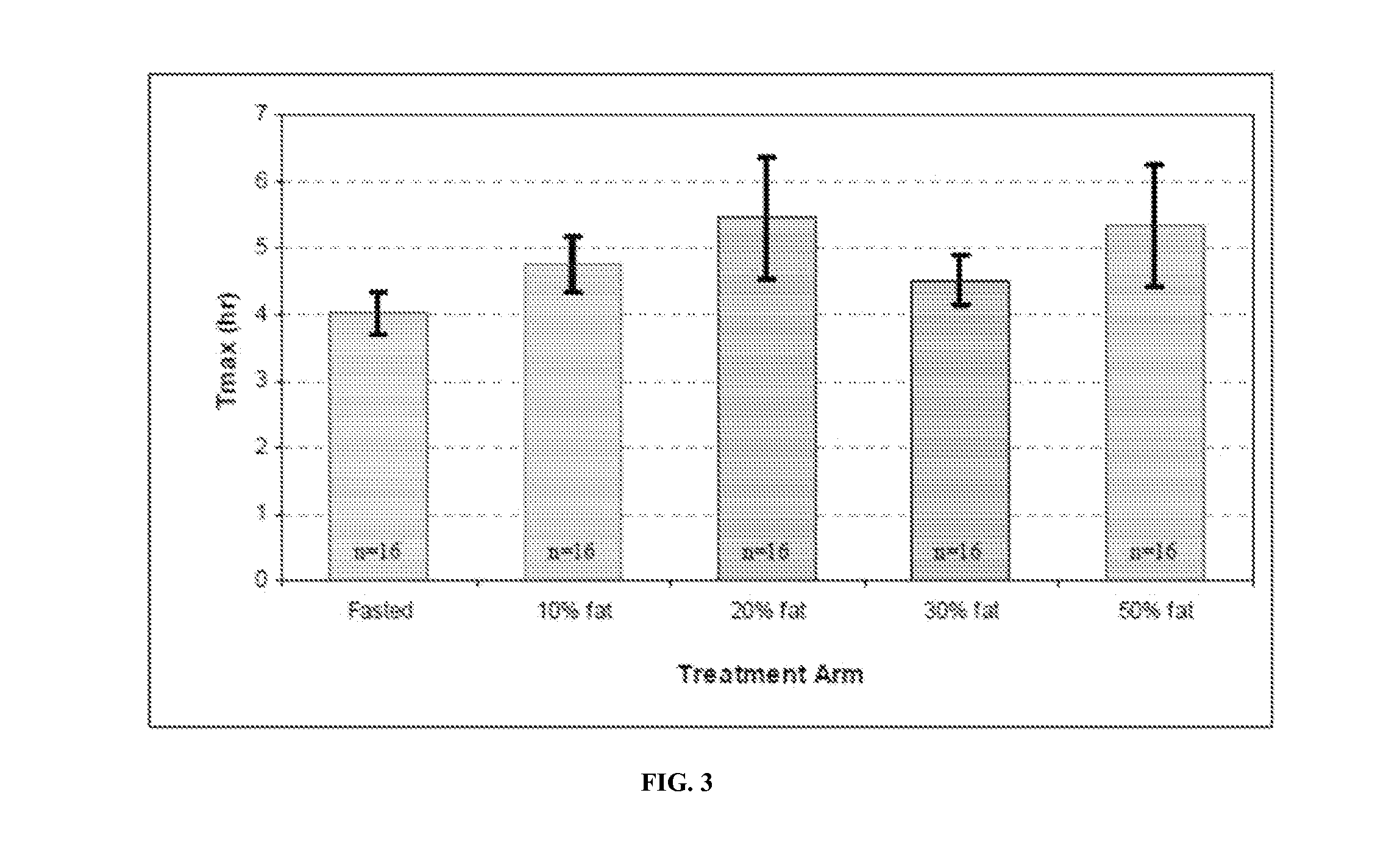
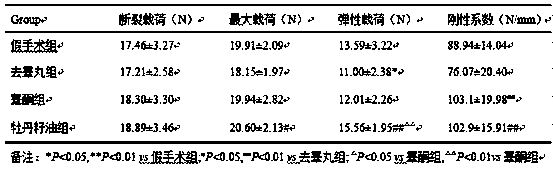
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Testosterone deficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Testosterone deficiency occurs when the body is unable to make enough testosterone. It is sometimes called hypogonadism. Testosterone deficiency can significantly affect a man's health and quality of life. Testosterone is the most important sex hormone in men.
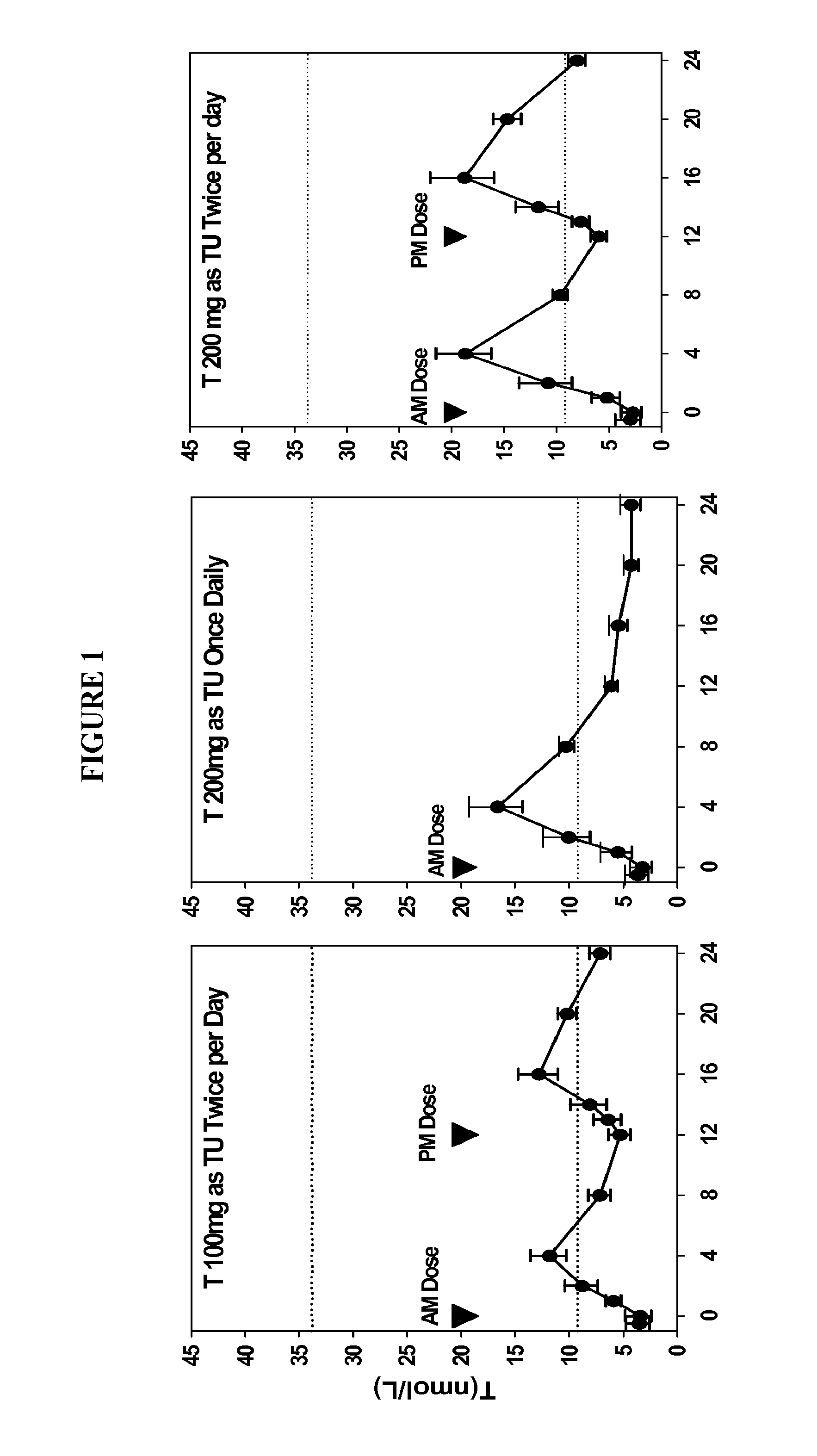
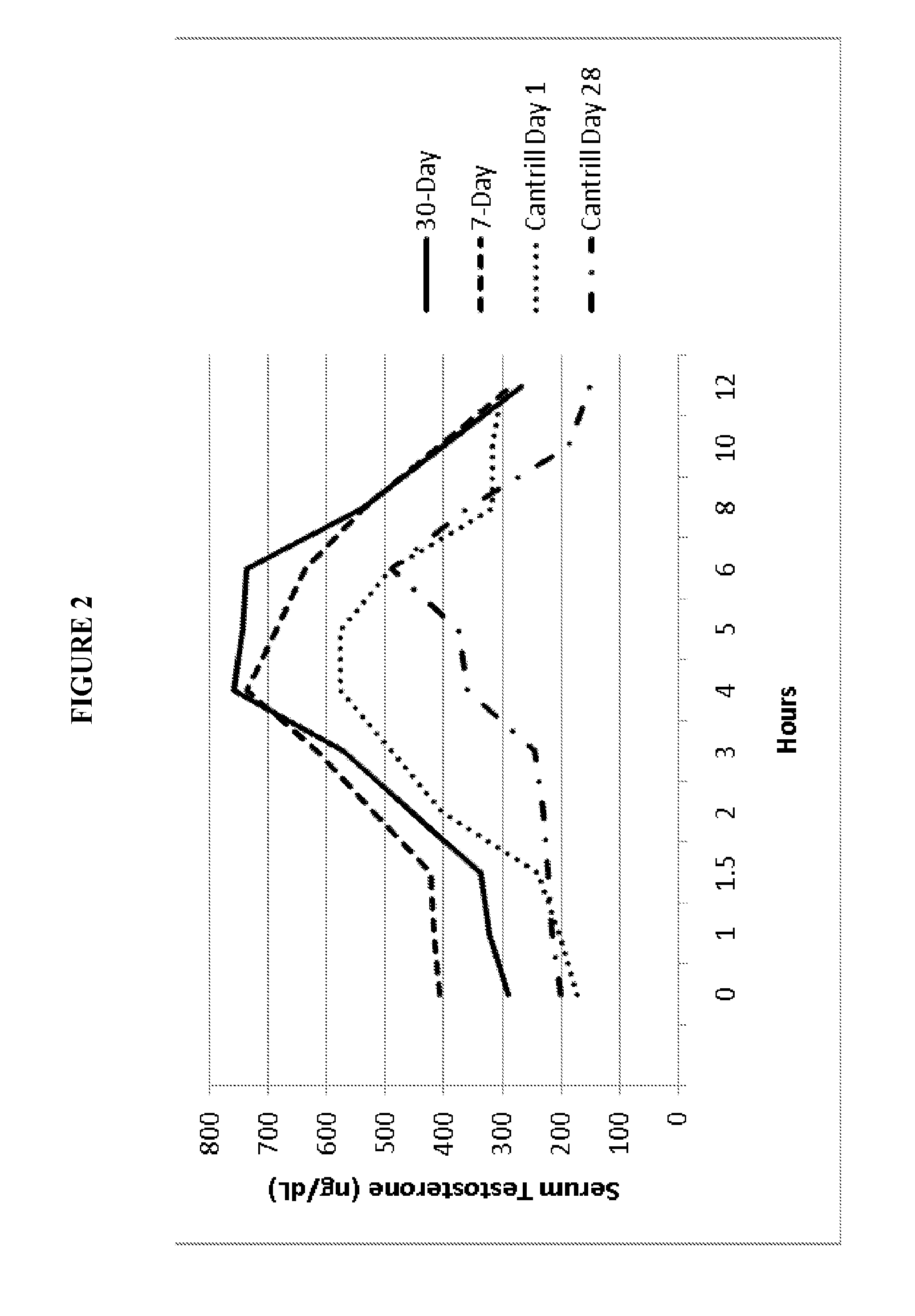
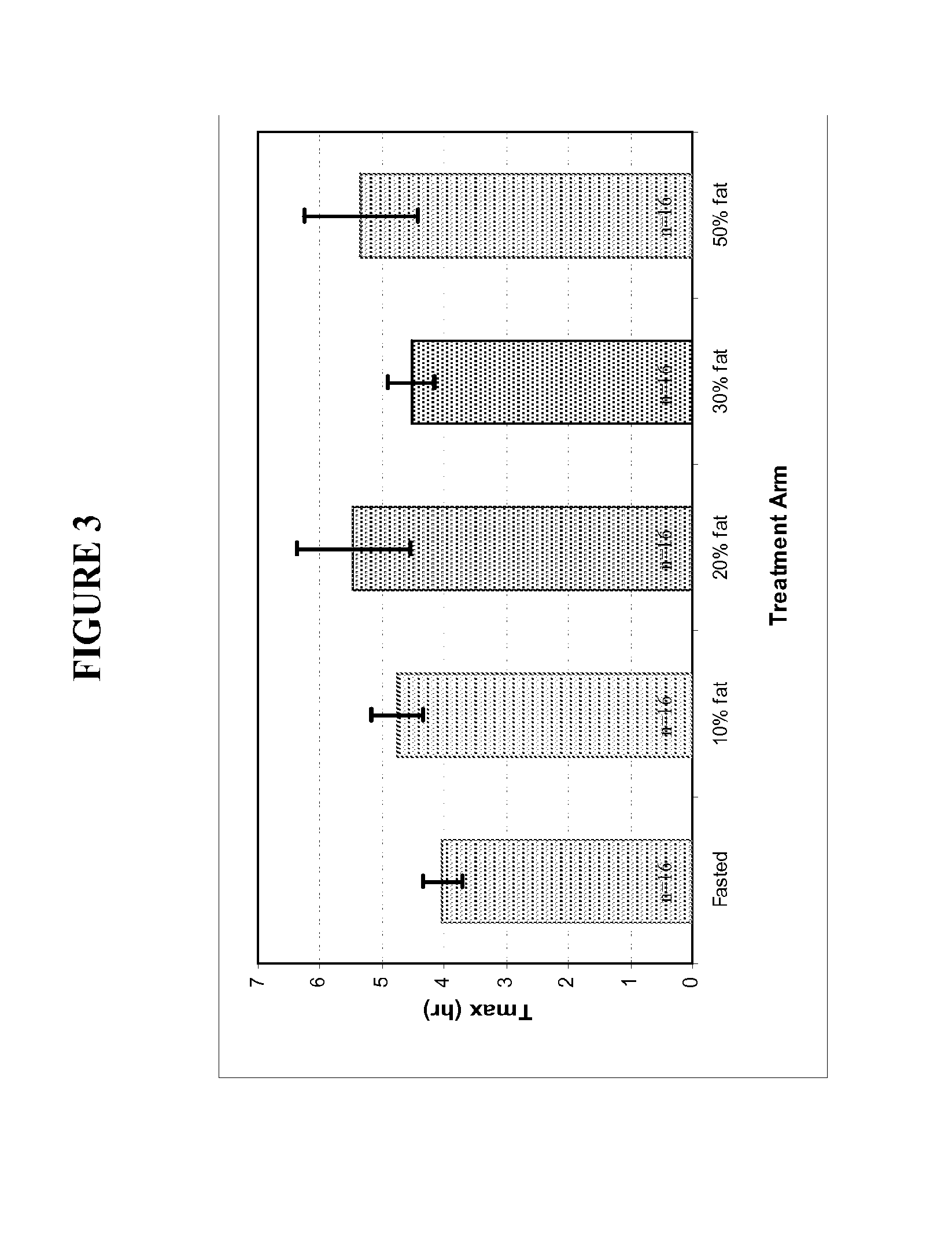
Oral testosterone ester formulations and methods of treating testosterone deficiency comprising same
ActiveUS20110251167A1Organic active ingredientsDispersion deliveryPhysiologyPharmaceutical formulation
A pharmaceutical formulation of testosterone undecanoate is provided. Methods of treating a testosterone deficiency or its symptoms with the inventive formulations are also provided.
Owner:TOLMAR INC
Transdermal delivery of medicaments with combinations of cetylated fatty ester penetrant complexes
This invention describes a topical delivery mechanism that contains a mixture of cetylated fatty esters that act as transdermal carriers of desired therapeutic molecules. The proposed cetyl fatty ester penetrant-complex (Base CFEP-complex) contains specific cetyl fatty esters, polar solvents, a carrier base (gel, cream, lotion, patch or stick gel), antioxidants and the desired pharmaceutical, cosmetic or antigenic response eliciting molecules that are efficaciously delivered by selectively varying component ratios in the complex. The invention proposes the use of transdermal delivery of medications such as those used in treatment of urinary incontinence, testosterone deficiency, arthritic and joint pain and other pains such as pain in the neck, lower back, back, knees, headaches, and other types of inflammatory pains, peripheral neuropathic pain, pain associated with repetitive strain injuries such as myofacial pain, rapid treatment of epileptic seizures, soluble antigens in the immuno-therapeutic treatment of allergies, actives in the treatment of foot cracks and elbow cracks, actives in the treatment of facial and other wrinkles in the form of anti-aging creams and gels and other topically delivered therapies.
Owner:CYMBIOTICS
Transdermal delivery of medicaments with combinations of cetylated fatty fatty ester penetrant complexes
InactiveUS20110065627A1Facilitate more efficacious permeationReduce resistanceCosmetic preparationsBiocideWrinkle skinAntioxidant
This invention describes a topical delivery mechanism that contains a mixture of cetylated fatty esters that act as transdermal carriers of desired therapeutic molecules. The proposed cetyl fatty ester penetrant-complex (Base CFEP-complex) contains specific cetyl fatty esters, polar solvents, a carrier base (gel, cream, lotion, patch or stick gel), antioxidants and the desired pharmaceutical, cosmetic or antigenic response eliciting molecules that are efficaciously delivered by selectively varying component ratios in the complex. The invention proposes the use of transdermal delivery of medications such as those used in treatment of urinary incontinence, testosterone deficiency, arthritic and joint pain and other pains such as pain in the neck, lower back, back, knees, headaches, and other types of inflammatory pains, peripheral neuropathic pain, pain associated with repetitive strain injuries such as myofacial pain, rapid treatment of epileptic seizures, soluble antigens in the immuno-therapeutic treatment of allergies, actives in the treatment of foot cracks and elbow cracks, actives in the treatment of facial and other wrinkles in the form of anti-aging creams and gels and other topically delivered therapies.
Owner:CYMBIOTICS
Oral testosterone ester formulations and methods of treating testosterone deficiency comprising same
ActiveUS20120309731A1Organic active ingredientsDispersion deliveryPhysiologyPharmaceutical formulation
A pharmaceutical formulation of testosterone undecanoate is provided. Methods of treating a testosterone deficiency or its symptoms with the inventive formulations are also provided.
Owner:TOLMAR INC
Oral Transmucosal Pharmaceutical Compositions Including Testosterone and a C-SERM
ActiveUS20160051564A1Improve the level ofRelieve symptomsOrganic active ingredientsBiocideSolid Dose FormMucoadhesion
Formulations for oral transmucosal compositions including a synergistic combination of low doses of testosterone with a clomiphene-like selective estrogen receptor modulator (C-SERM) that are combined with transmucosal absorption enhancers are disclosed. Oral transmucosal compositions can be for fast release or slow release, and can be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Oral transmucosal compositions include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms are: mucoadhesive liquids such as gel-forming liquids; gel-forming semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of testosterone and C-SERM. Oral transmucosal compositions will deliver testosterone and C-SERM directly into the patient's bloodstream, and provide high bioavailability of testosterone and C-SERM; therefore, the required doses are lower.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Oral pharmaceutical products and methods of use combining testosterone esters with hypolipidemic agents
ActiveUS20160317553A1No decline in steady state serum testosterone responsePowder deliveryOrganic active ingredientsPhysiologyTestosterone deficiency
Pharmaceutical products comprising a hypolipidemic agent and a testosterone ester such as testosterone undecanoate are provided. Methods of safely treating a testosterone deficiency or its symptoms with the inventive pharmaceutical products are also provided.
Owner:TOLMAR INC
Adult bone marrow cell transplantation to testes creation of transdifferentiated testes germ cells, leydig cells and sertoli cells
InactiveUS20070280907A1Increase productionIncreasing testosterone productionOrganic active ingredientsBiocideMale infertilityPlant Germ Cells
This invention pertains to the discovery that stem cells (e.g., bone marrow stem cells) transplanted directly into a testicular environment are transdifferentiated into bona fide Sertoli cells, and / or Leydig cells, and / or and germ cells. This provides a mechanism for the treatment of male infertility and / or testosterone deficiency. Thus, in one embodiment, this invention provides a method of treating infertility or testosterone deficiency in a male mammal. The method typically involves implanting stem cells into the testes of the mammal whereby the stem cells differentiate into germ cells and / or Sertoli cells and / or Leydig cells thereby reducing infertility and / or testosterone deficiency.
Owner:LOS ANGELES BIOMEDICAL RES INST
Controlled release nasal testosterone gels, methods and pre-filled multi-dose applicator systems for pernasal administration
InactiveCN103813784AEasy to useImprove toleranceOrganic active ingredientsNervous disorderNasal cavityDisease
The present invention relates to intranasal testosterone gels for the controlled release of testosterone into the systemic circulation of males and females for providing constant effective testosterone blood levels, without inducing undesired testosterone spike in blood levels, following pernasal administration. The intranasal testosterone gels of the present invention are safe, convenient to use, well tolerated, stable and easily and reproducibly manufactured on scale up. Moreover, because supra- normal and sub-normal testosterone blood levels are believed to be essentially kept to a minimum or avoided and the testosterone serum levels are believed to remain essentially constant during dose life, i.e., the intranasal testosterone gels of the present invention mimic or restore testosterone blood levels to normal physiologic daily rhythmic testosterone levels, the novel intranasal testosterone gels of the present invention are uniquely suited for testosterone replacement or supplemental therapy and effective for treating males diagnosed with, for example, male testosterone deficiency, such as, low sexual libido, low sexual drive, low sexual activity, low fertility, low spermatogenesis, aspermatogenesis, depression and / or hypogonadism, and females who are diagnosed with, for example, female sexual dysfunction, such as, low sexual libido, low sexual drive, low sexual activity, low amygdala reactivity, low sexual stimulation, hypoactive sexual desire disease ("HSDD"), female sexual arousal disorder and / or anorgasmia. The present invention also relates to methods and pre- filled multi-dose airless applicator systems for pernasal administration of the nasal testosterone gels of the present invention.
Owner:ACERUS PHARMA SRL
Method for inducing human umbilical cord mesenchymal stem cells into testicular interstitial cells and application thereof
InactiveCN102533659AImprove proliferation efficiencyLow immunogenicitySkeletal/connective tissue cellsViruses/bacteriophagesTesticular Interstitial CellsMesenchymal stem cell
The invention discloses a method for inducing human umbilical cord mesenchymal stem cells into testicular interstitial cells and application thereof. The method comprises the following steps: cloning mouse steroidogenic factor-1 (SF-1) gene into an adenovirus shuttle plasmid pAdtrack-CMV-EGFP to construct a recombinant vector pAdtrack-CMV-SF-1; recombining the recombinant vector pAdtrack-CMV-SF-1 and an adenovirus carrier plasmid pAdEasy-1 to obtain an adenovirus plasmid pAd-SF-1 carrying SF-1 gene; digesting and linearizing the adenovirus plasmid pAd-SF-1, and packaging with human embryonic kidney cells AD-293 to obtain adenovirus; adding the adenovirus into an induction culture medium to infect human umbilical cord mesenchymal stem cells, and carrying induced differentiation of the human umbilical cord mesenchymal stem cells to testicular interstitial cells. Through the induction using the method of the invention, human umbilical cord mesenchymal stem cells can in vitro differentiate into testicular interstitial cells only in one week, so as to provide an important cell source for treatment of testosterone deficiency using cell replacement or genetic method.
Owner:JINAN UNIVERSITY
Testosterone Solutions for the Treatment of Testosterone Deficiency
ActiveUS20130143851A1Lower testosterone levelsIncreased blood serum levelOrganic active ingredientsNervous disorderSerum testosterone levelTestosterone deficiency
Solutions of testosterone for oromucosal administration providing an increase in serum testosterone levels in subjects deficient in endogenous testosterone levels, and therapeutic methods for providing an increase in serum testosterone levels and methods for treating a disease or a symptom associated with deficient endogenous levels of testosterone.
Owner:INNOTESTO
Testosterone Booster Transdermal Compositions
InactiveUS20150065426A1Reduce riskPromote absorptionOrganic active ingredientsPeptide/protein ingredientsPhospholipidBULK ACTIVE INGREDIENT
Formulations and methods for transdermal drug delivery compositions that include synergistic combination of three pharmaceutical active ingredients (APIs), such as testosterone, anastrozole, and HCG are disclosed. TBTC of the present disclosure may be indicated for reducing symptoms of testosterone deficiency. Disclosed TBTC may include permeation enhancers that may improve penetration of testosterone, anastrozole, and HCG in human skin. Permeation enhancer compositions within TBTC may include oils from amazon rainforest such as Pracaxi oil, Plukenetia volubilis seed oil, Inaja oil, and Patauá oil, which includes behenic and oleic fatty acids that may provide penetration power. TBTC may include organic solvents as transdermal penetration enhancers. Additionally, TBTC may include physiological lipids, phospholipids, and one or more butters rich in linoleic acid and linolenic acid that may also provide penetration power with restorative benefits to the skin.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
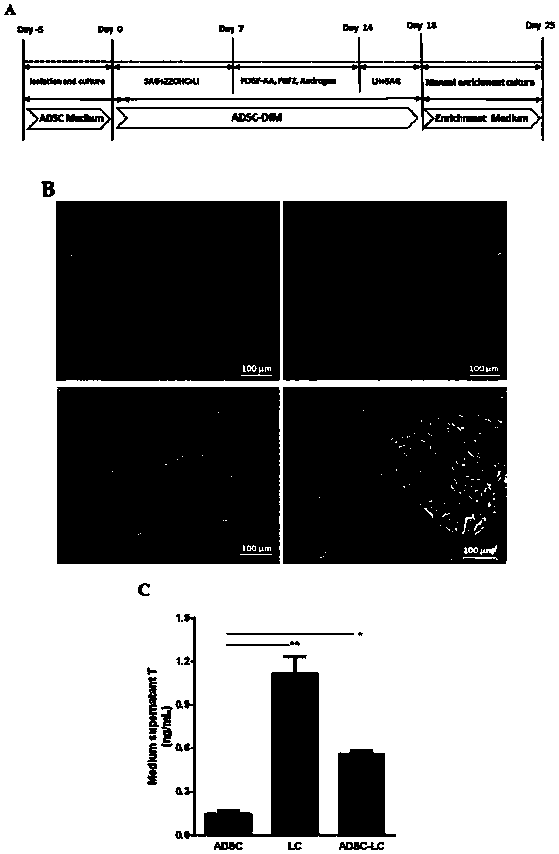
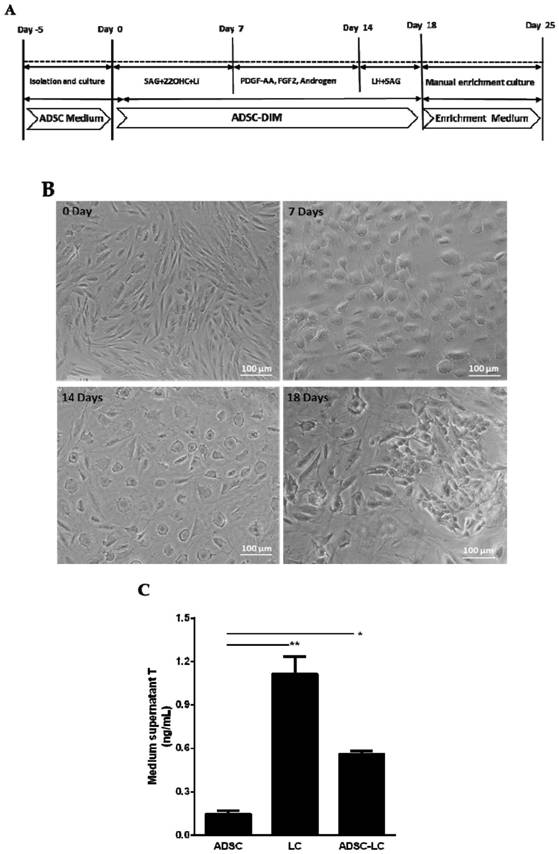
Method for inducing human ADSC (Adipose-Derived Stem Cells) to be differentiated into LCs (Leydig Cells) by micromolecules
ActiveCN108486039AImprove securityEasy to operateCulture processArtificial cell constructsAndrogenLeydig cell
The invention discloses a method for inducing human ADSC (Adipose-Derived Stem Cells) to be differentiated into LCs (Leydig Cells) by micromolecules. The method comprises the following steps: firstly,separating and extracting the human ADSC, and completing identification; then, inducing differentiation of the human ADSC by adopting a specific culture medium, and simultaneously adding a certain amount of micromolecules such as SAG, 22OHC, Li, PDGF-AA, FGF2, Androgen and LH in the special culture medium at different time points, thus promoting differentiation; finally, manually removing ALCs (Adult Leydig Cells), enriching and differentiating target cells ADSC-ALCs, and completing the identification. By inducing according to the method, the human ADSC can be successfully differentiated intothe LCs, so that a cell source is provided for treating testosterone deficiency by adopting cell transplantation.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Transdermal Delivery of Anastrozole for Systemic Effect
Formulations and methods for transdermal drug delivery compositions that include anastrozole are disclosed. Transdermal anastrozole compositions of the present disclosure may be indicated for treating testosterone deficiency. Disclosed transdermal anastrozole compositions may include permeation enhancers that may improve penetration of anastrozole in human skin. Permeation enhancers within transdermal anastrozole compositions may include oils from Amazon rainforest such as Pracaxi oil, Plukenetia volubilis seed oil, Inaja oil, and Patauá oil, which includes behenic and oleic fatty acids that may provide penetration power. Transdermal anastrozole may include organic solvents as transdermal penetration enhancers. Additionally, transdermal anastrozole compositions may include physiological lipids, phospholipids, and one or more butters rich in linoleic acid and linolenic acid that may also provide penetration power with restorative benefits to the skin.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Oral Transmucosal Compositions including Aromatase Inhibitors for Low Testosterone Levels in Men
InactiveUS20160022643A1Efficient skinEfficient tissue penetrationBiocideOintment deliveryOral mucous membraneImmediate release
Formulations for oral transmucosal compositions that include aromatase inhibitors (AIs) in combination with transmucosal absorption enhancers are disclosed. Disclosed oral transmucosal compositions may be for immediate release or slow release, and may be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Disclosed oral transmucosal compositions may include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms may include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms may be: mucoadhesive liquids such as gel-forming liquid; gel-forming; semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of AIs. Disclosed oral transmucosal compositions may allow the delivery of AIs directly into the patient's bloodstream, thus providing high bioavailability of AIs; therefore, required dose may be lower. Additionally, adjustments of AIs dosages may be achieved when using disclosed oral transmucosal compositions.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Testosterone Combined with Anastrozole Injection Solutions
ActiveUS20140371186A1Improve the level ofOrganic active ingredientsBiocideAnastrozoleIntramuscular injection
Compositions and methods for testosterone booster injection solutions (TBIS) that includes testosterone cypionate in synergistic combination with anastrozole are disclosed. Disclosed TBIS may be administered for treating testosterone deficiency. Disclosed TBIS is an intramuscular injection. The therapeutic dosage and protocol varies, according to the individual person. Different formulations may be designed to provide higher or lower testosterone doses.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Establishing method of monkey testosterone deficiency model
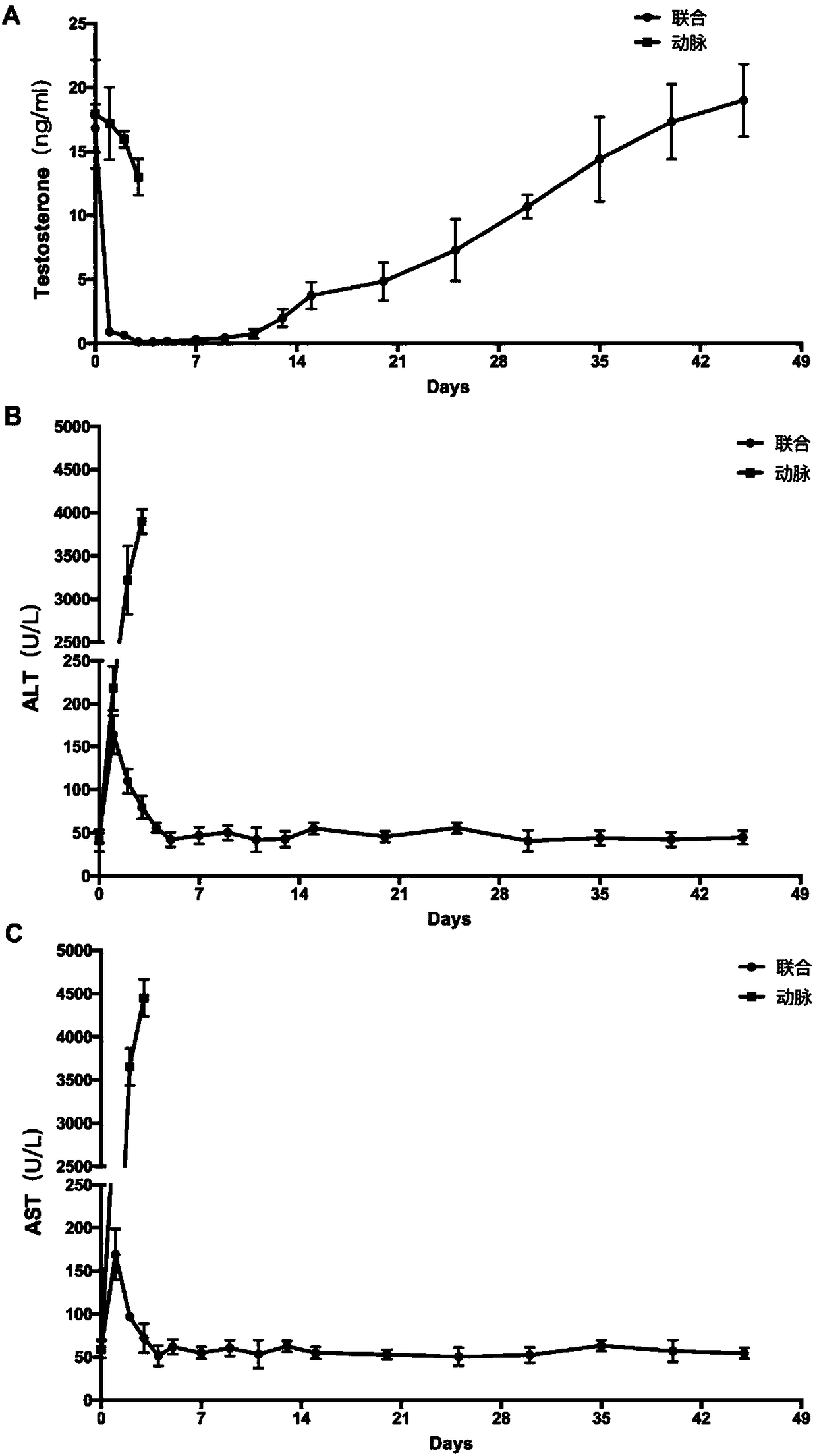
InactiveCN108524485AEfficient removalStable model carrierSulfur/selenium/tellurium active ingredientsAnimal husbandryPhysiologyTesticle
The invention provides an establishing method of a monkey testosterone deficiency model, which adopts a way of arterially injecting dimethyl sulfonate ethane to testicle of an adult monkey and blocking the spermatic cord blood flow. By adopting the method, a safe and stable testosterone deficiency animal model can be provided.
Owner:SUN YAT SEN UNIV
Oral pharmaceutical products and methods of use combining testosterone esters with hypolipidemic agents
Pharmaceutical products comprising a hypolipidemic agent and a testosterone ester such as testosterone undecanoate are provided. Methods of safely treating a testosterone deficiency or its symptoms with the inventive pharmaceutical products are also provided.
Owner:TOLMAR INC
Testosterone combined with anastrozole injection solutions
ActiveUS10201549B2Improve the level ofOrganic active ingredientsPharmaceutical delivery mechanismIntramuscular injectionAnastrozole
Compositions and methods for testosterone booster injection solutions (TBIS) that includes testosterone cypionate in synergistic combination with anastrozole are disclosed. Disclosed TBIS may be administered for treating testosterone deficiency. Disclosed TBIS is an intramuscular injection. The therapeutic dosage and protocol varies, according to the individual person. Different formulations may be designed to provide higher or lower testosterone doses.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Transdermal Pharmaceutical Compositions Including Testosterone and a C-SERM
InactiveUS20160051498A1Improve the level ofRelieve symptomsOrganic active ingredientsBiocideRegimenLotion
Formulations for transdermal pharmaceutical compositions including a synergistic combination of low doses of testosterone with a selective estrogen receptor modulator (C-SERM) that are combined with transdermal permeation enhancers are disclosed. Transdermal pharmaceutical compositions can be designed with various release rates, and can be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Transdermal pharmaceutical compositions include liquid dosage forms, such as, for example solutions, liquid sprays, lotions, and the like. Additionally, transdermal pharmaceutical compositions include semi-solid dosage forms, such as, for example emulsions, creams, gels, pastes, ointments, and the like. Transdermal pharmaceutical compositions will deliver testosterone and C-SERM through the skin and directly into the patient's bloodstream, thereby providing high bioavailability of testosterone and C-SERM. The dosage regimen of the transdermal pharmaceutical compositions can be easily tailored for individual patients according to the baseline blood levels of testosterone and estradiol.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Controlled release topical testosterone formulations and methods
InactiveUS20160113946A1Constant effective testosterone blood levelsEasy to useOrganic active ingredientsInorganic non-active ingredientsDiseaseFemale Sexual Arousal Disorder
The present invention relates to testosterone topical formulations, especially high testosterone concentration formulations, such as between about 6% to about 15% w / w or higher, for the controlled release of testosterone into the systemic circulation of males and females for providing constant effective testosterone blood levels, without inducing undesired testosterone spike in blood levels or testosterone transference, following topical administration. The testosterone topical formulations of the present invention are safe, convenient to use, well tolerated, stable and easily and reproducibly manufactured on scale up. Moreover, because supra-normal and sub-normal testosterone blood levels are believed to be essentially kept to a minimum or avoided and the testosterone serum levels are believed to remain essentially constant during dose life, i.e., the testosterone topical formulation of the present invention mimic or restore testosterone blood levels to normal physiologic daily rhythmic testosterone levels, the novel testosterone topical formulation of the present invention are uniquely suited for testosterone replacement or supplemental therapy and effective for treating males diagnosed with, for example, male testosterone deficiency, such as, low sexual libido, low sexual drive, low sexual activity, low fertility, low spermatogenesis, aspermatogenesis, depression and / or hypogonadism, and females who are diagnosed with, for example, female sexual dysfunction, such as, low sexual libido, low sexual drive, low sexual activity, low amygdala reactivity, low sexual stimulation, hypoactive sexual desire disease (“HSDD”), female sexual arousal disorder and / or anorgasmia. The present invention also relates to methods and pre-filled multi-dose airless applicator systems for pernasal administration of the nasal testosterone gels of the present invention.
Owner:ACERUS BIOPHARMA INC
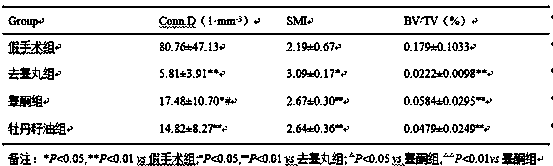
Application of peony seed oil in preparation of drugs and health food for improving male bone biomechanical properties
With the increase of age, the testosterone level of middle-aged and elderly men gradually decreases, the bone biomechanical properties decrease, and fracture and osteoporosis are easy to occur. Testosterone deficiency is believed to be one of the main causes of osteoporosis in men. Our research found that peony seed oil has the same effect as testosterone, has a certain antagonistic effect on thedecrease of bone biomechanical properties and osteoporosis caused by testis-removed mice. The peony seed oil provided by the invention can be made into soft capsules, hard capsules, tablets, granules,oral liquids and beverages that can be used for clinical application, and is used for preventing and treating male osteoporosis patients and people whose bone biomechanical properties are reduced dueto various reasons. The invention also discloses formulas and preparation technologies of peony seed oil soft capsules, peony seed oil vitamin D calcium soft capsules, peony seed oil estrogen soft capsules and peony seed oil aspirin soft capsules, which have good effects of preventing and treating low bone biomechanical function and osteoporosis of various populations.
Owner:GUANGDONG MEDICAL UNIV +1
Oral transmucosal compositions including aromatase inhibitors for low testosterone levels in men
InactiveUS20170209366A1Effective penetrationEfficient skinOrganic active ingredientsOintment deliveryOral mucous membraneImmediate release
Formulations for oral transmucosal compositions that include aromatase inhibitors (AIs) in combination with transmucosal absorption enhancers are disclosed. Disclosed oral transmucosal compositions may be for immediate release or slow release, and may be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Disclosed oral transmucosal compositions may include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms may include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms may be: mucoadhesive liquids such as gel-forming liquid; gel-forming; semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of AIs. Disclosed oral transmucosal compositions may allow the delivery of AIs directly into the patient's bloodstream, thus providing high bioavailability of AIs; therefore, required dose may be lower. Additionally, adjustments of AIs dosages may be achieved when using disclosed oral transmucosal compositions.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Stable pharmaceutical composition comprising testosterone undecanoate
PendingCN112638365AImprove ease of useImprove stabilityOrganic active ingredientsInorganic non-active ingredientsTestosterone deficiencyInternal medicine
The present invention relates to an injectable composition of testosterone ester for the treatment of testosterone deficiency. Particularly, the present invention relates to a pharmaceutical composition comprising testosterone undecanoate, which is capable of increasing the convenience for use in injection with the low viscosity and injection force, and has improved stability.
Owner:CHONG KUN DONG CORP
A method for inducing human adipose stem cells to differentiate into Leydig cells with small molecules
ActiveCN108486039BImprove securityEasy to operateCulture processArtificial cell constructsMedicineLeydig cell
The invention discloses a method for fully utilizing small molecules to induce human fat stem cells to differentiate into Leydig cells (LCs). The steps of the method are as follows. First, human adipose stem cells are isolated and identified; then, a specific medium is used to induce the differentiation of human adipose stem cells, and a certain amount of small molecules are added to the medium at different time points, including: SAG, 22OHC, Li, PDGF‑ AA, FGF2, Androgen, and LH promote differentiation; finally, the non-testis Leydig cells (ALCs)-like cells are manually removed to enrich the differentiation target cells (ADSC‑ALCs), and the identification is completed. Induced according to the method of the present invention, human adipose stem cells can be successfully differentiated into Leydig cells of the testis, thereby providing a cell source for the treatment of testosterone deficiency by cell transplantation.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Oral Transmucosal Compositions including C-SERMs for Low Testosterone Levels in Men
InactiveUS20160051495A1High percentage of bioavailability of C-SERMsEffective penetrationBiocideOrganic active ingredientsFast releaseBioavailability
Formulations for oral transmucosal compositions that include clomiphene-like selective estrogen receptor modulators (C-SERMs) in combination with transmucosal absorption enhancers are disclosed. Oral transmucosal compositions can be for fast release or slow release, and can be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Oral transmucosal compositions include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms are: mucoadhesive liquids such as gel-forming liquid; gel-forming; semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of C-SERMs. Oral transmucosal compositions allow the delivery of C-SERMs directly into the patient's bloodstream, thereby providing high bioavailability of C-SERMs; therefore, required dose is lower.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Controlled release nasal testosterone gel, method for nasal administration, and pre-filled multi-dose applicator system
The present invention relates to a controlled release nasal testosterone gel, a method for nasal administration, and a pre-filled multi-dose applicator system. Specifically, the present invention relates to an intranasal testosterone gel for controlled release of testosterone into the systemic circulatory system of males and females, thereby providing a constant effective testosterone blood levelafter nasal administration without causing an undesirable peak in testosterone blood level. The intranasal testosterone gel of the present invention is safe, convenient to use, good in tolerance, stable, and easy and reproducible for large-scale production. The novel intranasal testosterone gel of the present invention is uniquely suitable for testosterone replacement or supplemental treatment andis effective in treating males diagnosed with, for example, male testosterone deficiency and females diagnosed with, for example, female sexual dysfunction. The invention also relates to a method fornasal administration of the nasal testosterone gel of the invention and a pre-filled multi-dose airless applicator system.
Owner:ACERUS BIOPHARMA INC
Oral transmucosal pharmaceutical compositions including testosterone and an aromatase inhibitor
ActiveUS10213440B2Improve the level ofRelieve symptomsOrganic active ingredientsPill deliveryMucoadhesionChewing gum
Formulations for oral transmucosal compositions including a synergistic combination of low doses of testosterone with an aromatase inhibitor (AI) that are combined with transmucosal absorption enhancers are disclosed. Oral transmucosal compositions can be for fast release or slow release, and can be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Oral transmucosal compositions include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms are: mucoadhesive liquids such as gel-forming liquids; gel-forming semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of testosterone and AI. Oral transmucosal compositions will deliver testosterone and AI directly into the patient's bloodstream, and provide high bioavailability of testosterone and AI; therefore, the required doses are lower.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Transdermal pharmaceutical compositions including C-SERMs for low testosterone levels in men
ActiveUS9814687B2Effective penetrationLower API dosageOrganic active ingredientsAerosol deliveryRegimenMale infertility
Formulations for transdermal pharmaceutical compositions including clomiphene-like selective estrogen receptor modulators (C-SERMs) in combination with transdermal penetration enhancers are disclosed. Transdermal pharmaceutical compositions can be designed with various release rates, and are administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency in male hypogonadism or male infertility. Transdermal pharmaceutical compositions include a range of dosage forms, such as, for example solutions, liquid sprays, lotions, emulsions, creams, pastes, and ointments, among other dosage forms that exhibit transdermal properties, and provide transdermal delivery of C-SERMs. Transdermal pharmaceutical compositions will deliver C-SERMs through the skin and directly into the patient's bloodstream, thereby providing high bioavailability of C-SERMs. The dosage regimen of the transdermal pharmaceutical compositions can be easily tailored for individual patients according to the baseline blood levels of testosterone and estradiol.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Testosterone solutions for the treatment of testosterone deficiency
ActiveUS9498436B2Lower testosterone levelsImprove the level ofOrganic active ingredientsNervous disorderSerum testosterone levelTestosterone deficiency
Solutions of testosterone for oromucosal administration providing an increase in serum testosterone levels in subjects deficient in endogenous testosterone levels, and therapeutic methods for providing an increase in serum testosterone levels and methods for treating a disease or a symptom associated with deficient endogenous levels of testosterone.
Owner:INNOTESTO BVBA
Oral transmucosal pharmaceutical compositions including testosterone and a C-SERM
ActiveUS9452174B2Improve the level ofRelieve symptomsBiocideOrganic active ingredientsMucoadhesionChewing gum
Formulations for oral transmucosal compositions including a synergistic combination of low doses of testosterone with a clomiphene-like selective estrogen receptor modulator (C-SERM) that are combined with transmucosal absorption enhancers are disclosed. Oral transmucosal compositions can be for fast release or slow release, and can be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Oral transmucosal compositions include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms are: mucoadhesive liquids such as gel-forming liquids; gel-forming semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of testosterone and C-SERM. Oral transmucosal compositions will deliver testosterone and C-SERM directly into the patient's bloodstream, and provide high bioavailability of testosterone and C-SERM; therefore, the required doses are lower.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com