Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
276results about How to "Maintain level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
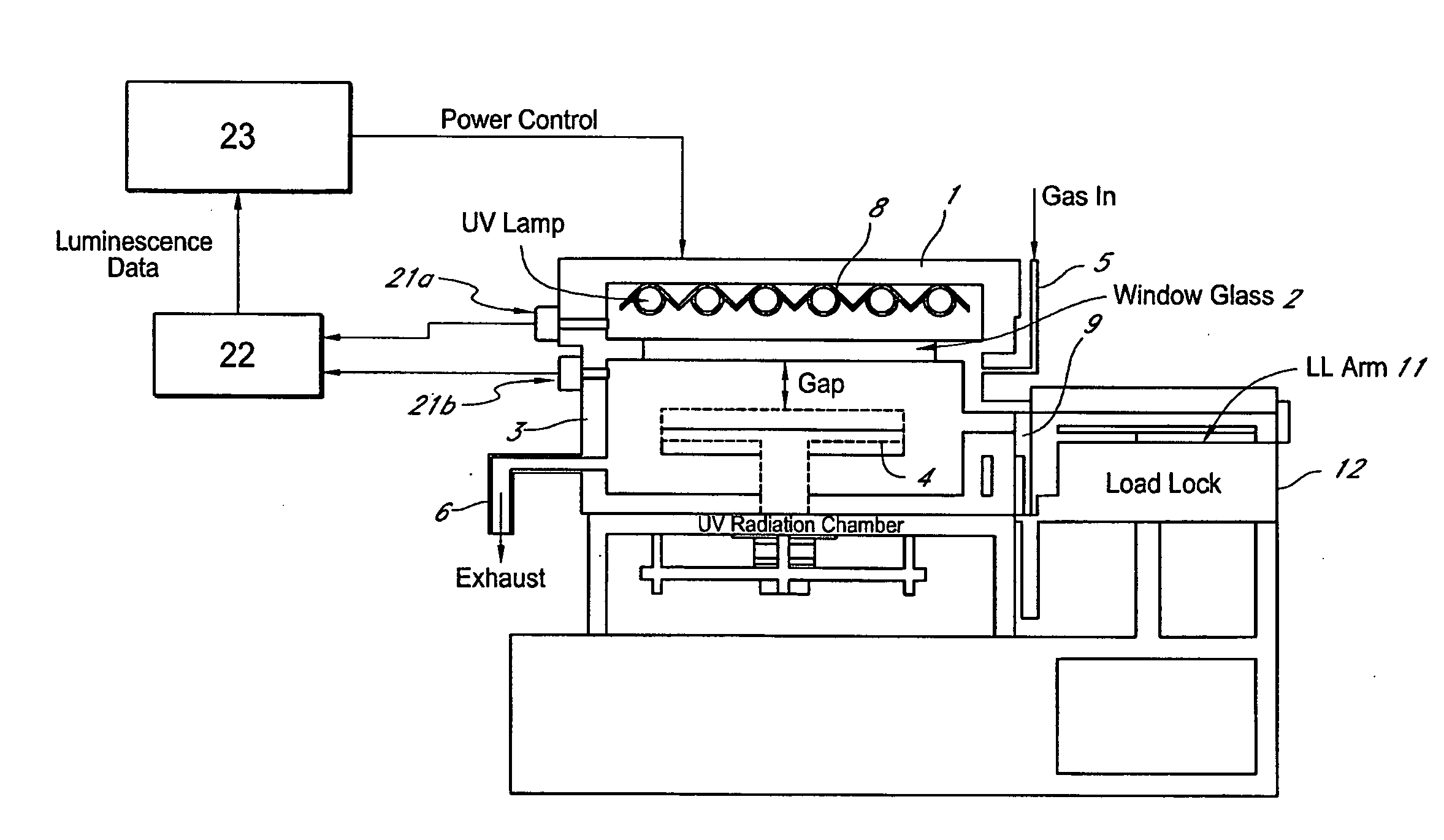
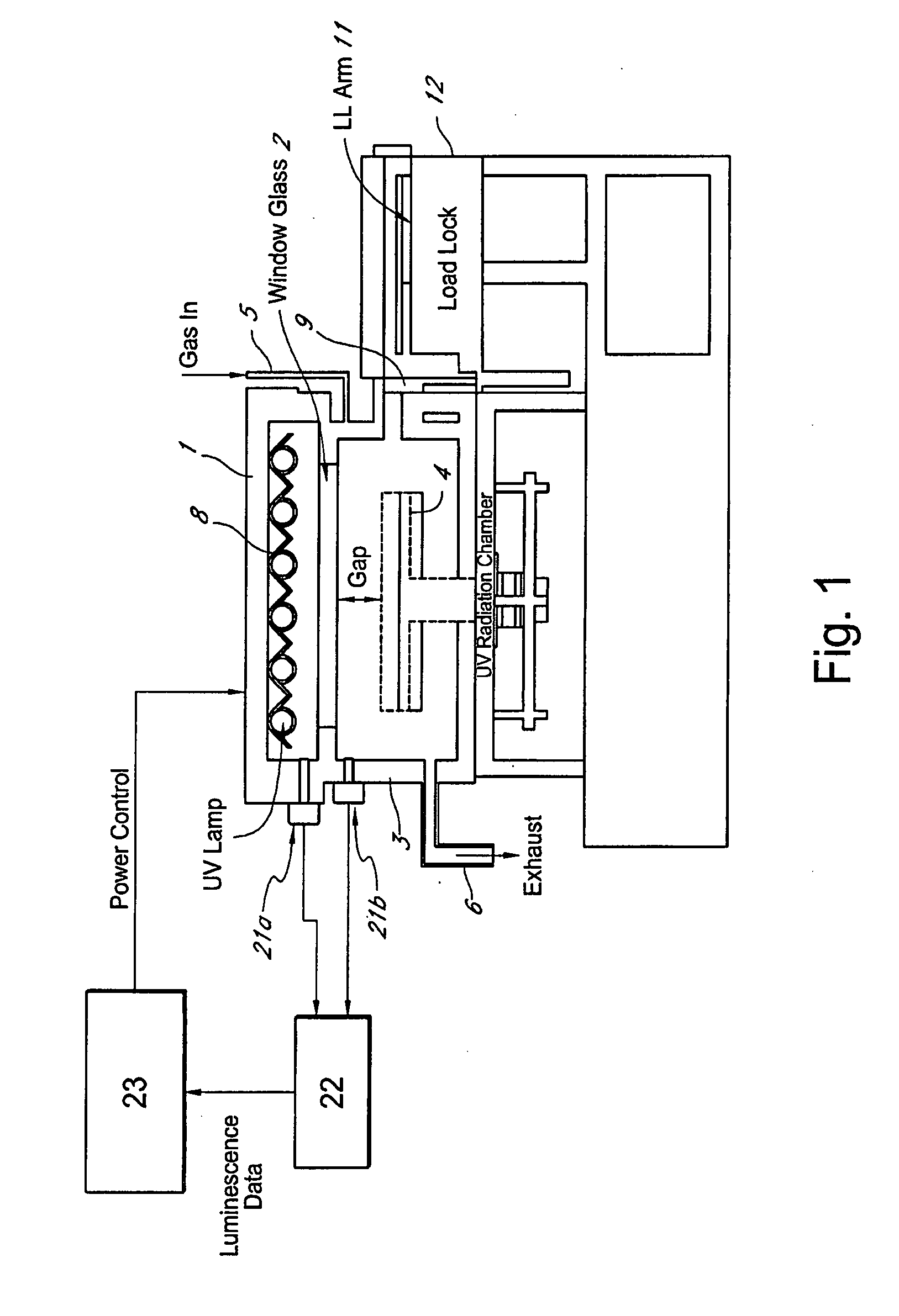
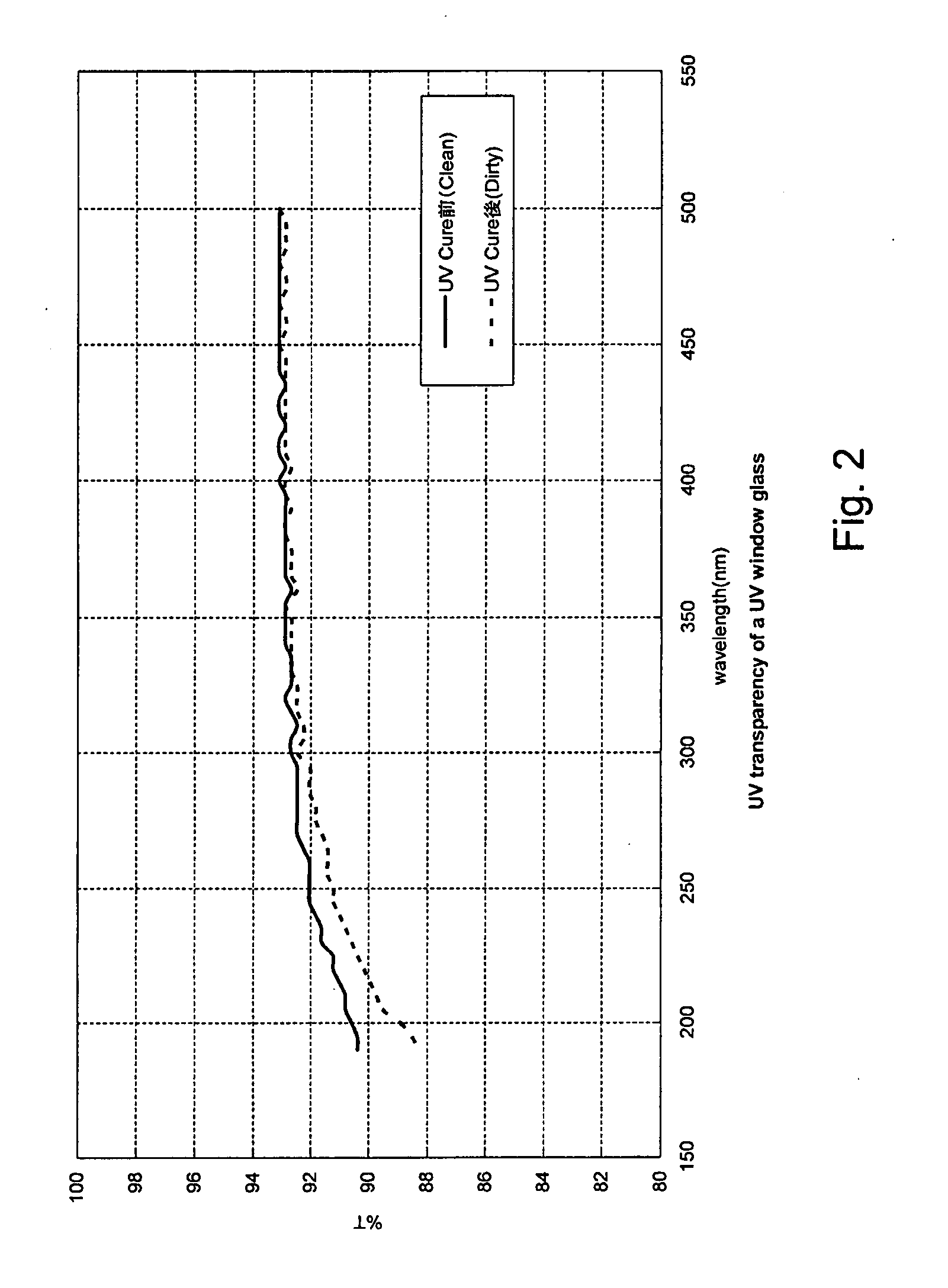
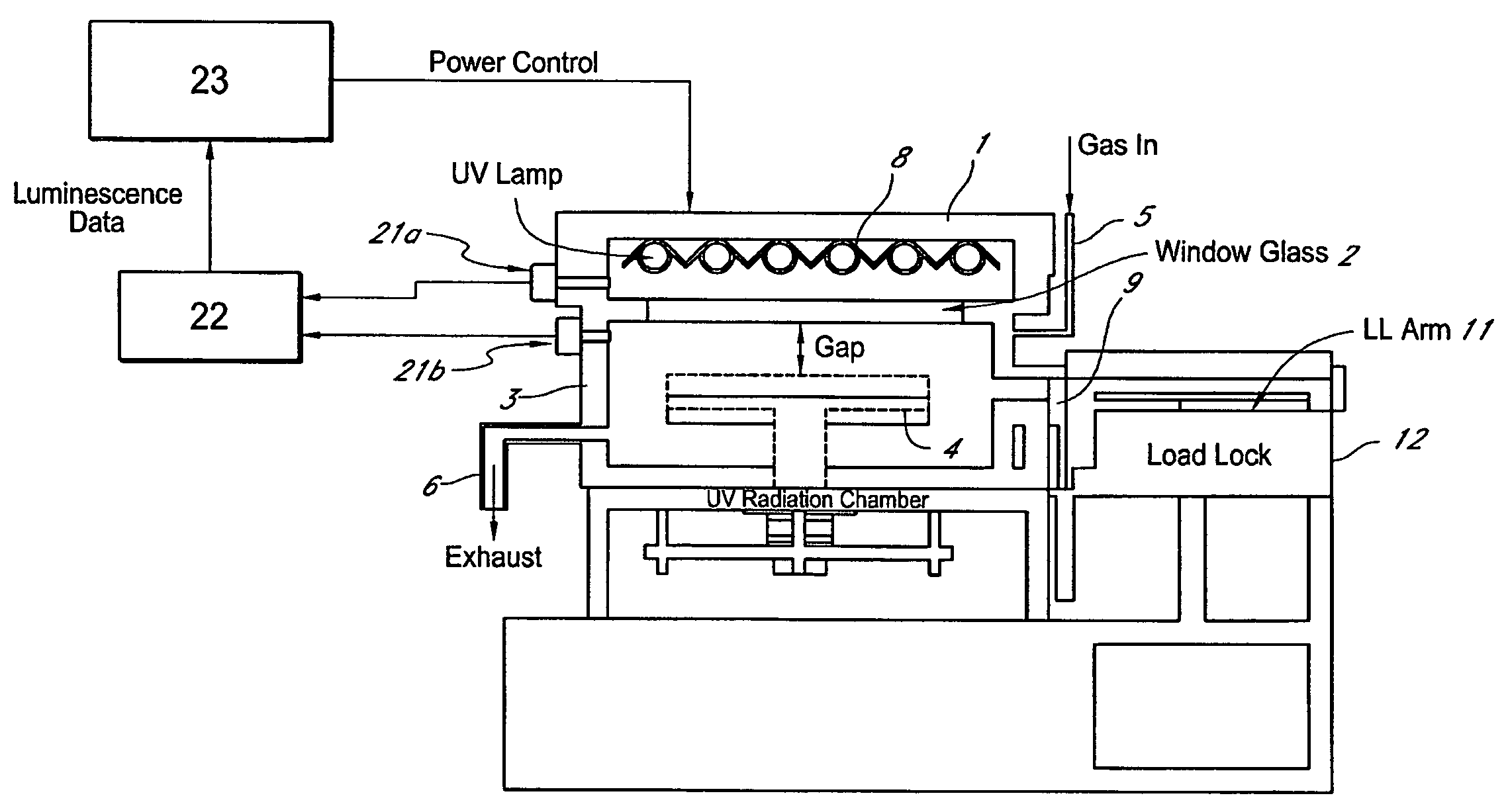
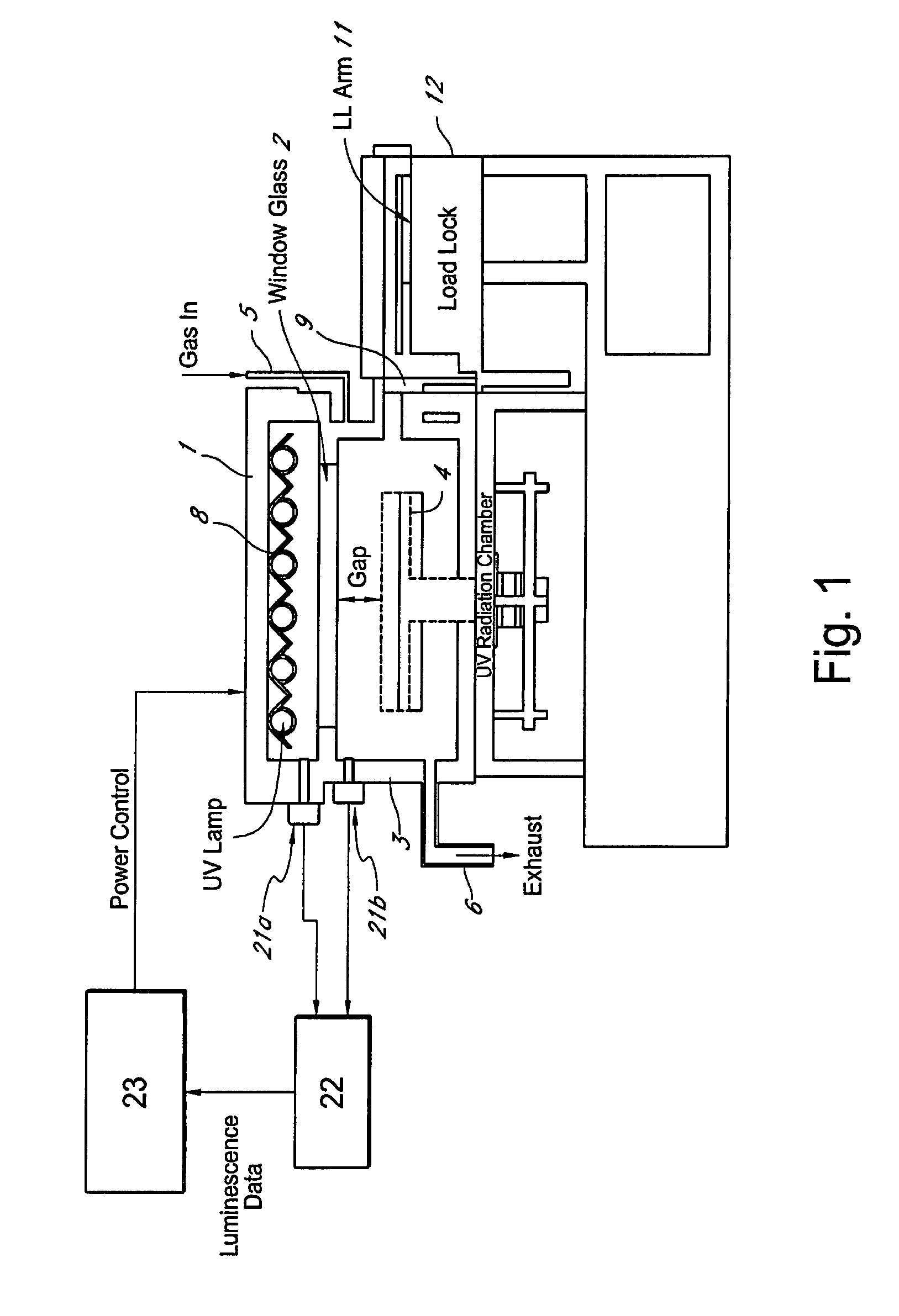
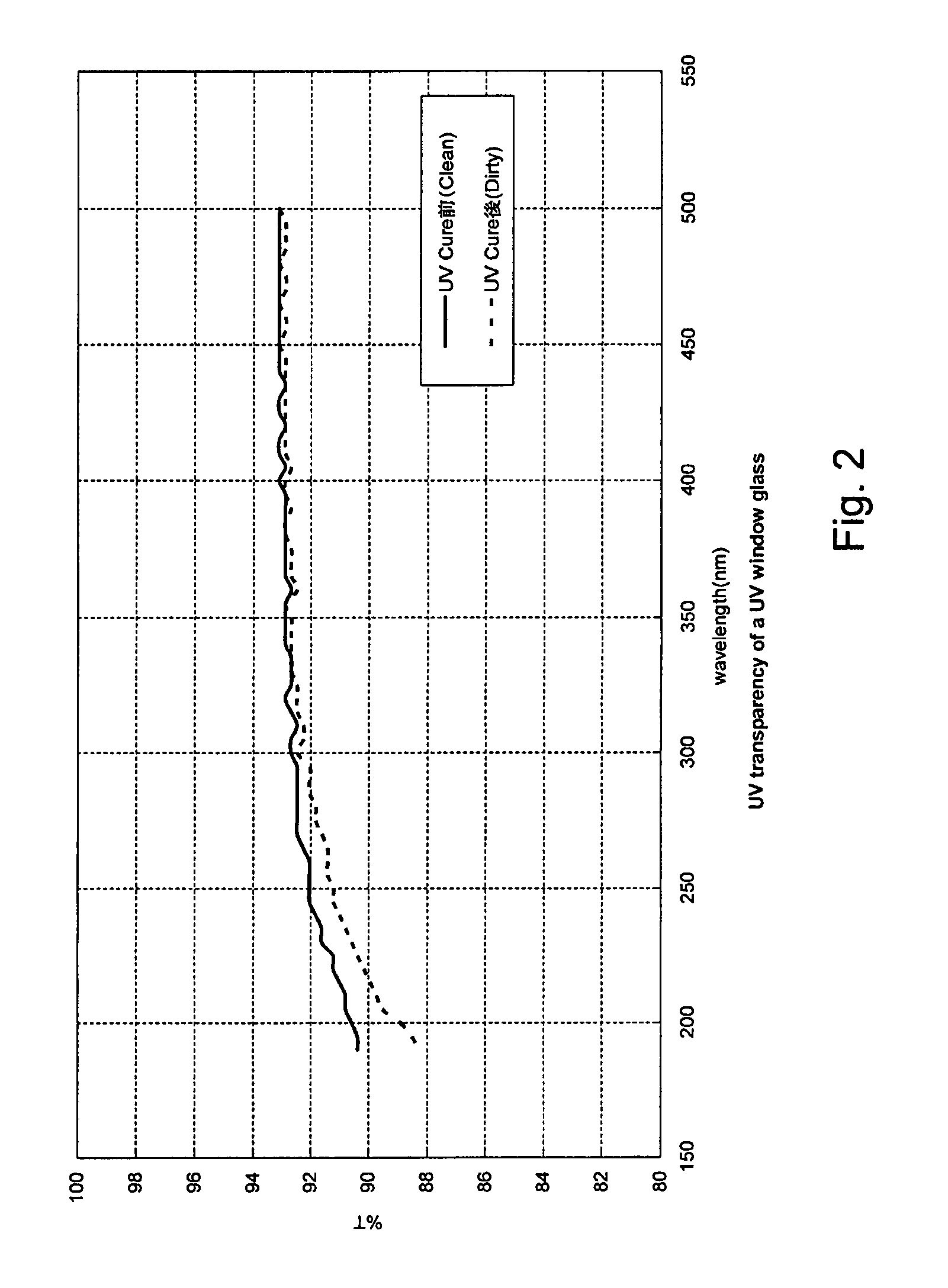
Method for managing UV irradiation for curing semiconductor substrate
ActiveUS20090023229A1Automatic controlMaintain levelSemiconductor/solid-state device testing/measurementSemiconductor/solid-state device manufacturingIlluminanceEngineering
A method for managing UV irradiation for curing a semiconductor substrate, includes: passing UV light through a transmission glass window provided in a chamber for curing a semiconductor substrate placed in the chamber; monitoring an illuminance upstream of the transmission glass window and an illuminance downstream of the transmission glass window; determining a timing and / or duration of cleaning of the transmission glass window, a timing of replacing the transmission glass window, a timing of replacing a UV lamp, and / or an output of the UV light based on the monitored illuminances.
Owner:ASM JAPAN
Method for managing UV irradiation for curing semiconductor substrate
ActiveUS7501292B2Maintain levelAutomatic controlSemiconductor/solid-state device testing/measurementSemiconductor/solid-state device manufacturingIlluminanceEngineering
A method for managing UV irradiation for curing a semiconductor substrate, includes: passing UV light through a transmission glass window provided in a chamber for curing a semiconductor substrate placed in the chamber; monitoring an illuminance upstream of the transmission glass window and an illuminance downstream of the transmission glass window; determining a timing and / or duration of cleaning of the transmission glass window, a timing of replacing the transmission glass window, a timing of replacing a UV lamp, and / or an output of the UV light based on the monitored illuminances.
Owner:ASM JAPAN
Computer resource distribution method based on prediciton
InactiveUS20090113056A1Reduce in quantityReduce maintenance costsResource allocationDigital computer detailsComputer resourcesDistribution method
Owner:HITACHI LTD
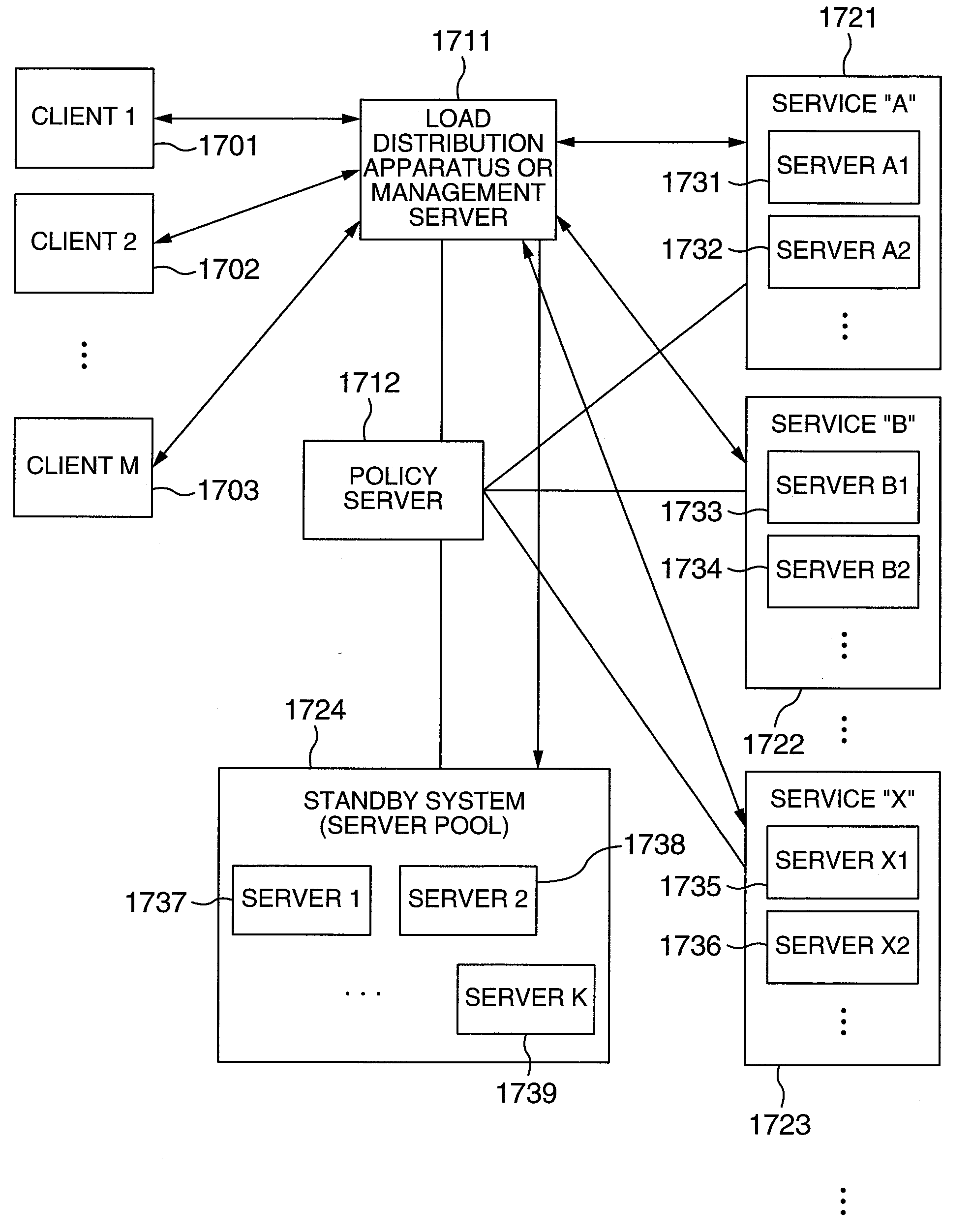
Provisioning system, method, and program
ActiveUS20100058342A1Addressing slow performanceEasy to useResource allocationSoftware simulation/interpretation/emulationResource allocationComputer science
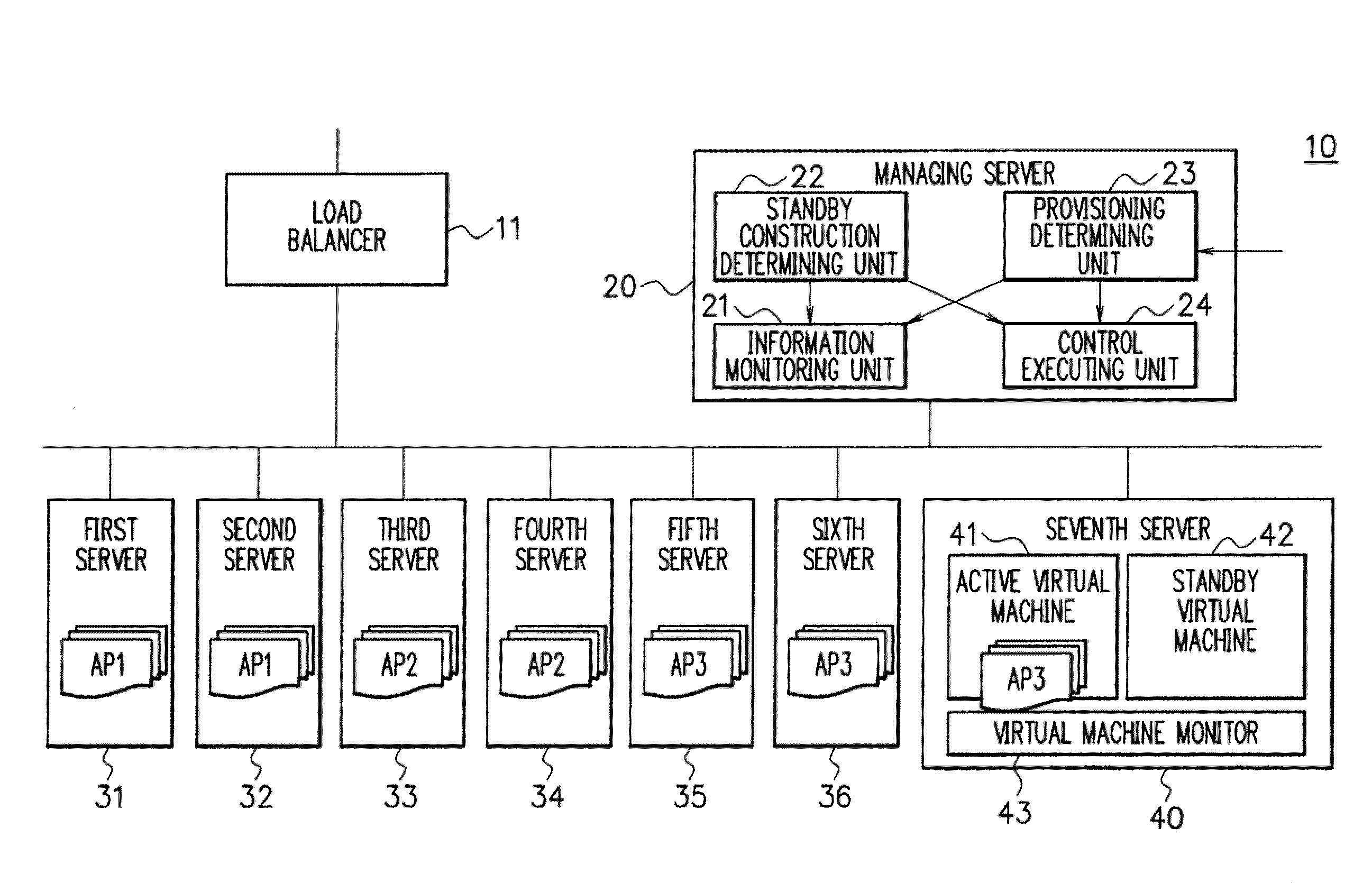
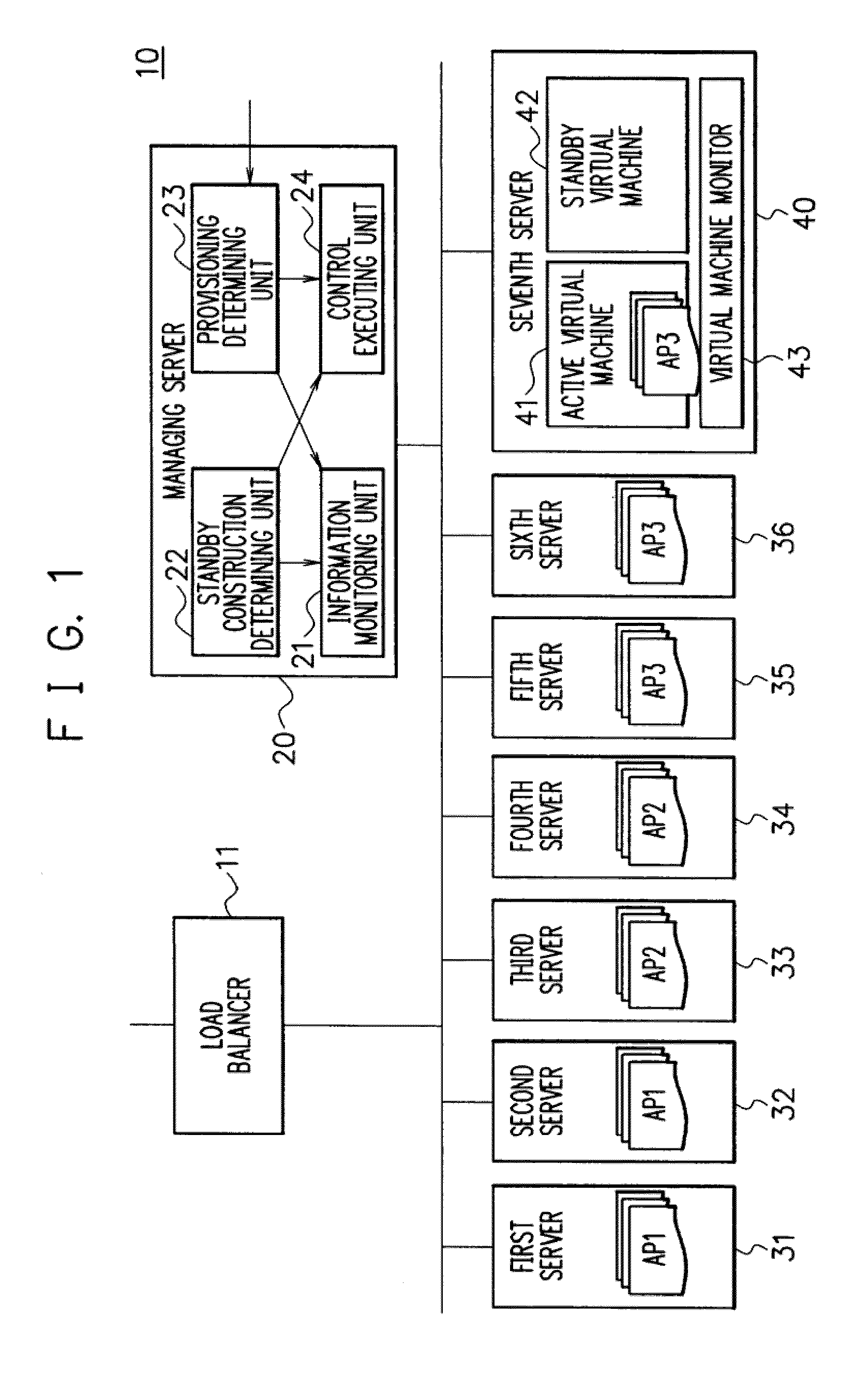
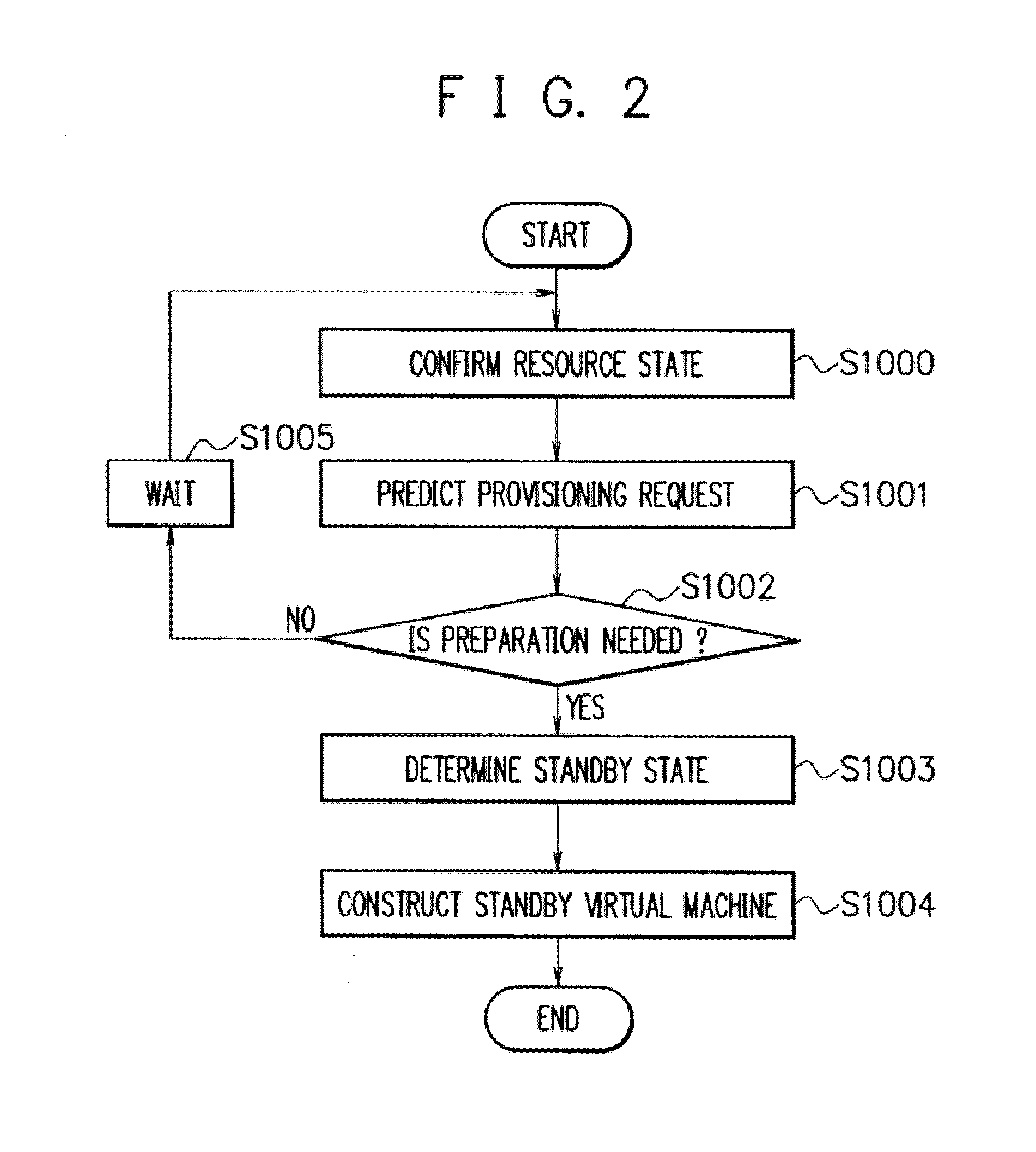
A shared server 40 includes an active virtual machine 41 where a sufficient amount of resources are allocated to an operation of an application system and a standby virtual machine 42 that starts with a minimum amount of resources. When it is predicted that a provisioning request is generated, a standby construction determining unit 22 previously executes provisioning on a standby virtual machine 42, and performs a start of an OS and an application or a setting change of a network apparatus. A provisioning determining unit 23 changes the resource allocation amounts of the active virtual machine 41 and the standby virtual machine 42, allocates a sufficient amount of resources to the standby virtual machine 42, registers the standby virtual machine 42 as a target of load balancing in a load balancer 11, and executes provisioning.
Owner:NEC CORP
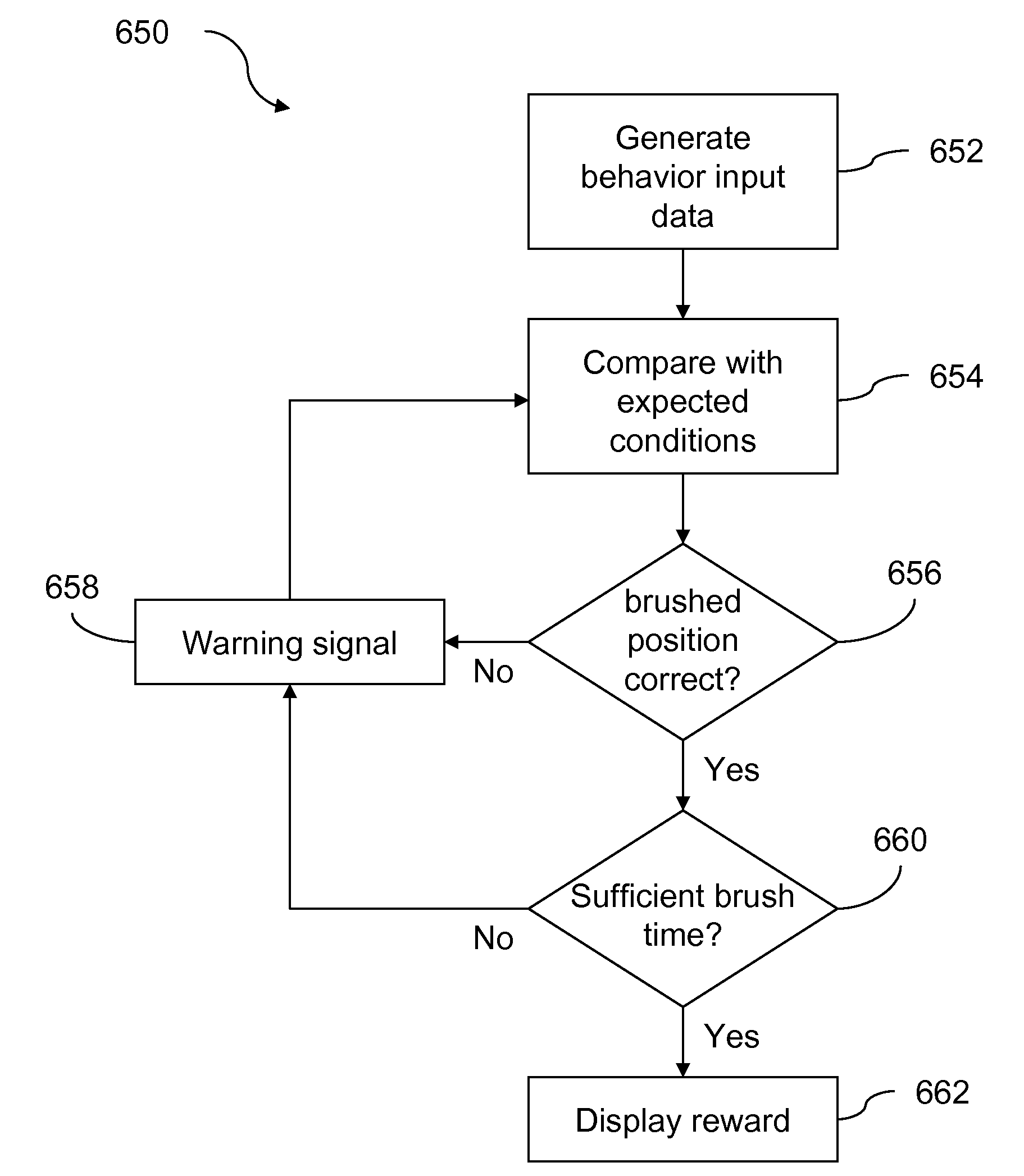
Method and Apparatus for Developing a Proper Tooth Brushing Technique
One embodiment of the present invention includes a signaling device capable of assisting a child to develop a tooth brushing technique. The signaling device includes a toothbrush and an extension device, which is affixed to the tooth brush. The extension device is coded with a plurality of patterns that can be converted into motion and orientation information associated with the tooth brush in a tooth brushing session, and the motion and orientation information is further compared to a predetermined set of expected conditions that correspond to the tooth brushing technique to determine a reward tailored to promote learning of the tooth brushing technique.
Owner:RAINDROP NETWORK
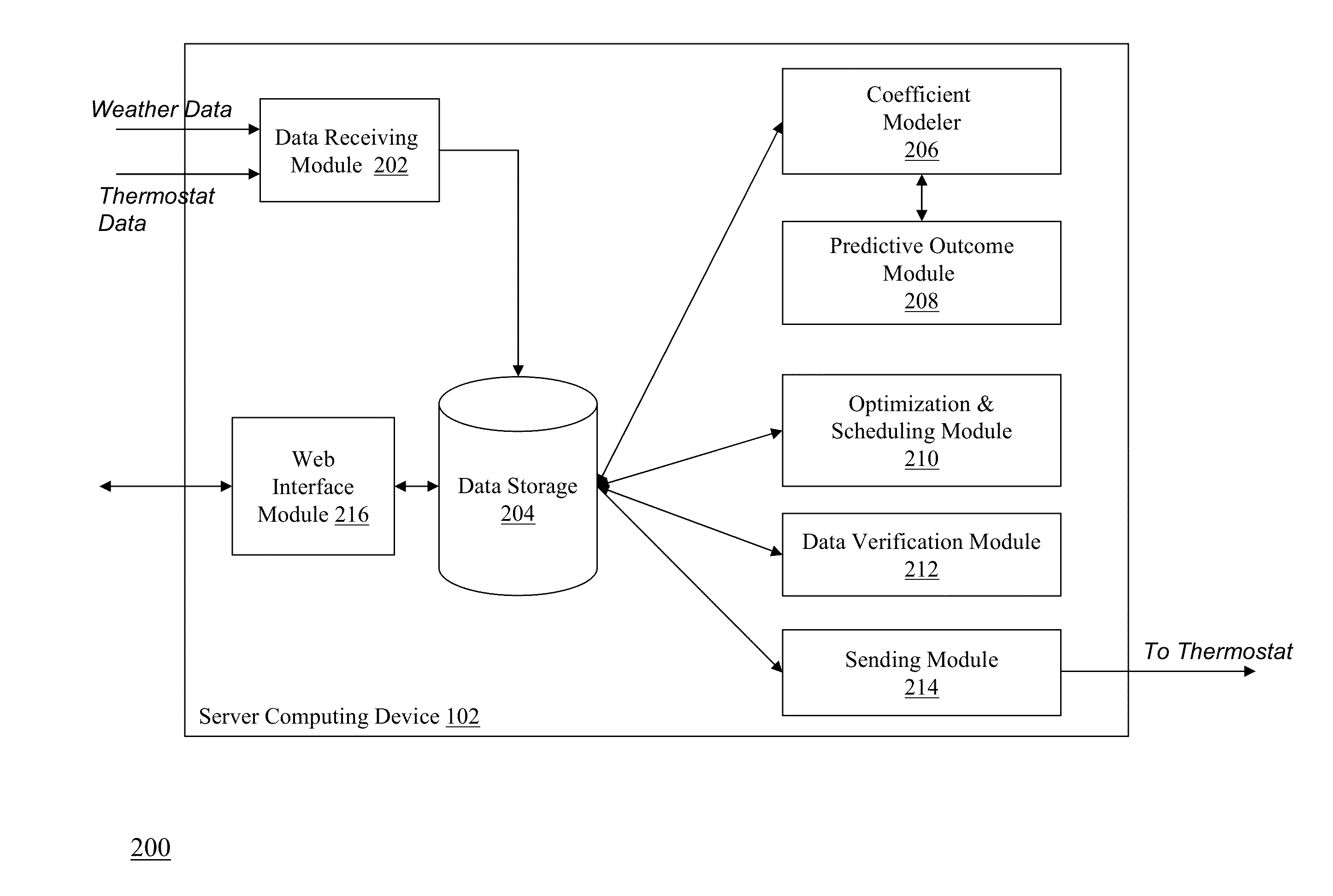
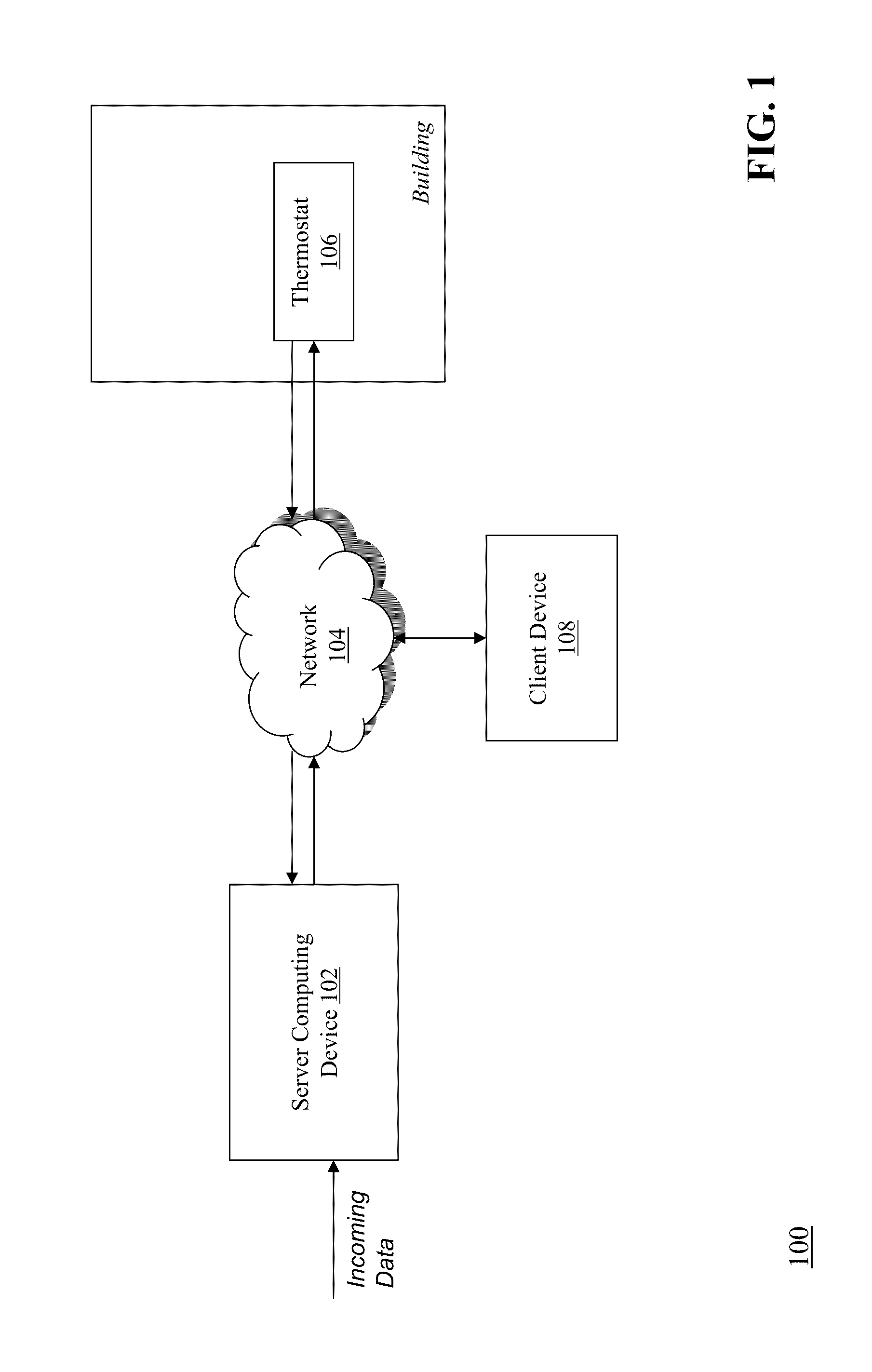
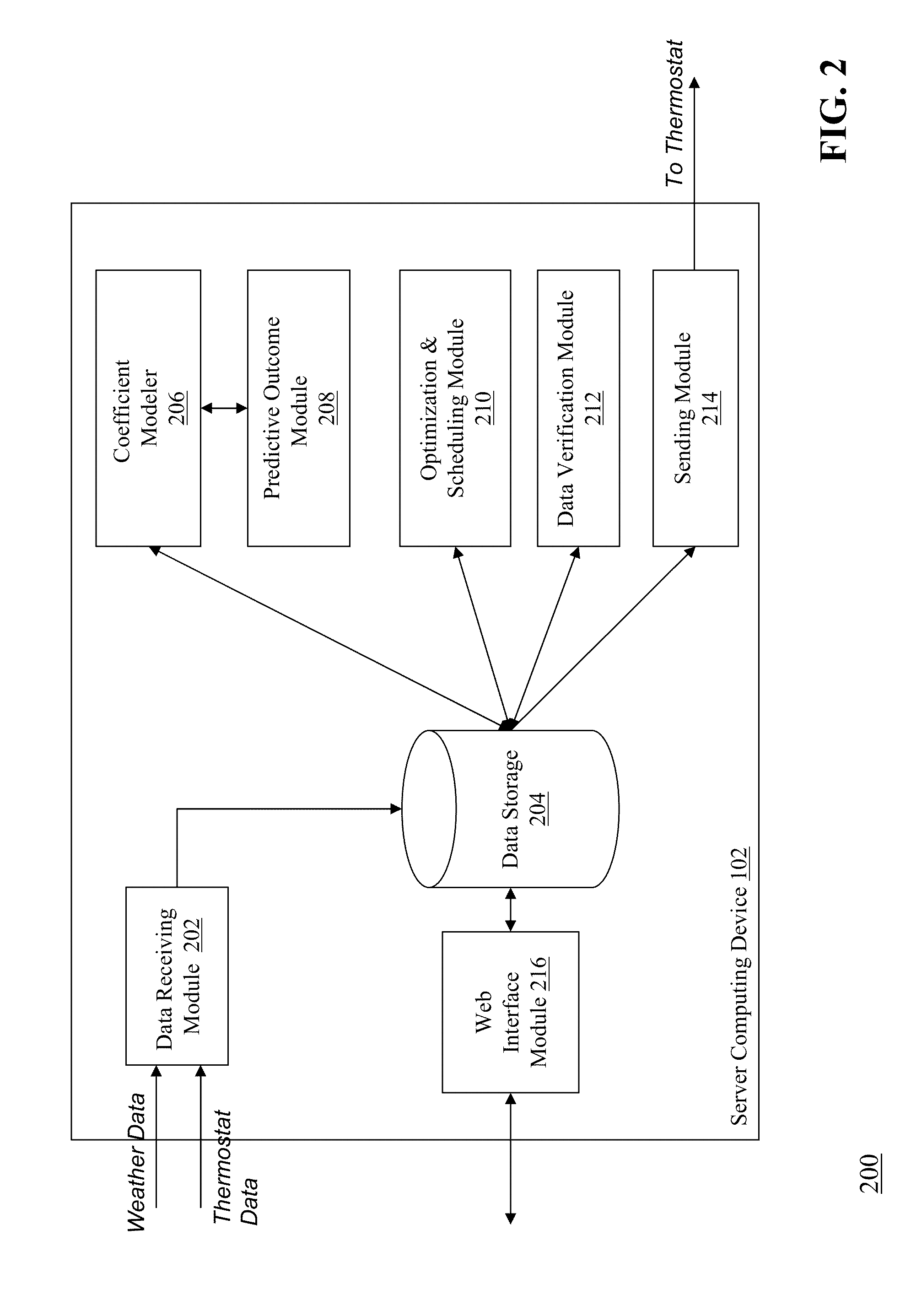
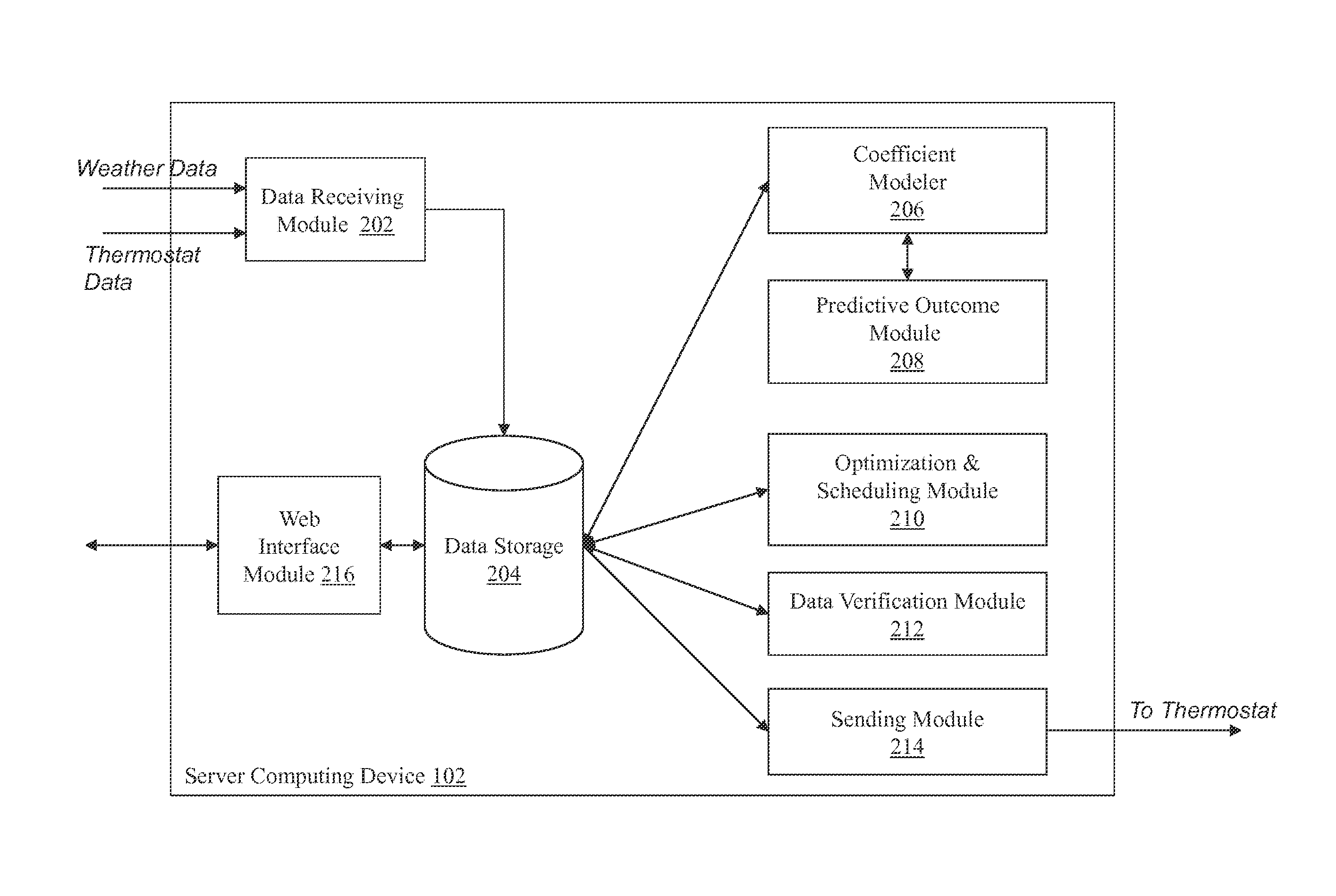
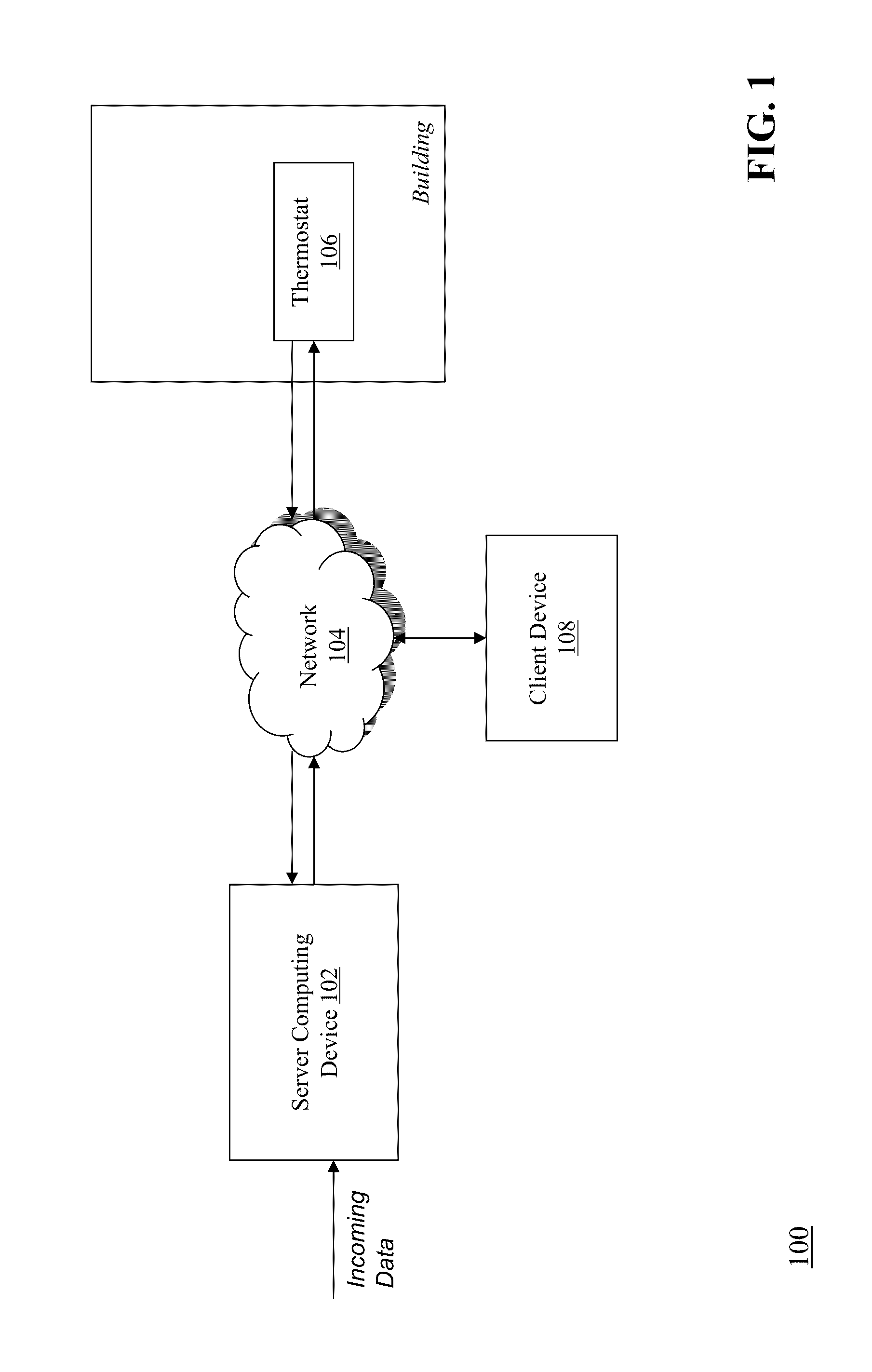
Optimizing and controlling the energy consumption of a building
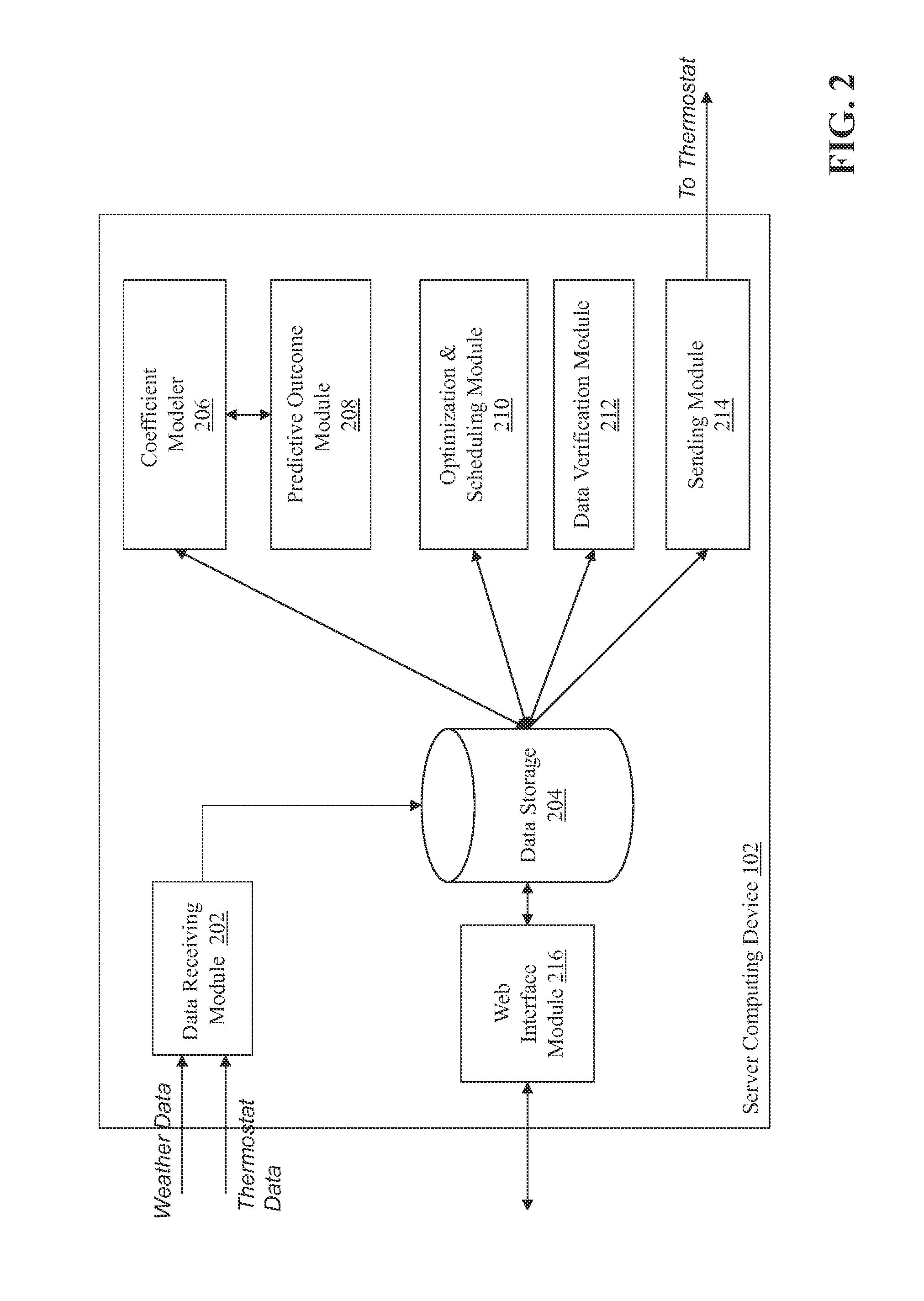
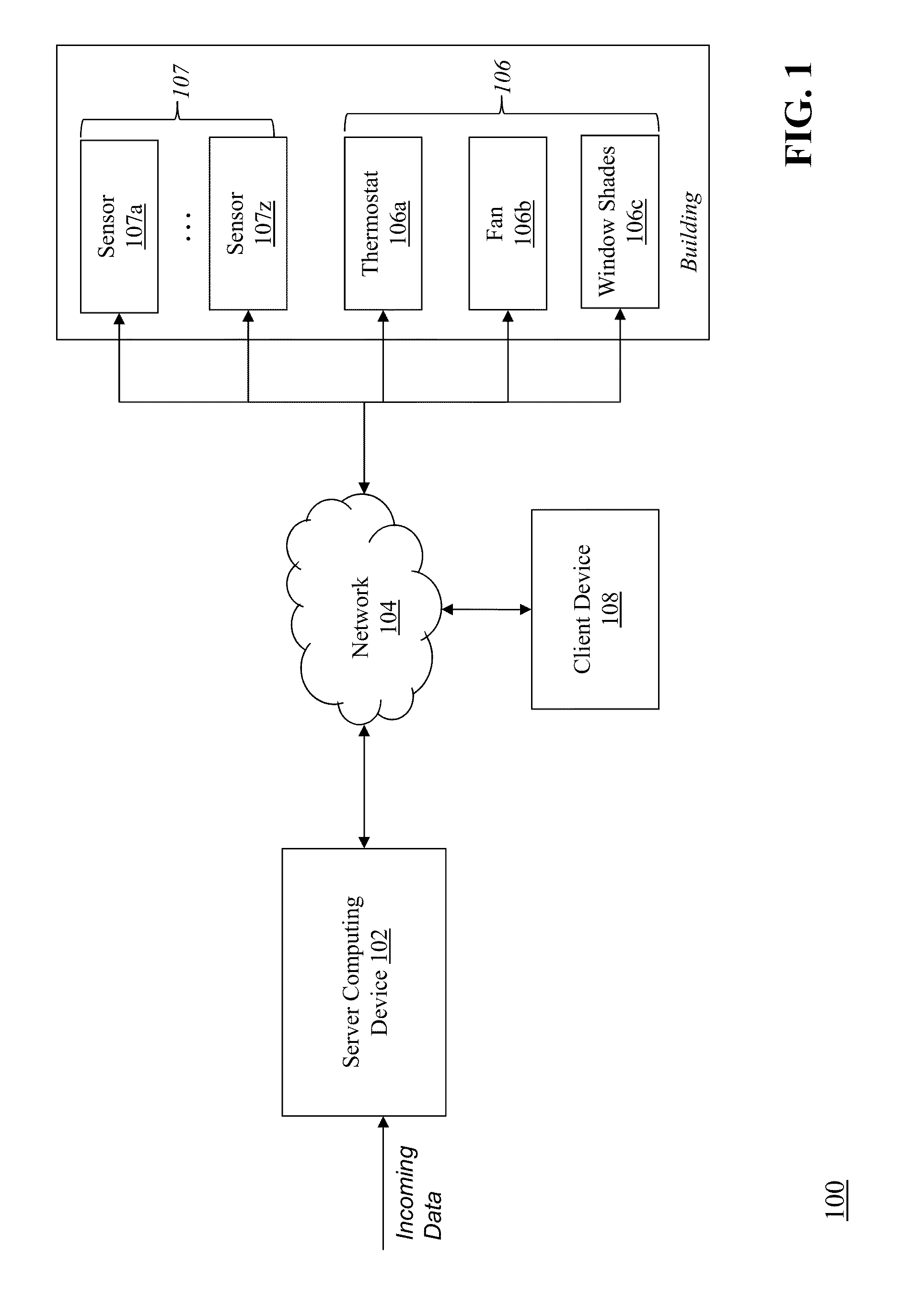
ActiveUS20130190940A1Optimize energy useLevel of comfortProgramme controlMechanical power/torque controlEnergy basedEngineering
Described herein are methods and systems, including computer program products, for optimizing and controlling the energy consumption of a building. A first computing device generates a set of thermal response coefficients for the building based on energy characteristics of the building and weather data associated with the location of the building. The first computing device predicts an energy response of the building based on the set of thermal response coefficients and forecasted weather associated with the location of the building. The first computing device selects minimal energy requirements of the building based on an energy consumption cost associated with the building. The first computing device determines one or more temperature set points for the building based on the energy response and the minimal energy requirements. The first computing device transmits the one or more temperature set points to a thermostat of the building.
Owner:UNIV OF MARYLAND +1
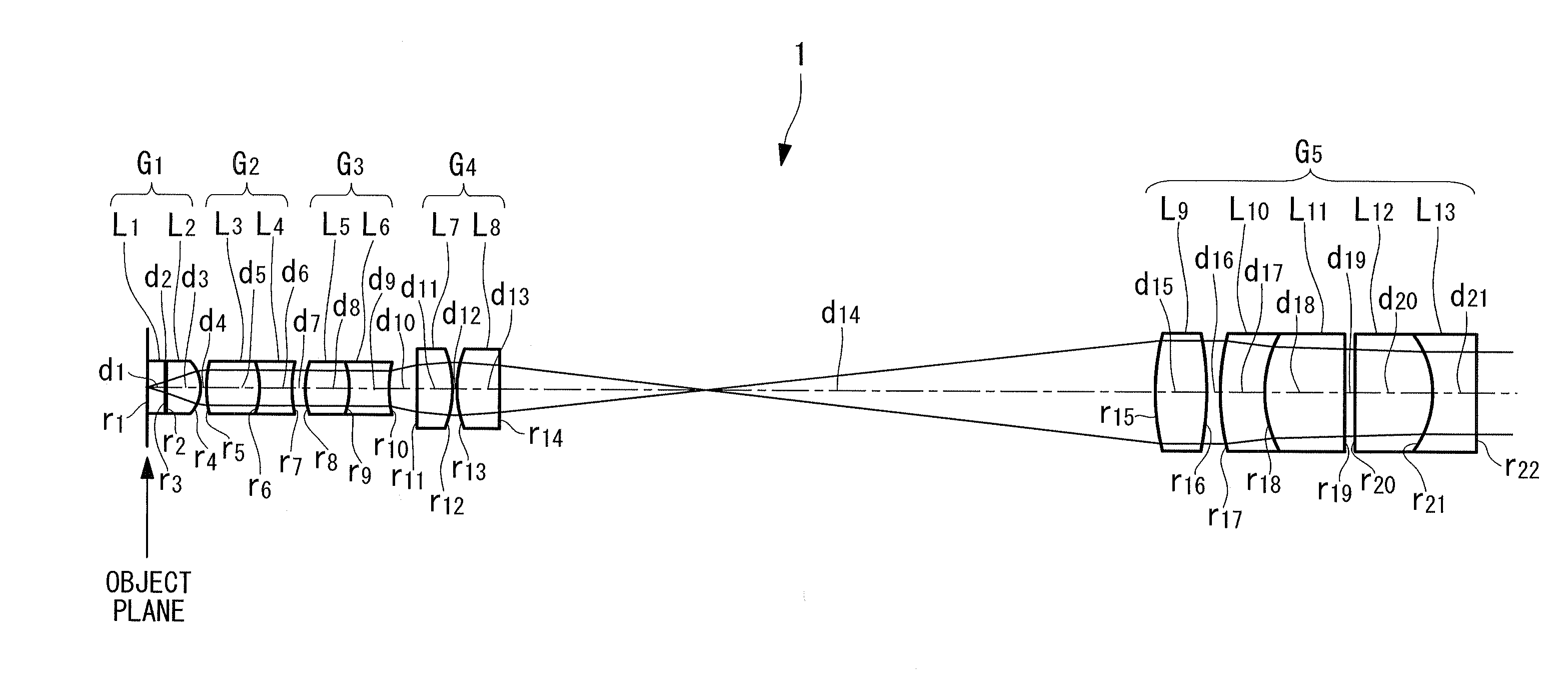
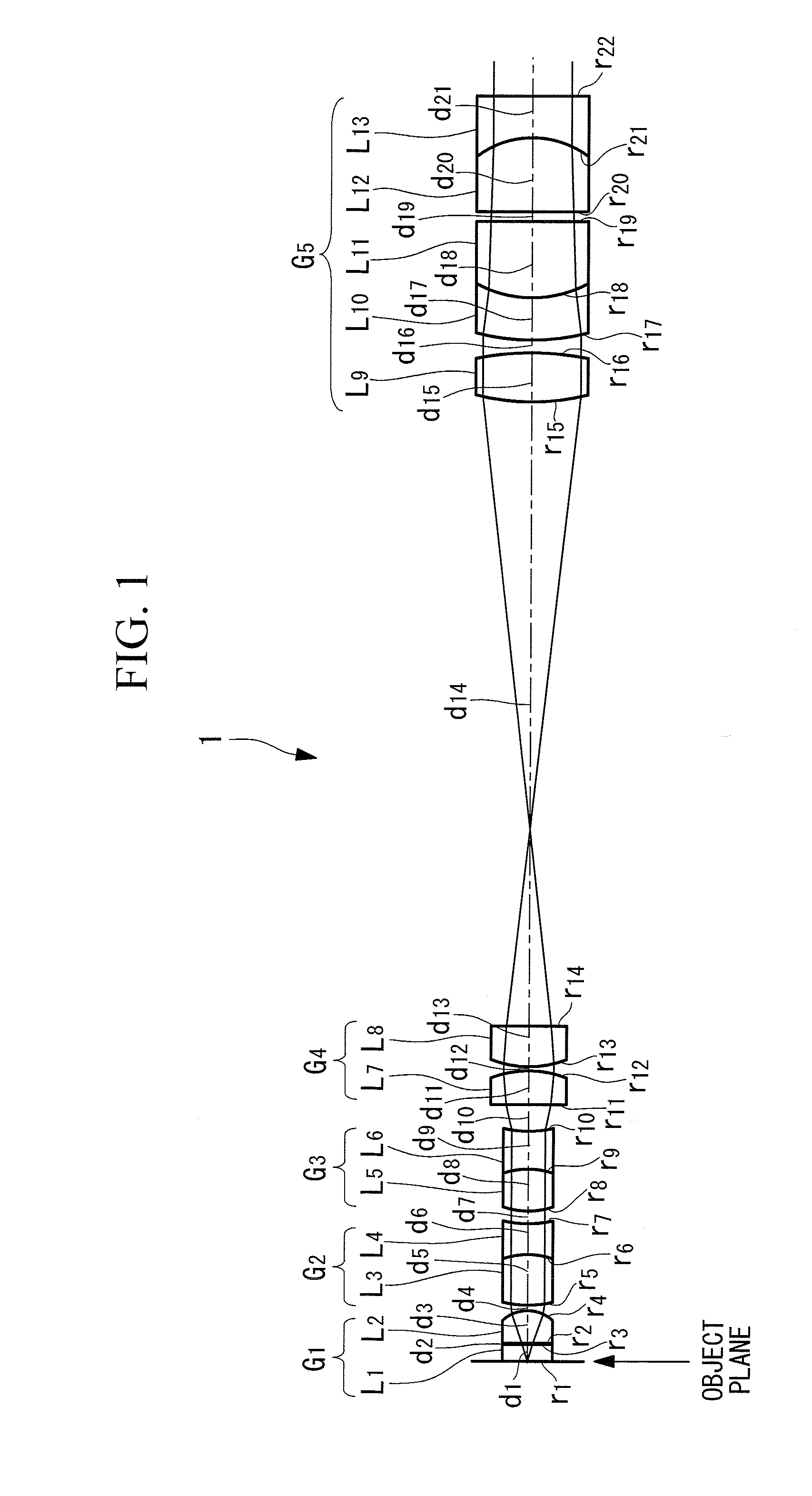
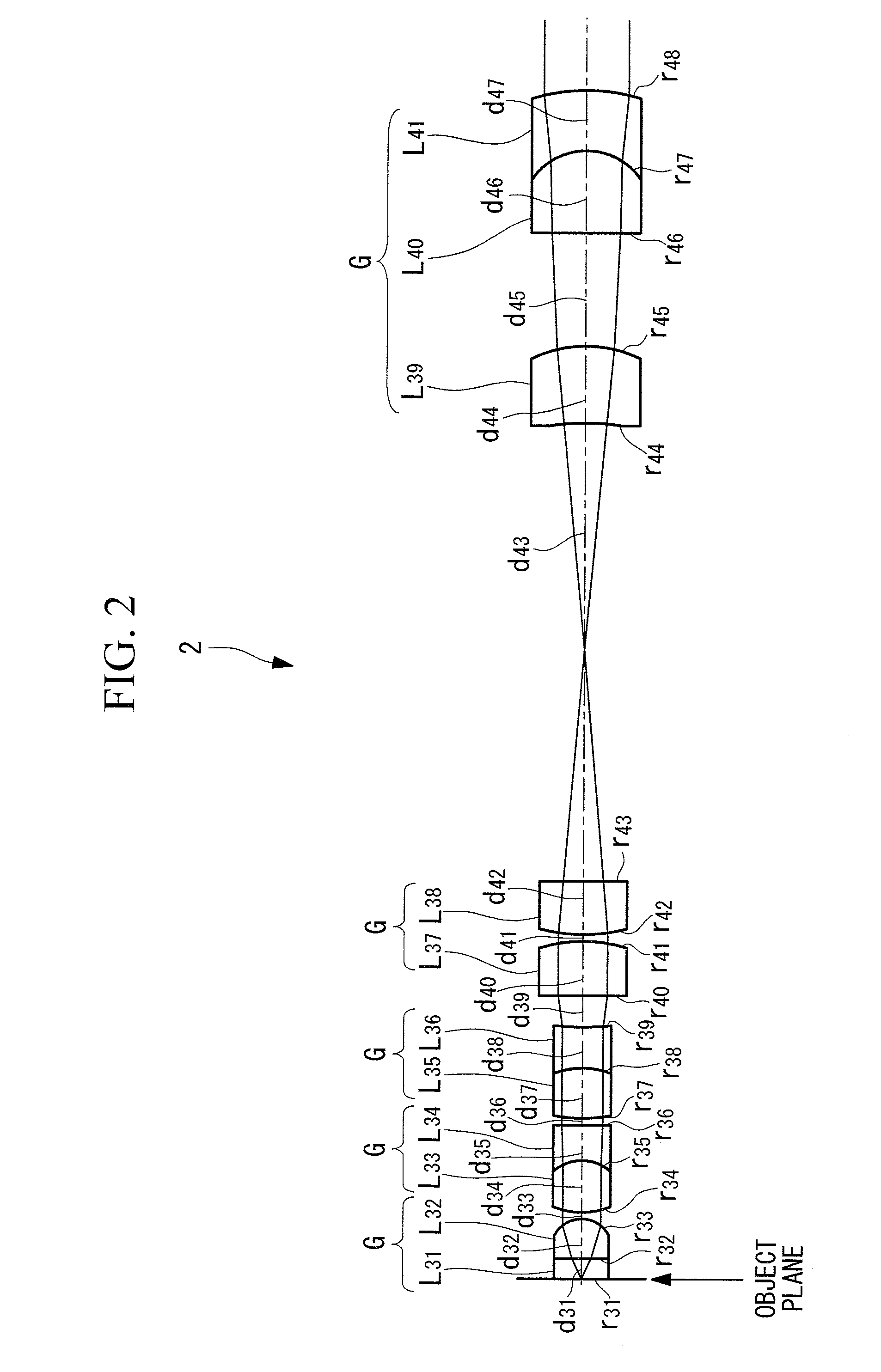
Objective optical system
ActiveUS20100123950A1Minimally invasiveReduce the overall diameterEndoscopesLaproscopesPhysicsConcave surface
An objective optical system includes a first group having positive refractive power and a piano-convex lens with the convex surface facing the image side; a second group having positive refractive power and a lens whose extreme-object-side lens surface is a convex surface facing the object side; a third group having negative refractive power and a lens whose extreme-image-side lens surface is a concave surface facing the image side; a fourth group having positive refractive power and a lens disposed on the extreme object side, whose image-side lens surface is a convex surface facing the image side and a lens disposed on the extreme image side, whose object-side lens surface is a convex surface facing the object side; and a fifth group having positive refractive power and a combined lens formed by joining a convex lens and a concave lens, the joined surface having negative refractive power.
Owner:EVIDENT CORP
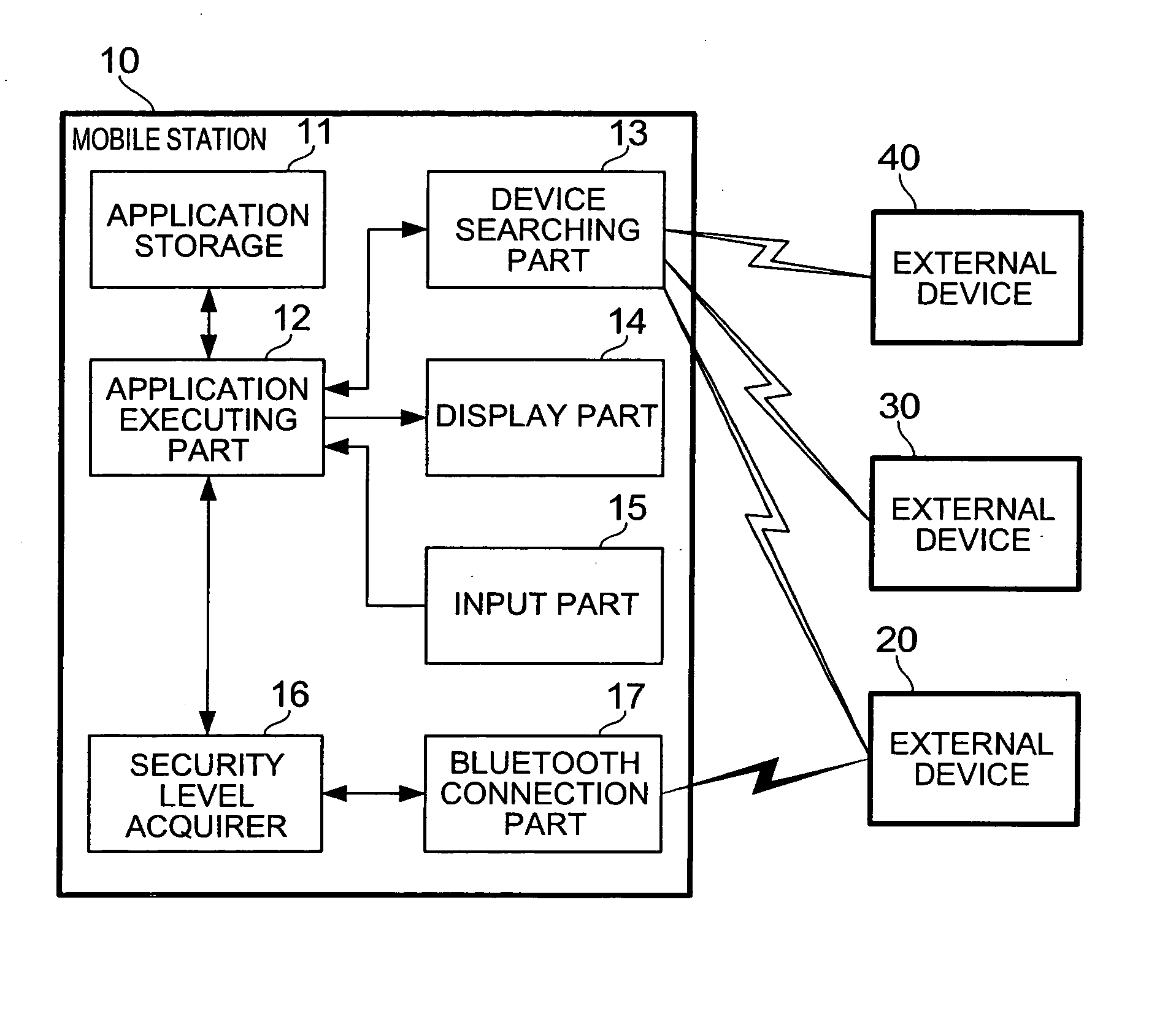
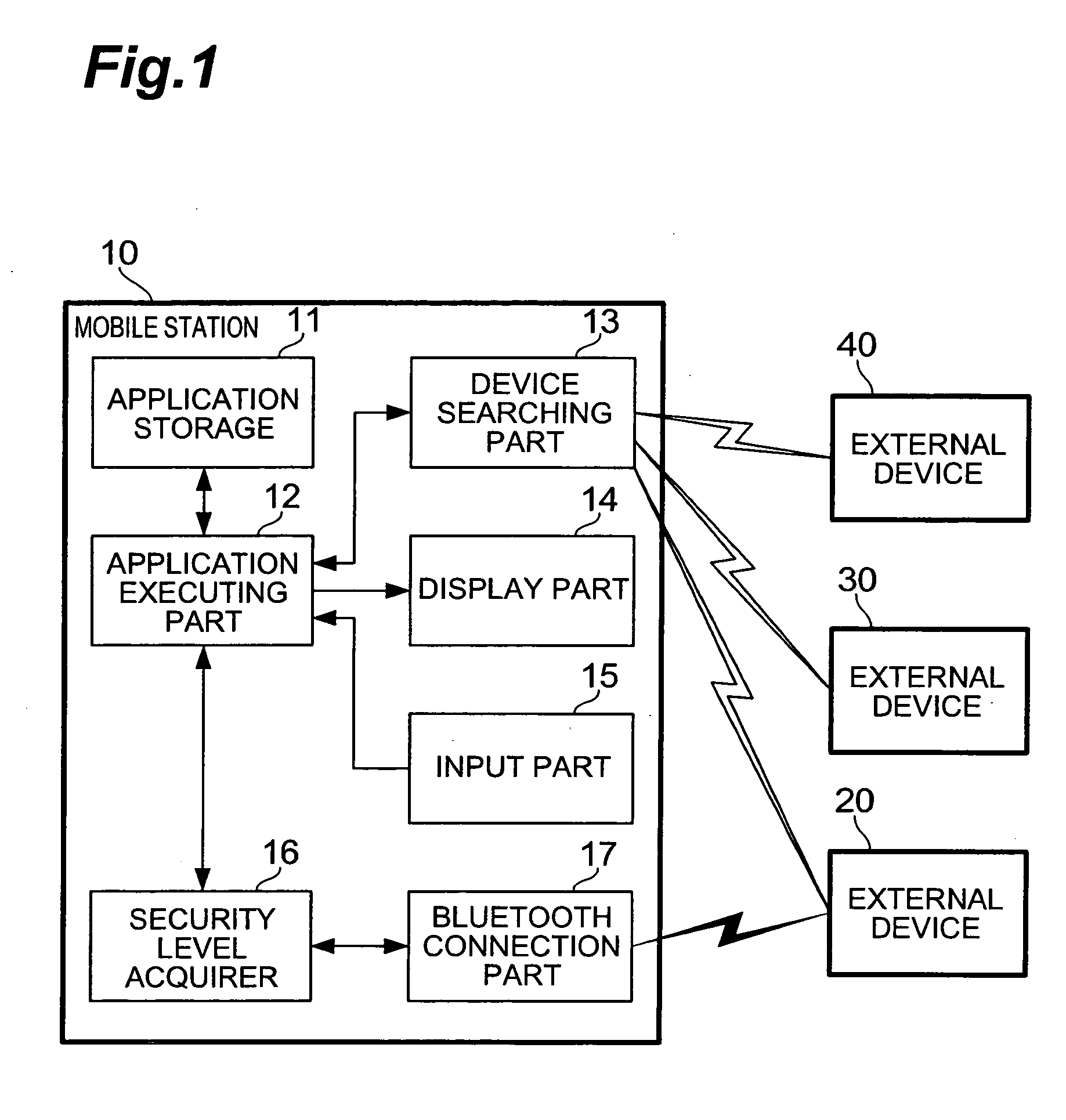
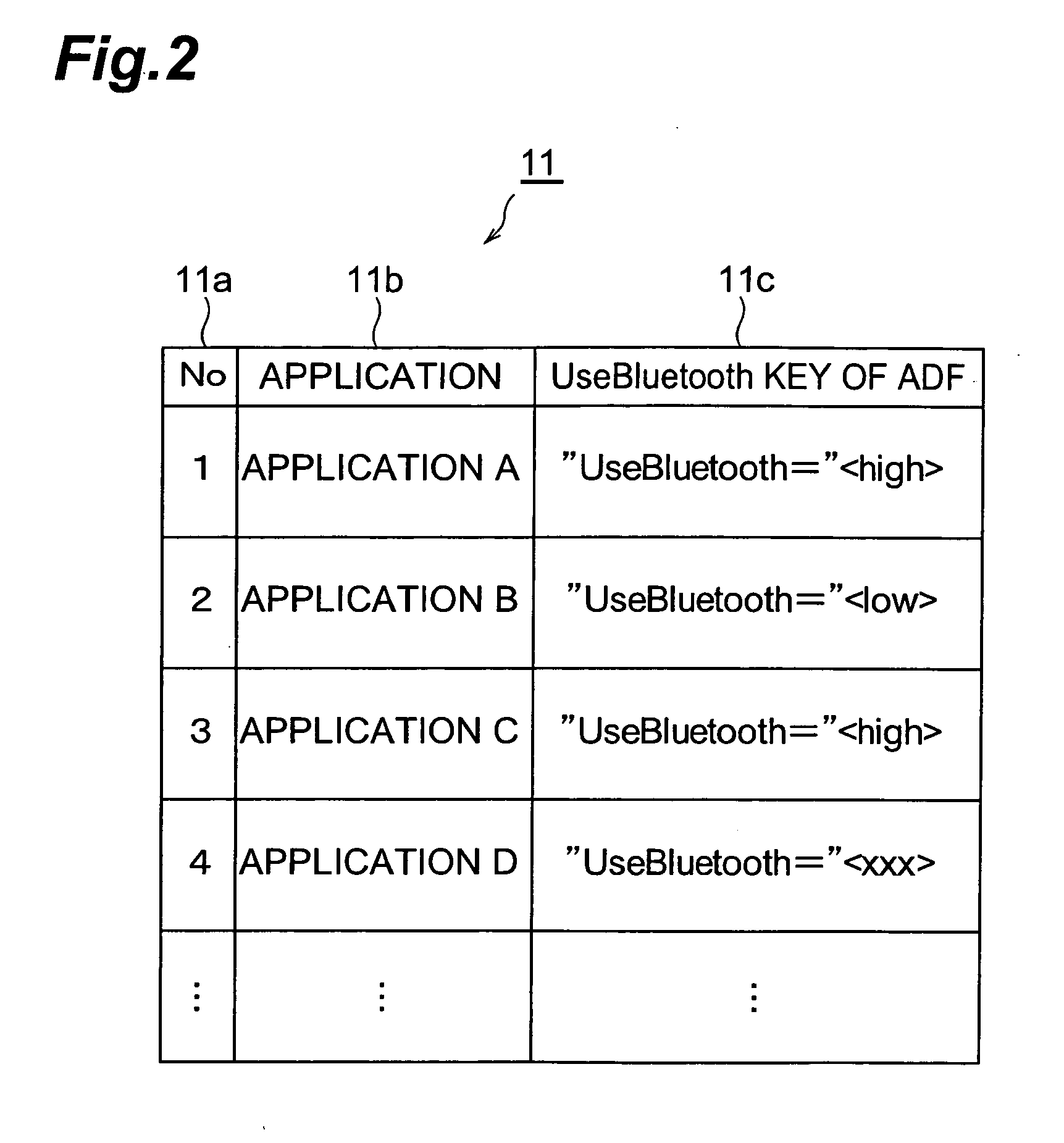
Mobile station and communication control method
InactiveUS20050257052A1Maintain securityFast communicationDigital data processing detailsUser identity/authority verificationMobile stationCommunication control
When an application is activated, a mobile station 10 detects external devices 20, 30, 40 as candidates for a BLUETOOTH connection out of external devices existing in the surrounding area, and presents a list of the devices to a user. When the user selects from the list an external device 20 with which a connection is to be attempted to make, a link is established by a connection procedure according to a security level described in an ADF of the application. Namely, when “high” is described as the security level, an authentication process and an encryption process requiring input of a PIN are executed prior to the establishment of the link. On the other hand, when “low” is described as the security level, a link is established without executing these processes.
Owner:NTT DOCOMO INC
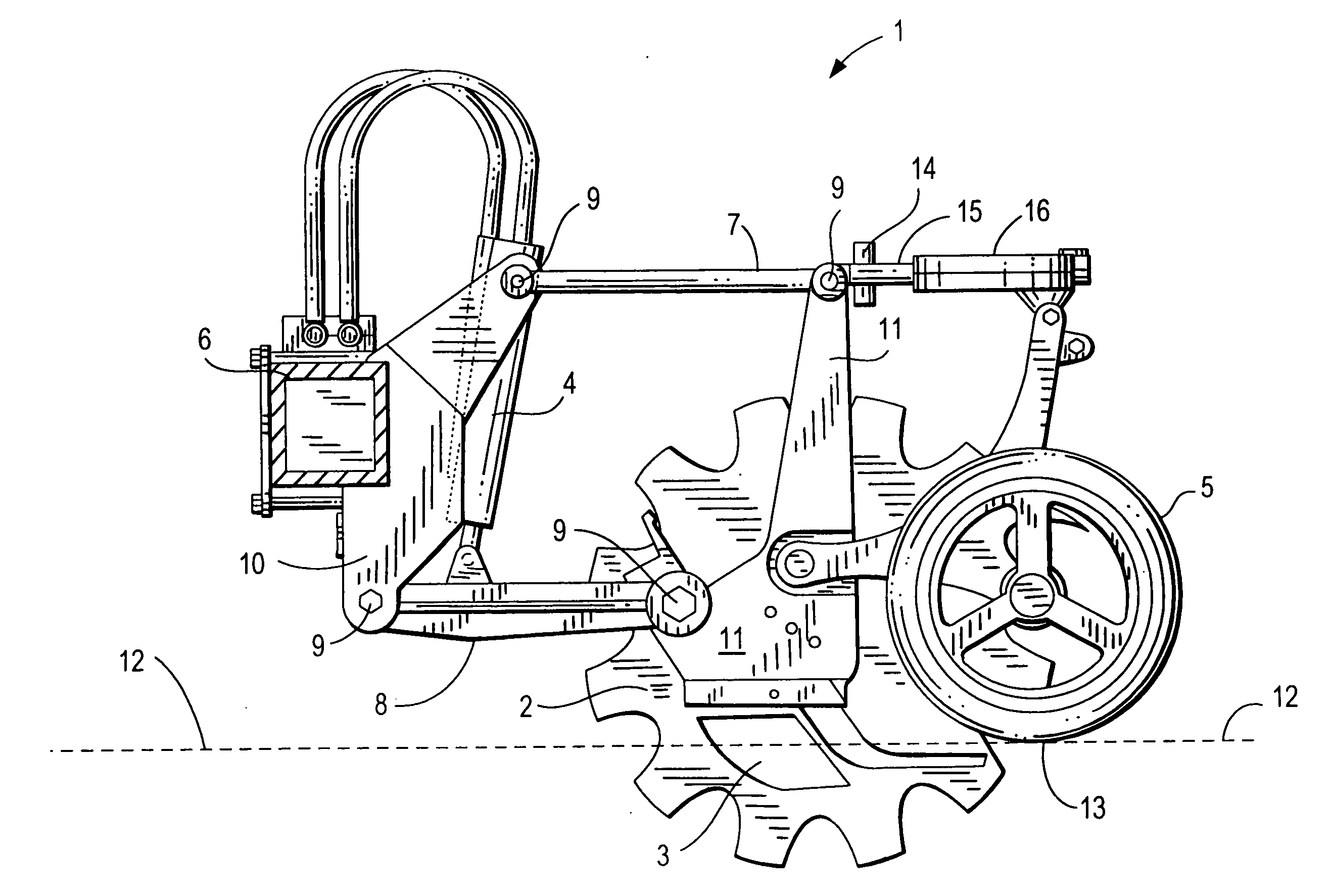
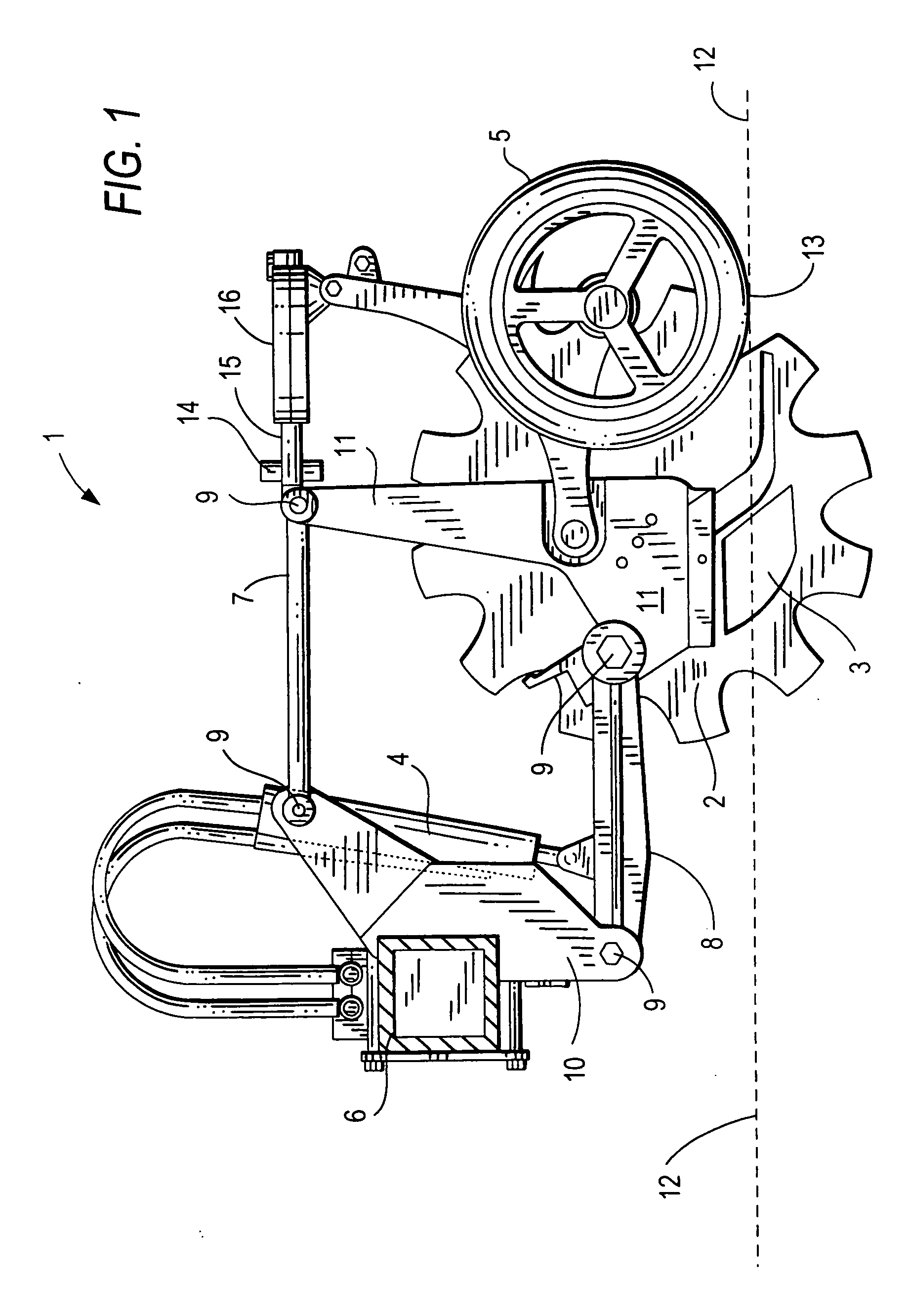
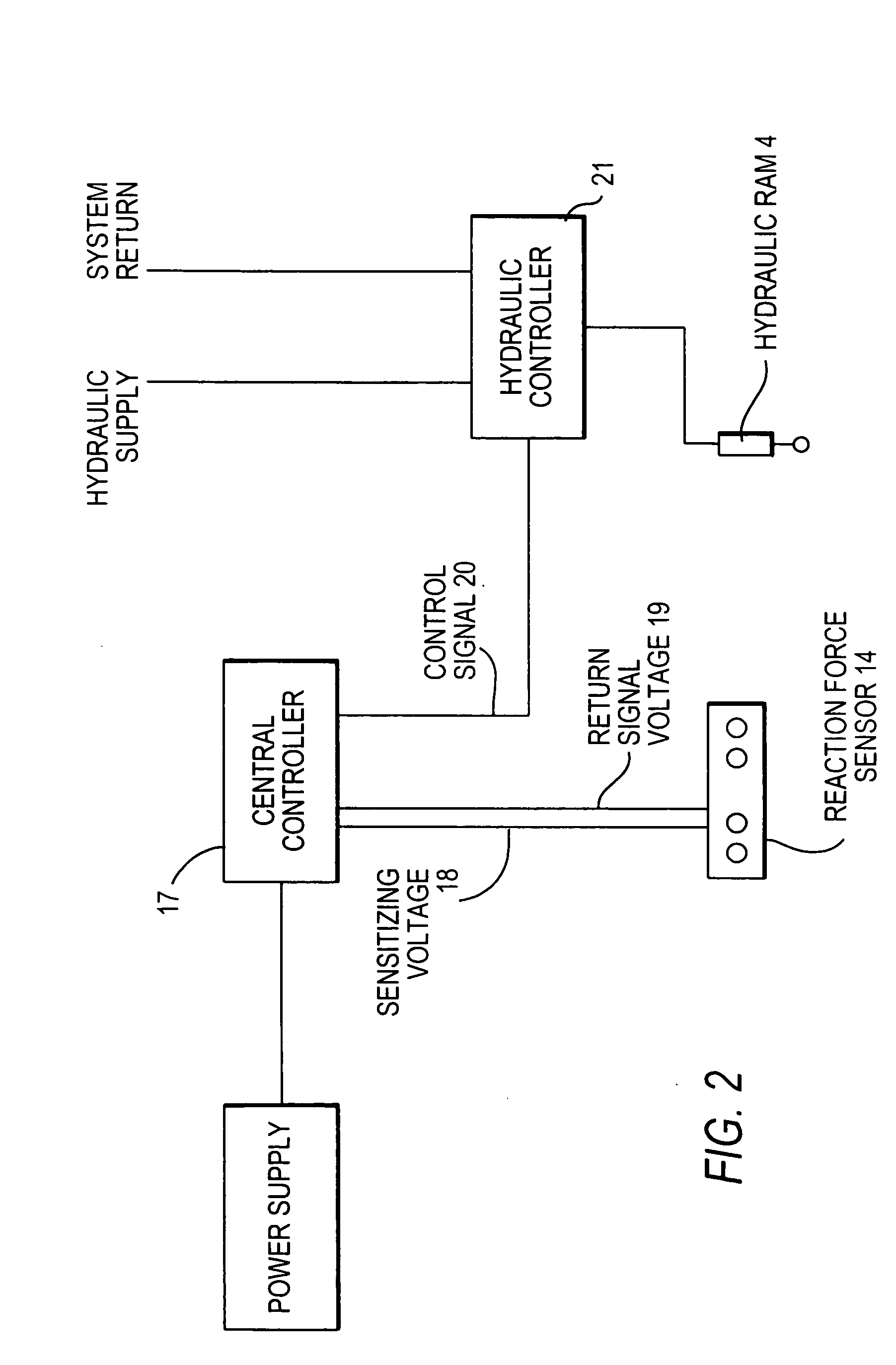
Ground opening device
InactiveUS20070272134A1Adjustable loadImprove variationPlantingAdjusting devicesEngineeringForce sensor
A ground opening device includes a ground-penetrating element configured to penetrate a ground surface, a down drive element configured to apply a downward force to the ground-penetrating element, a reaction force sensor configured to sense a ground reaction force in response to the action of the ground-penetrating element, and a controller configured to adjust the downward force on the ground-penetrating element in response to the sensed ground reaction force.
Owner:BAKER
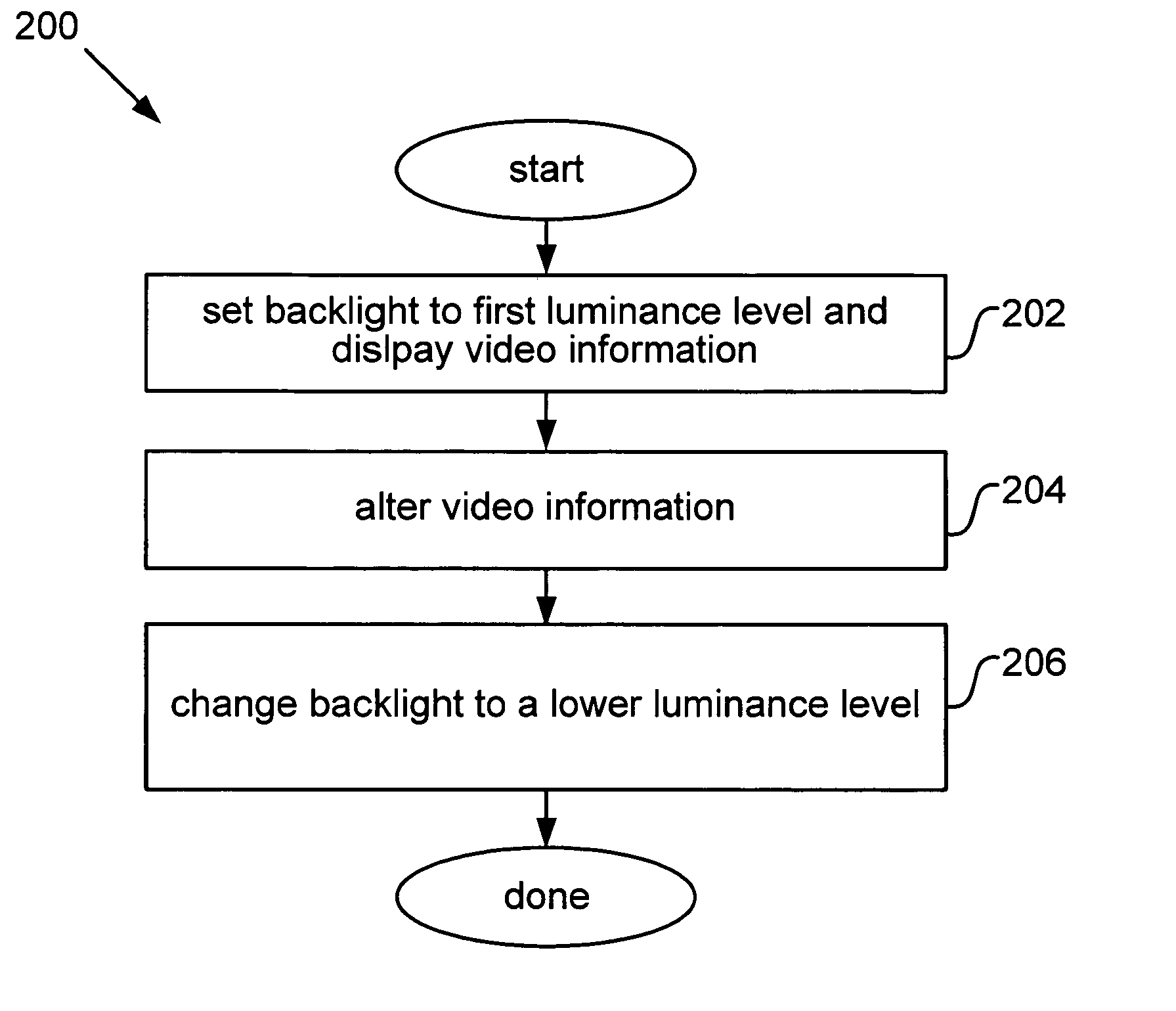
LCD plateau power conservation
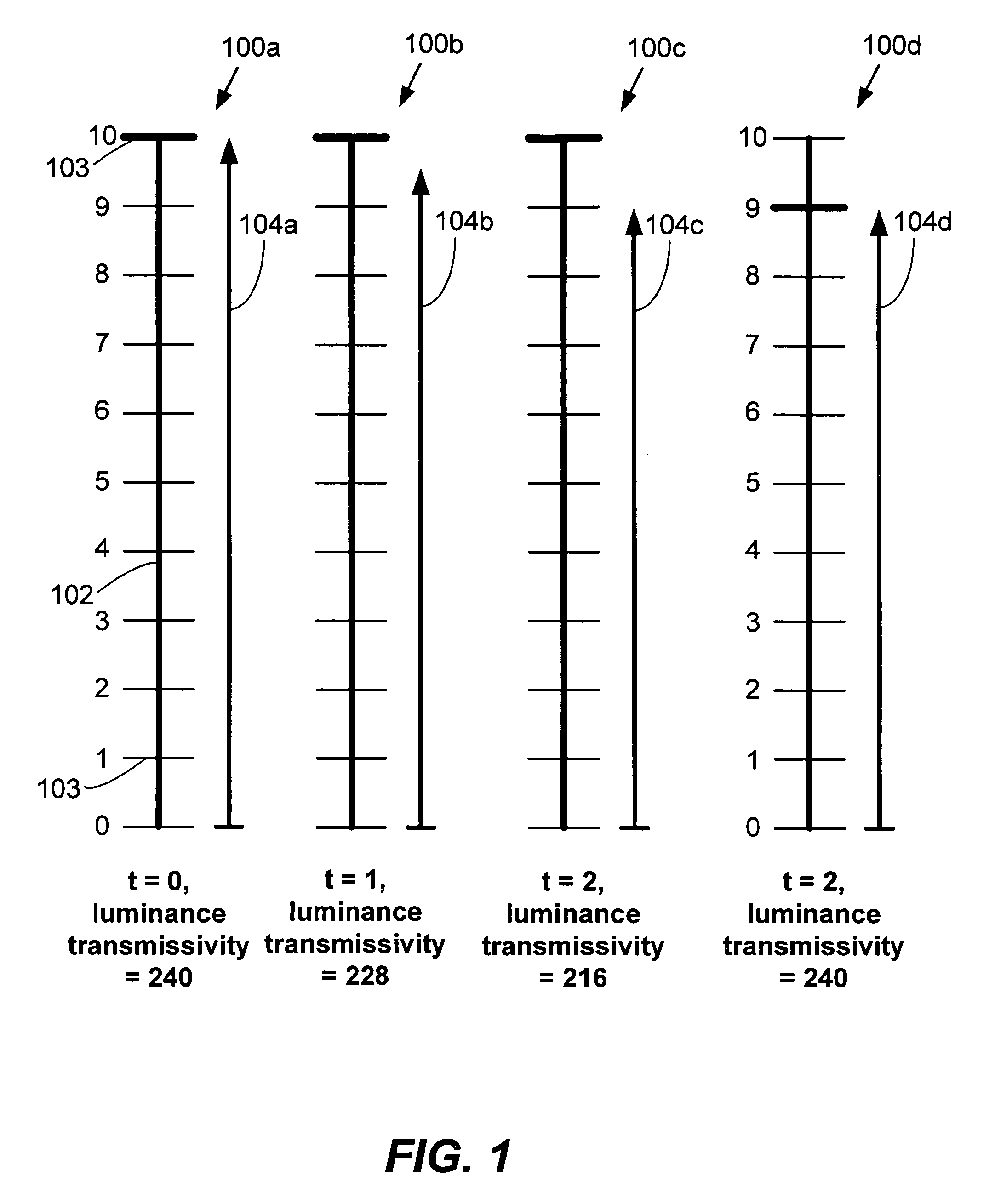
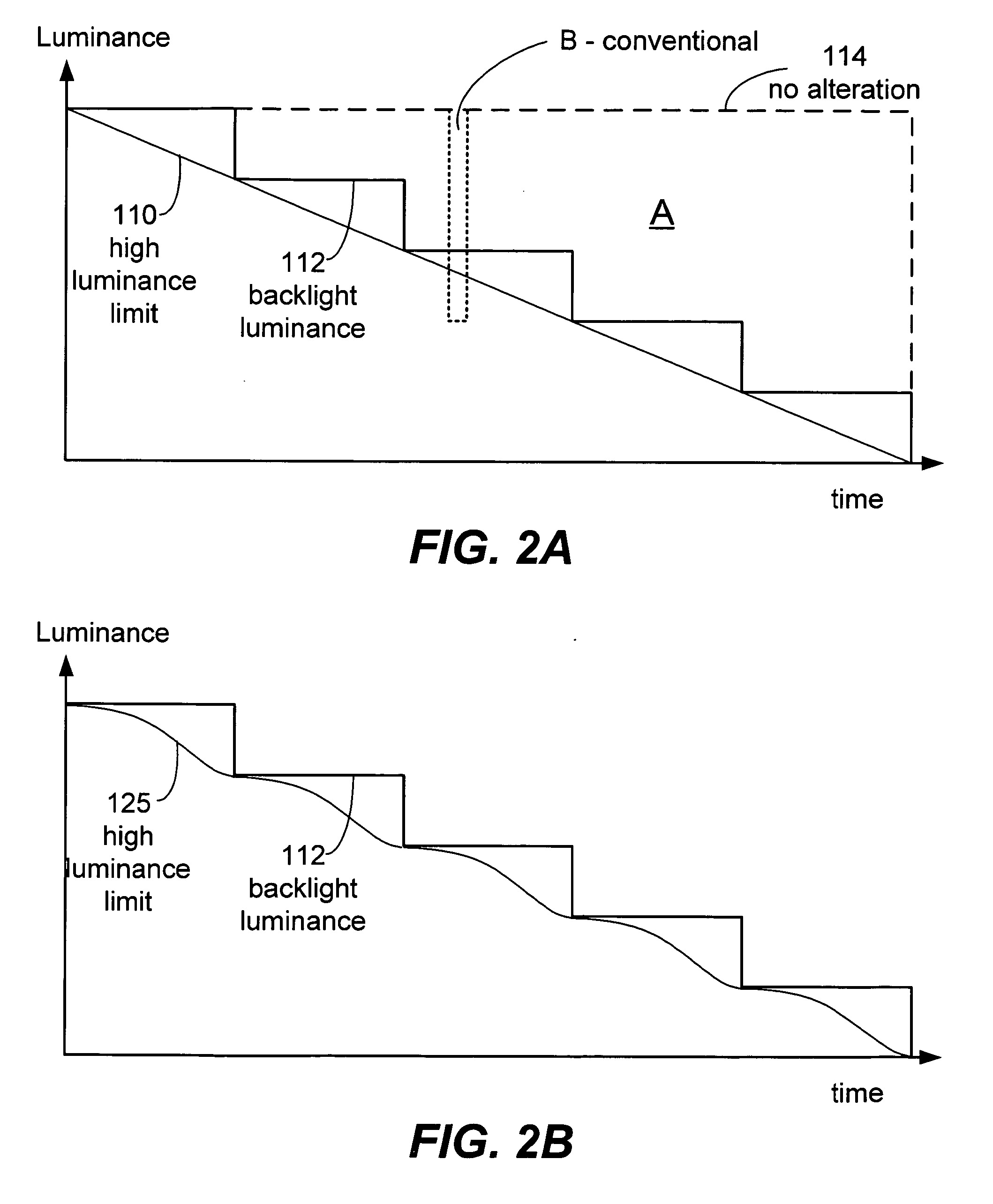
ActiveUS20050270265A1Reduce power consumptionDecrease in luminanceEnergy efficient ICTCathode-ray tube indicatorsLiquid-crystal displayPlateau
Described herein are power conservation systems and methods that reduce power consumption for an electronics device including a liquid crystal display (LCD). The LCD includes a backlight that offers multiple luminance levels, where each level consumes a different amount of power. The systems and methods alter video information while the backlight remains at a backlight luminance level. The alteration reduces luminance for the video information to produce new video information that can be presented at a lower backlight luminance level. Change to the lower backlight luminance level may then occur without significantly affecting aggregate luminance of the new video information, as perceived by a user. The LCD and electronics device consume less power at the lower luminance level.
Owner:SAMSUNG ELECTRONICS CO LTD
Computer resource distribution method based on prediction
InactiveUS20050102674A1Reduce in quantityReduce maintenance costsResource allocationDigital computer detailsComputer resourcesDistribution method
A resource distribution method capable of lending surplus resources among a plurality of services and reducing the maintenance cost of the surplus resources is provided. Computer resources in the standby system have a dead standby state in which at least an application is not installed. A plurality of services or a plurality of users share the computer resources in the standby system. As a result, improvement of the utilization factor of idle computer resources and server integration are implemented, and the cost required to maintain the computer resources is reduced. Furthermore, load prediction is conducted as regards individual services by using past operation history. Idle computer resources secured from services having surplus and maintained are thrown in according to a result of the prediction.
Owner:HITACHI LTD
Computer resource distribution method based on prediction
InactiveUS7500001B2Reduce in quantityReduce maintenance costsResource allocationDigital computer detailsComputer resourcesDistribution method
A resource distribution method capable of lending surplus resources among a plurality of services and reducing the maintenance cost of the surplus resources is provided. Computer resources in the standby system have a dead standby state in which at least an application is not installed. A plurality of services or a plurality of users share the computer resources in the standby system. As a result, improvement of the utilization factor of idle computer resources and server integration are implemented, and the cost required to maintain the computer resources is reduced. Furthermore, load prediction is conducted as regards individual services by using past operation history. Idle computer resources secured from services having surplus and maintained are thrown in according to a result of the prediction.
Owner:HITACHI LTD
Controlled release formulations using intelligent polymers
InactiveUS6893661B1Promote absorptionMaintenance of therapeutically effective blood levelPowder deliveryOrganic active ingredientsSmart polymerWater contact
An extended release dosage composition of pharmaceutically active substances that have a water contact angle (θ) such that cos θ is between +0.9848 and −0.9848 presented as a matrix tablet containing the said pharmaceutically active substances, with / without suitable pharmaceutical excipients in intimate mixture with two groups of intelligent polymers having opposing wettability characteristics, one demonstrating a stronger tendency towards hydrophobicity and the other a stronger tendency towards hydrophilicity, the polymer combination being between the ratios of 1:50 and 50:1 amounts effective to control the release of said pharmaceutically active substances in a mathematically predictable manner, wherein the polymer demonstrating a stronger tendency towards hydrophobicity is not less than 5% wt / wt and preferably between 5-70% wt / wt of the final formulation composition. The intelligent polymers being ethylcellulose (EC) as a more strongly hydrophobic and hydroxyethylcellulose (HEC) and / or hydroxypropyl methylcellulose (HPMC) as more strongly hydrophilic (the ratio of HEC to HPMC being between 1:100 and 100:1). The matrix tablet is optionally coated with an enteric coat, 0-5%-15% wt / wt to prevent the initial burst effect seen in such systems and to impart gastrointestinal tract (GIT) “stealth” characteristics especially in the presence of food.
Owner:VALEANT INT BERMUDA
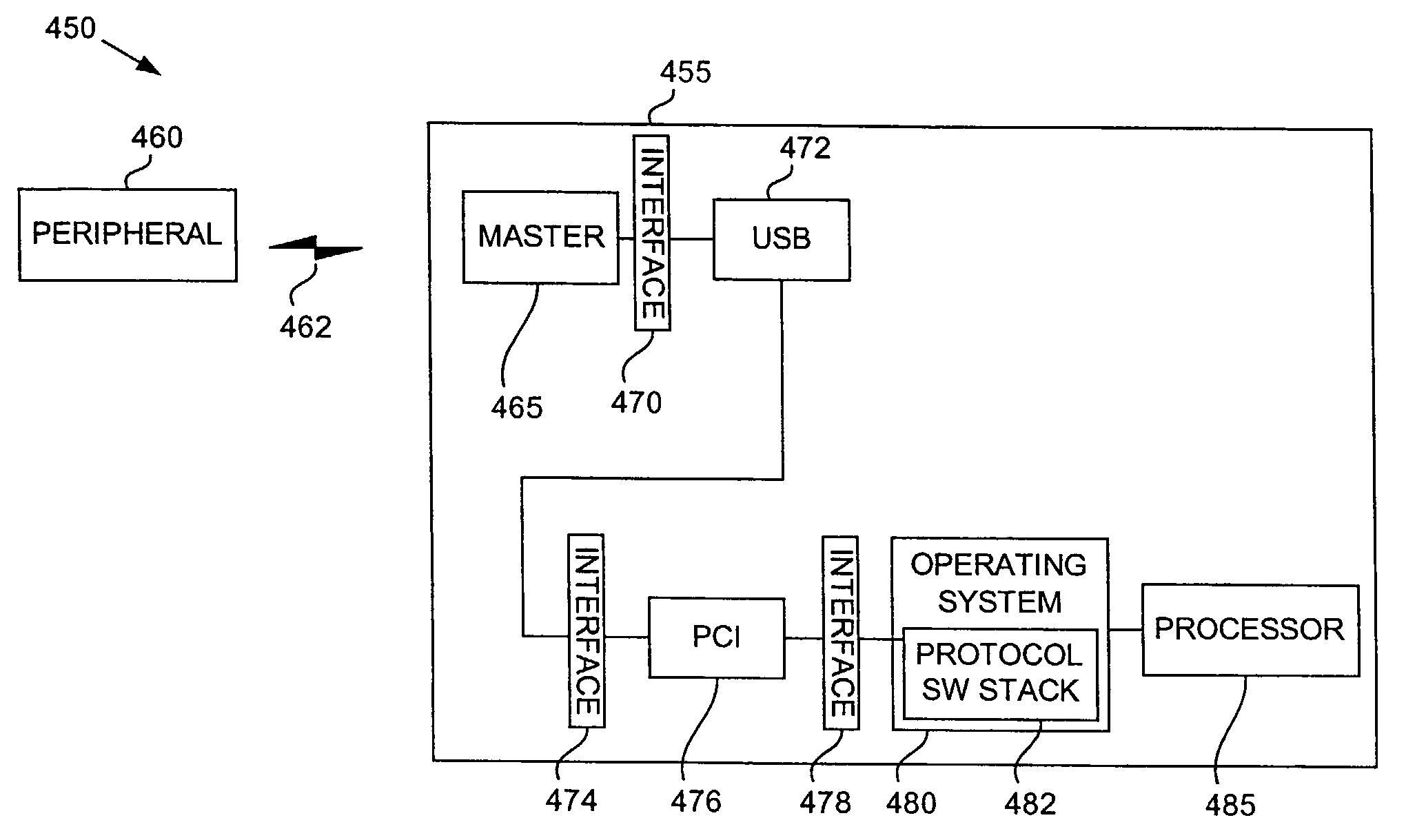
Bluetooth transparent bridge
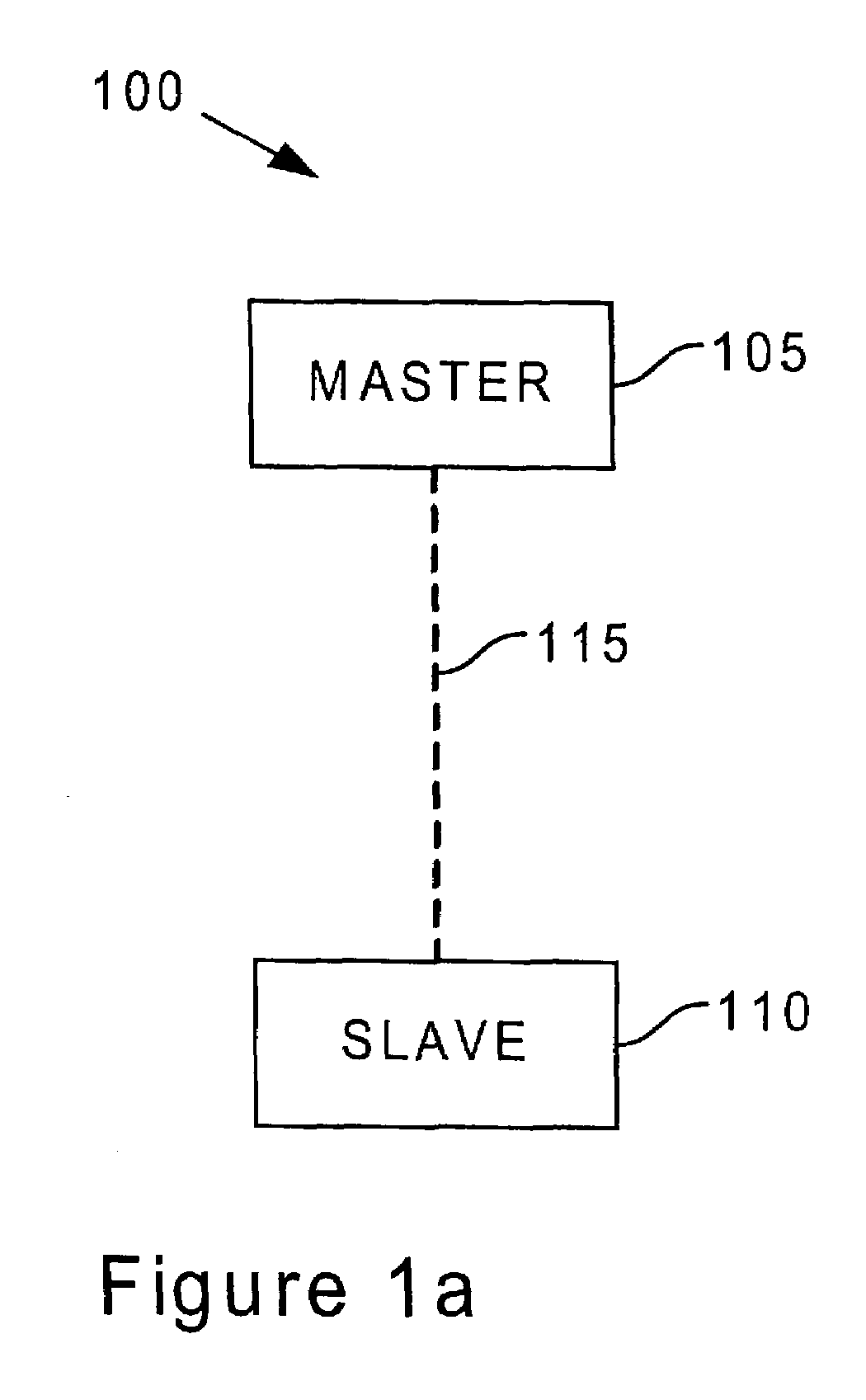
ActiveUS7164886B2Spread quicklyIssue reliabilityNear-field transmissionAntenna supports/mountingsComputerized systemBluetooth
System and method for transparently attaching wireless peripherals to a computer using a Bluetooth wireless network. A preferred embodiment comprises an interface (for example, interface 630), a communications bus (for example, a USB 620), and a Bluetooth wireless network adapter (for example, a master unit 610). The interface translates messages from either the communications bus or the Bluetooth wireless network adapter so that a software stack is not needed to perform the translation at a later time. This helps to maintain the computer's reliability and performance. The system and method also affords wireless connectivity without the presence of a computer system.
Owner:TEXAS INSTR INC
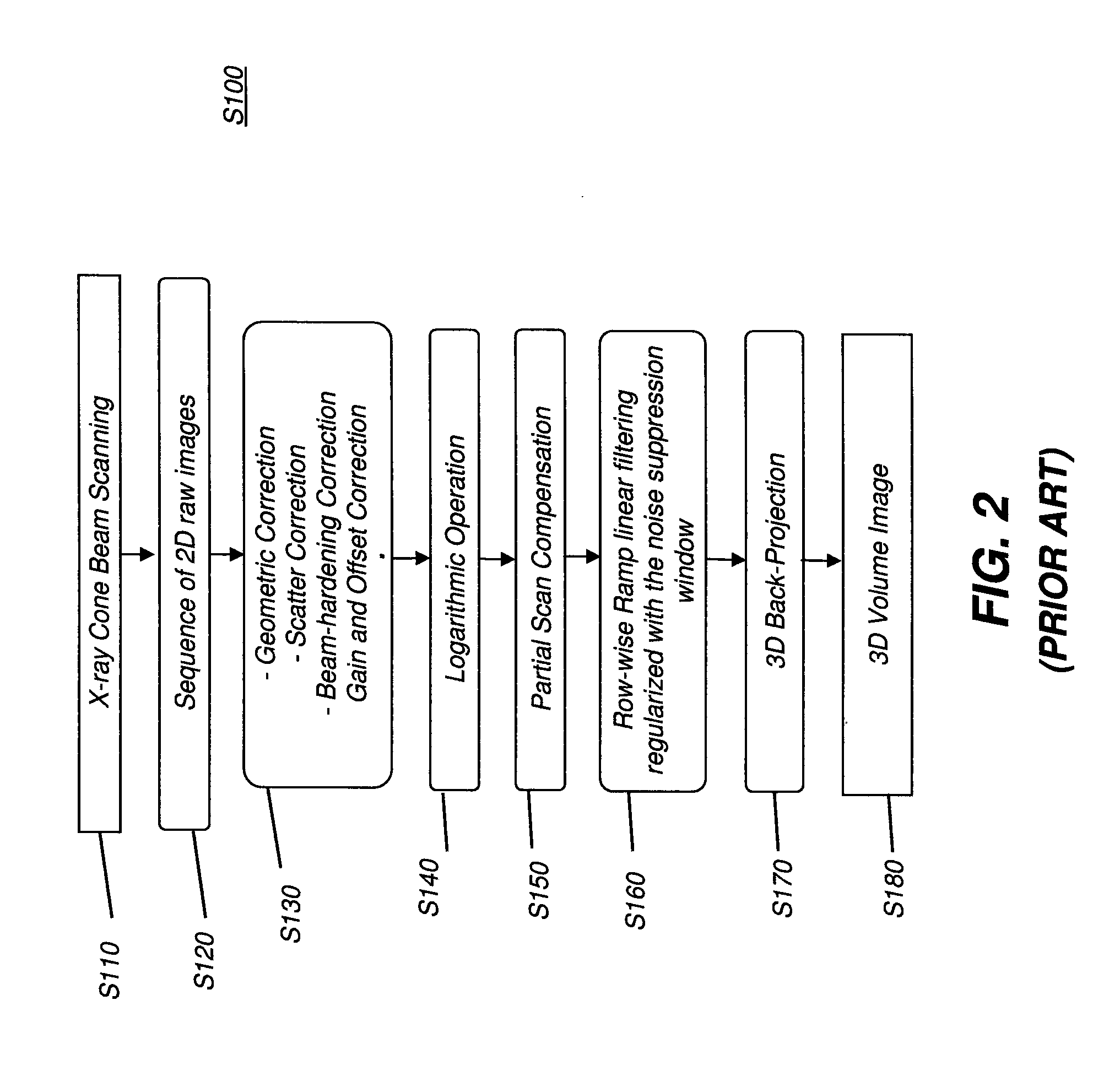
Methods and apparatus for super resolution scanning for cbct system and cone-beam image reconstruction
InactiveUS20130051519A1High resolutionQuality improvementReconstruction from projectionMaterial analysis using wave/particle radiationProjection imageImage resolution
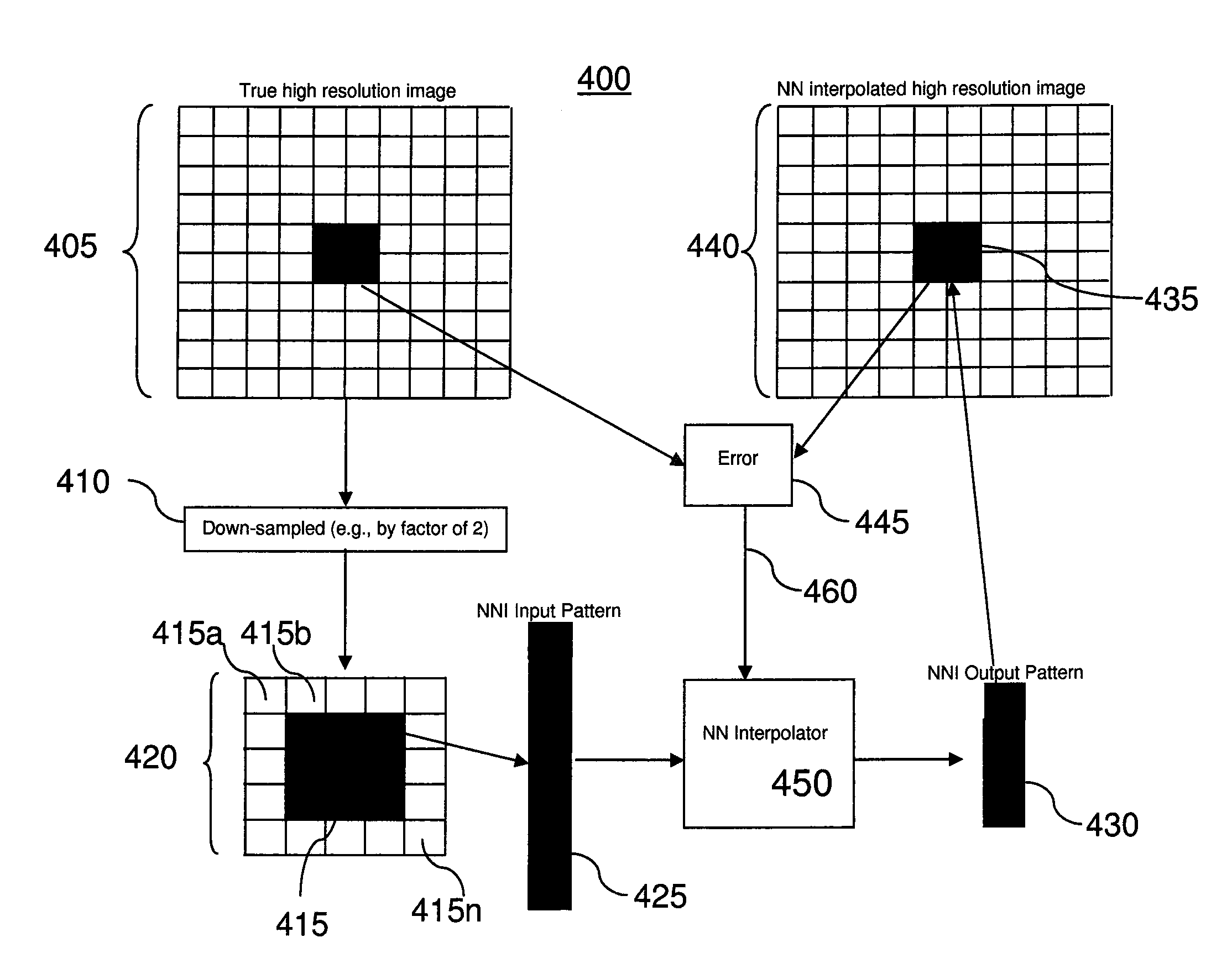
Embodiments of methods and / or apparatus for 3-D volume image reconstruction of a subject, executed at least in part on a computer for use with a digital radiographic apparatus can obtain image data for 2-D projection images over a range of scan angles. For each of the plurality of projection images, an enhanced projection image can be generated. In one embodiment, through the application of a resolution increasing interpolator, a prescribed CBCT routine scanning mode with preset binning can increase a spatial resolution, Nyquist frequency or MTF.
Owner:CARESTREAM HEALTH INC
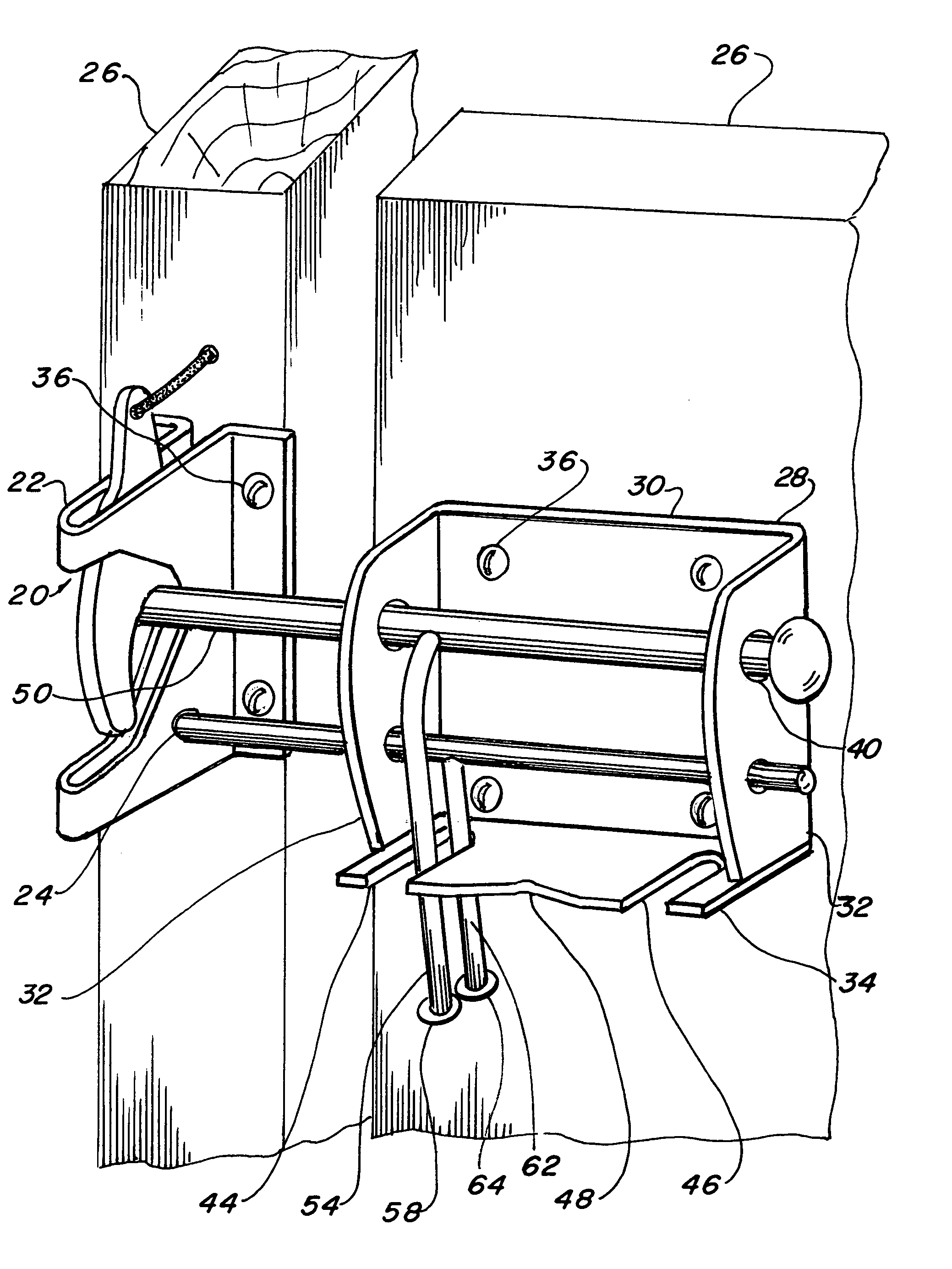
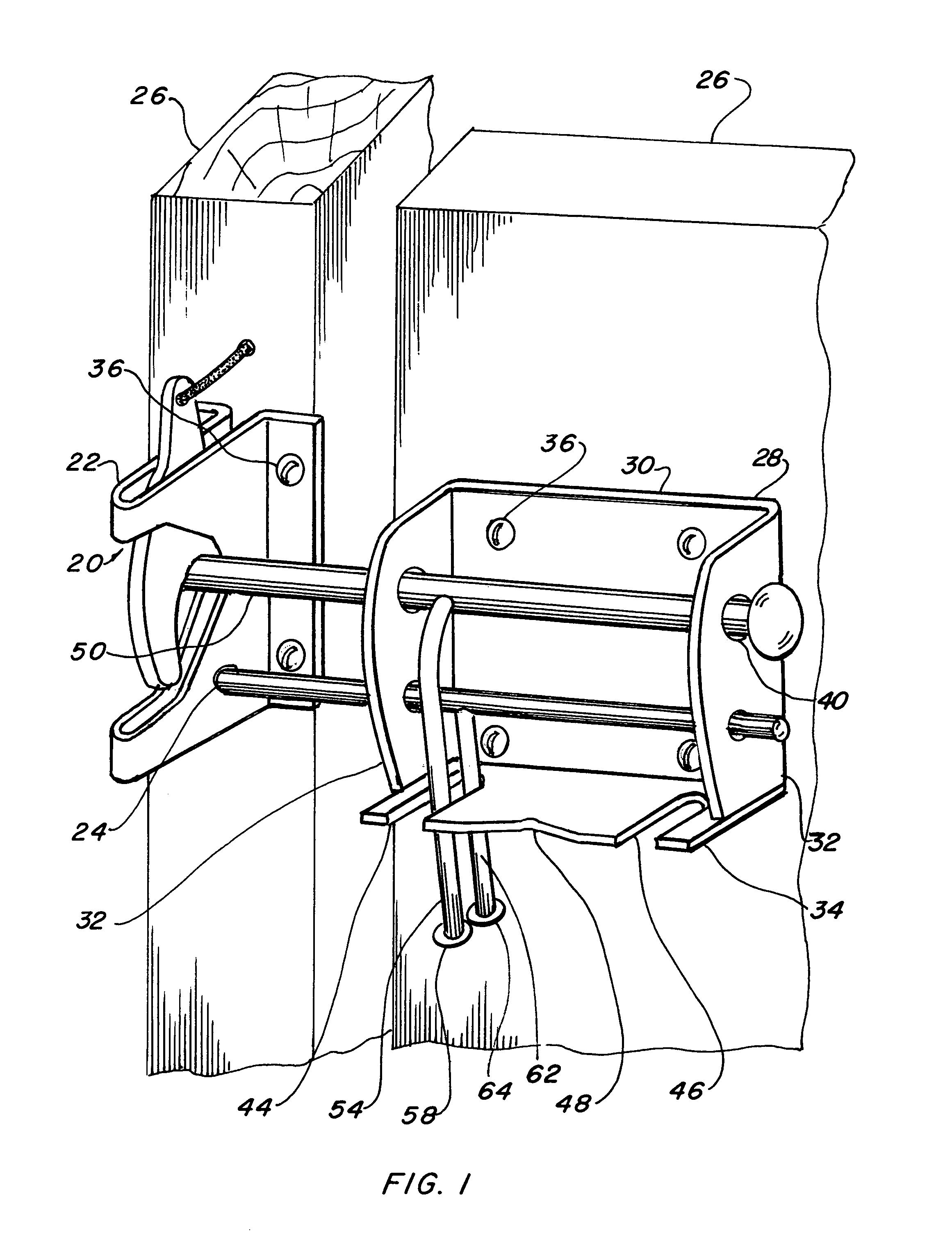
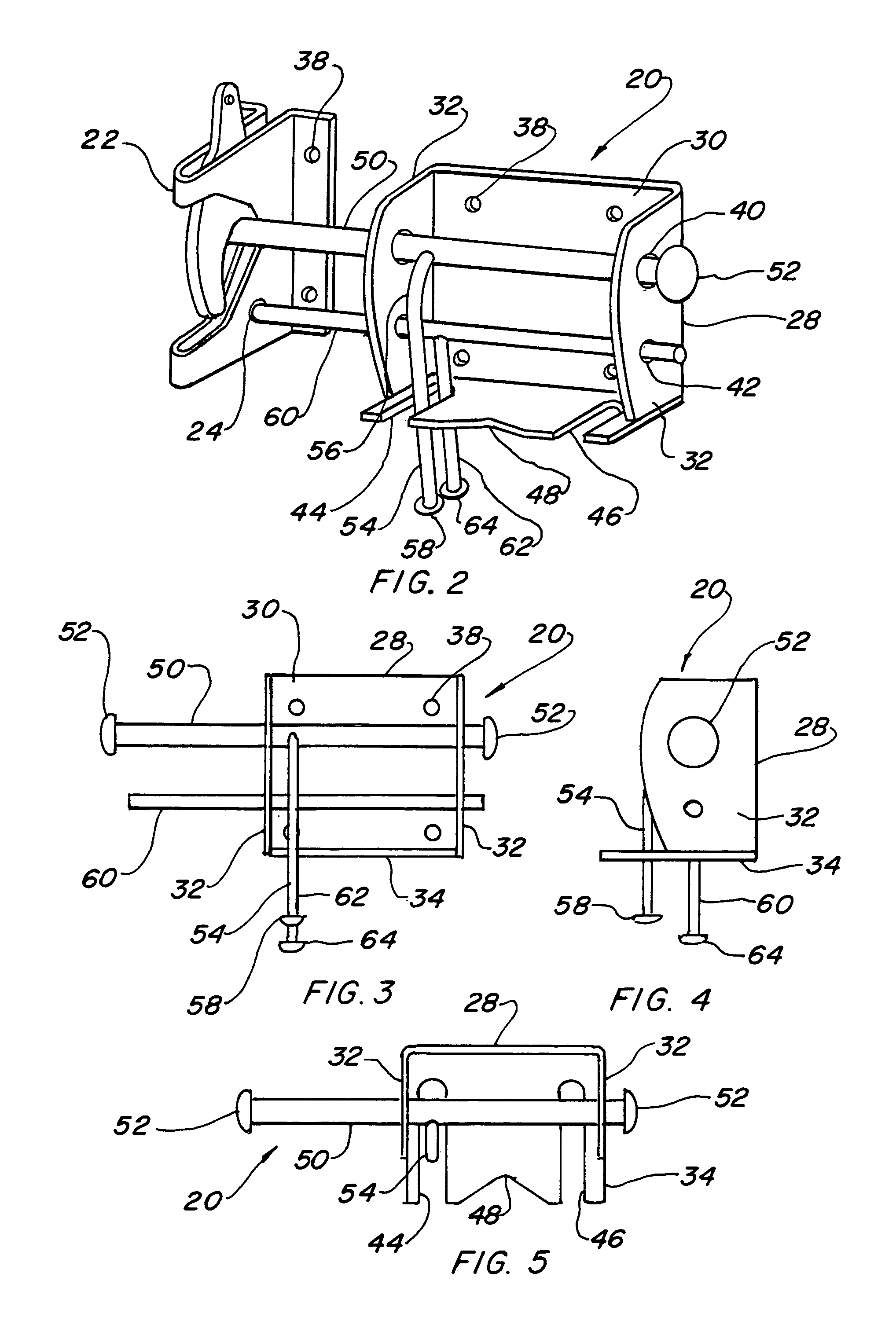
Four position gate latch assembly
InactiveUS7021678B1Lower initial costEasy to produceBuilding locksFencingElectrical and Electronics engineering
A four position gate latch (20) that is used to fasten and lock a gate. The gate latch includes a self fastening gravity gate latch (22) that is fastened to one face of the gate. A gate latch bracket (28) is fastened to the gate on an opposed face, mating with said gate latch. A latch striker bar slideably extends from the bracket such that the striker bar interfaces with the gate latch and links the gate portions together. A locking bolt (60) slideably extends though the bracket, allowing it to interface with the gate latch and provide positive locking capabilities. The gate latch is capable of being placed in a self fastening gravity gate latch conventional position, a locked position with the locking bolt penetrating a padlock hole, an unlatched position that permits the gate to swing inward on the gate latch side without interference, and a free-swinging position that permits the gate to pivot in both directions.
Owner:RAOULT PHILIPPE P
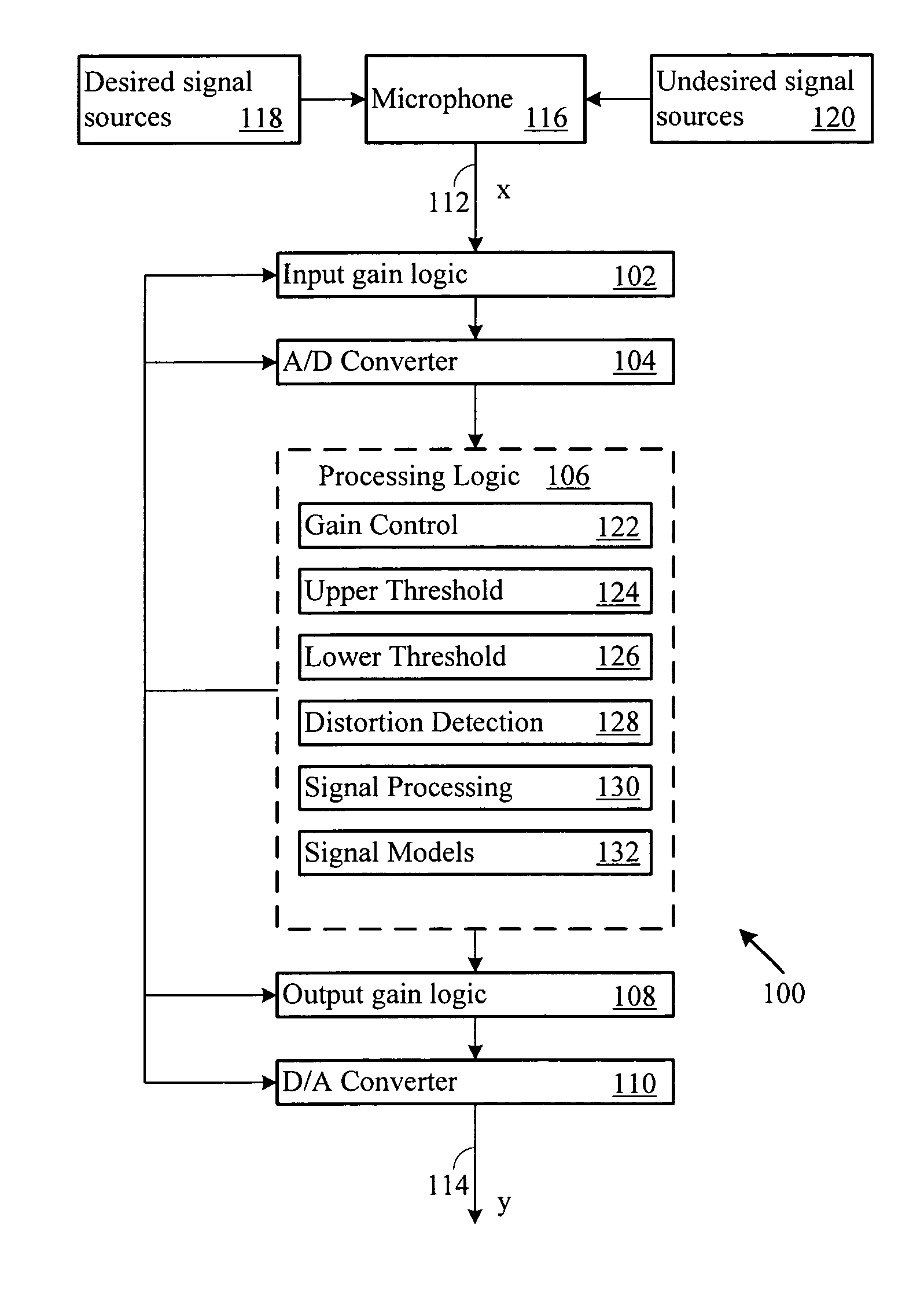
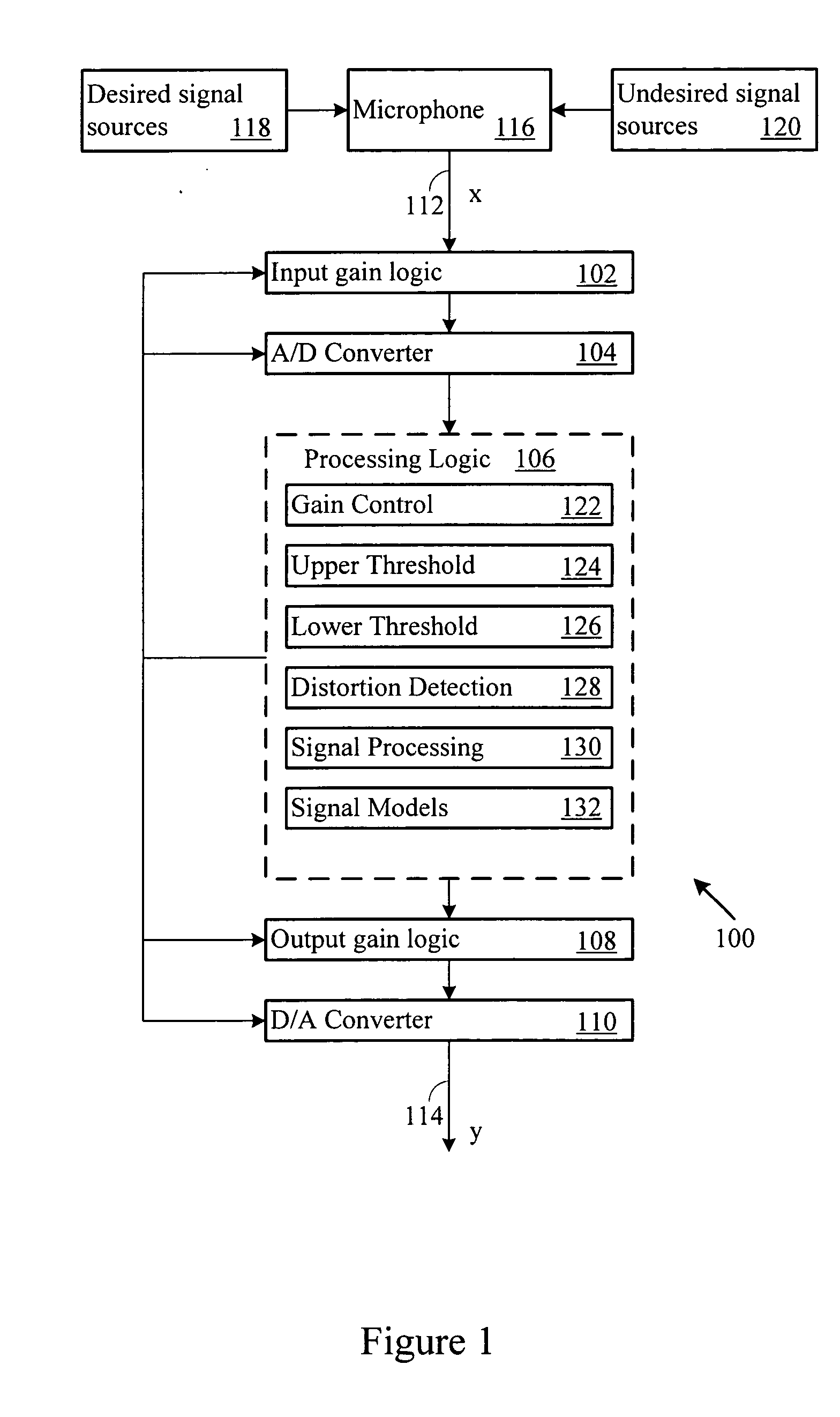
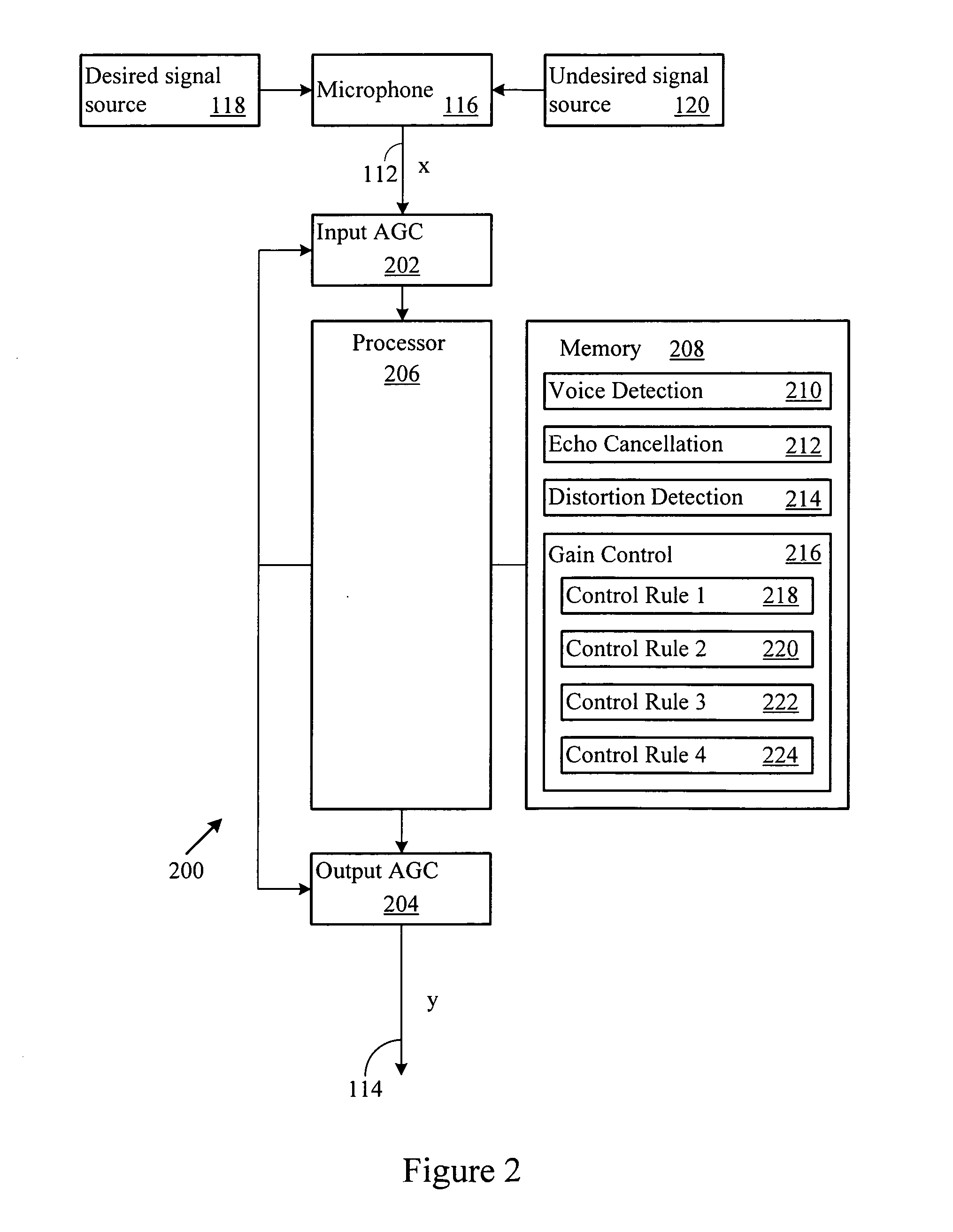
Adaptive gain control system
ActiveUS20070003078A1Maintain levelGain controlVolume compression/expansion in digital/coded amplifiersSelf adaptiveEngineering
An automatic gain control system maintains desired signal content level, such as voice, in an output signal. The system includes automatic gain control over an input signal, and compensates the output signal based on input signal content. When the input signal level exceeds an upper or lower processing threshold level, or is distorted (e.g., clipped), the system applies a gain to the input signal level. The system may compensate for the gain in the output signal when the input signal includes desired signal content.
Owner:BLACKBERRY LTD
Controlled extended drug release technology
ActiveUS20060003007A1Longer resident timeEasy to controlCapsule deliveryCoatingsActive agentWater insoluble
A controlled extended drug release technology for the controlled extended release of hydrophobic or hydrophilic drugs or therapeutically active agents consisting of a homogeneous blend of one or more therapeutic agents, gas generators and surrounded by one or more layers of coat made of thermoplastic water insoluble cellulose derivatives, acrylic polymers, superdisintegrants and optionally an oil, antioxidants and electrolytes. The technology platform is capable of releasing therapeutic agents via zero, first or pseudo first order release.
Owner:INTELLIPHARMACEUTICS
Direct backplane connector
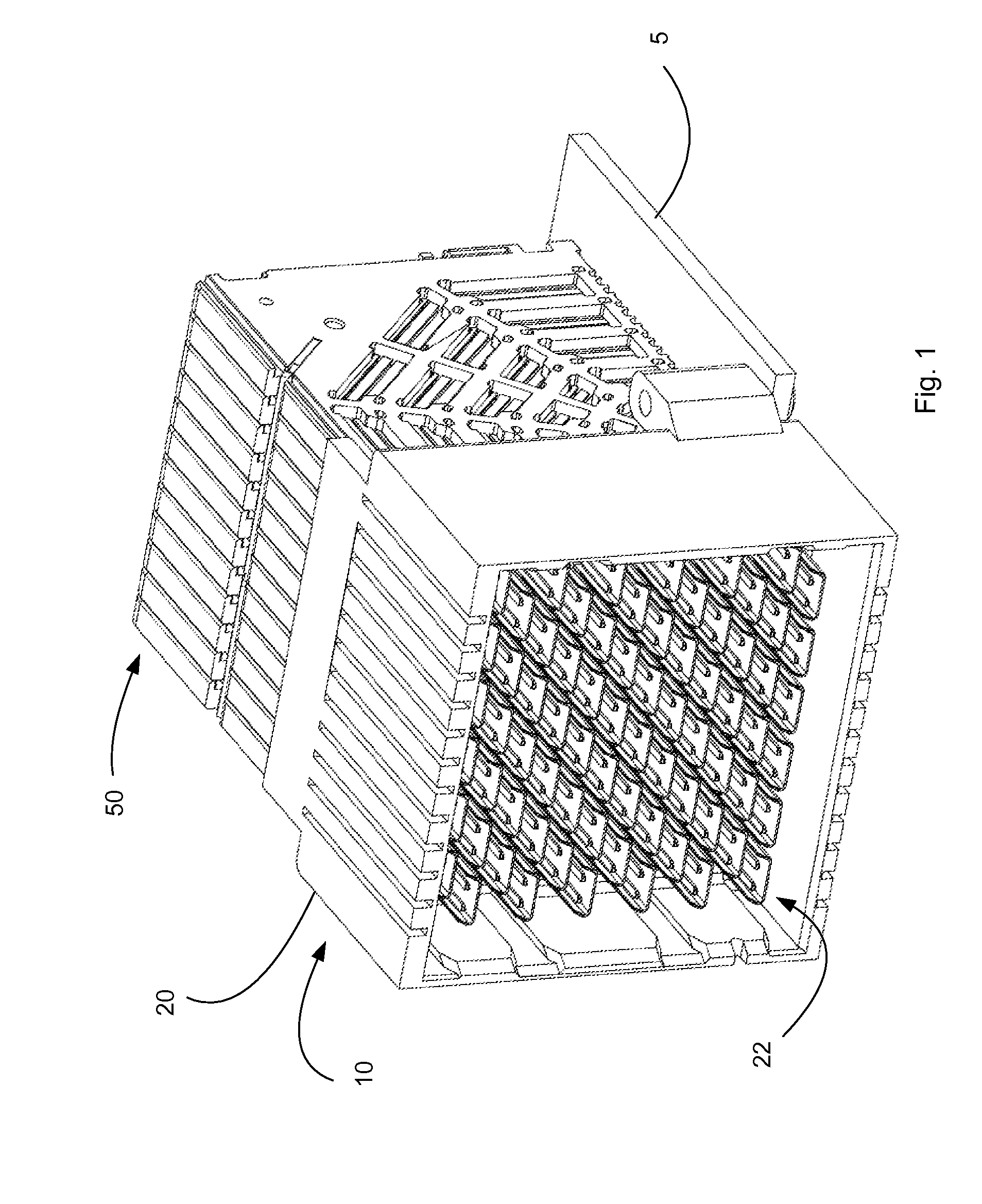
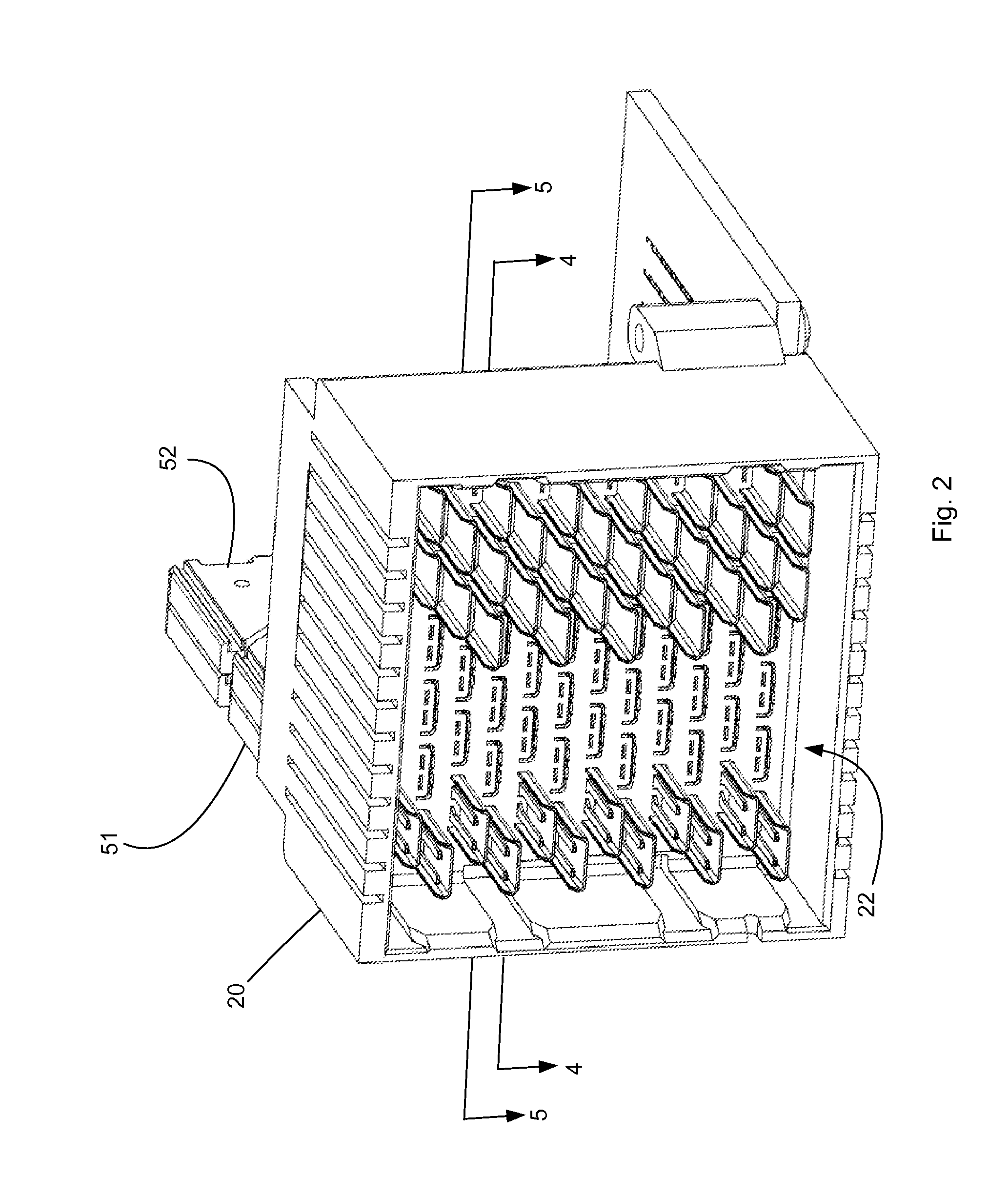
ActiveUS20160181732A1Maintain impedanceMaintain levelSecuring/insulating coupling contact membersCoupling protective earth/shielding arrangementsEngineeringContact position
A connector is configured to provide a mating side that includes a 90 degree rotation about two different axis when compared to a mounting. The connector, when mounted on a first circuit board is thus suitable for directly mating to a right-angle connector that is mounted on a second circuit board, the second circuit board at a being at a 90 angle to the first circuit board. The connector can include a shroud that supports a u-shield that partially shields contacts positioned in the mating side.
Owner:MOLEX INC
Guaranteed memory card performance to end-of-life
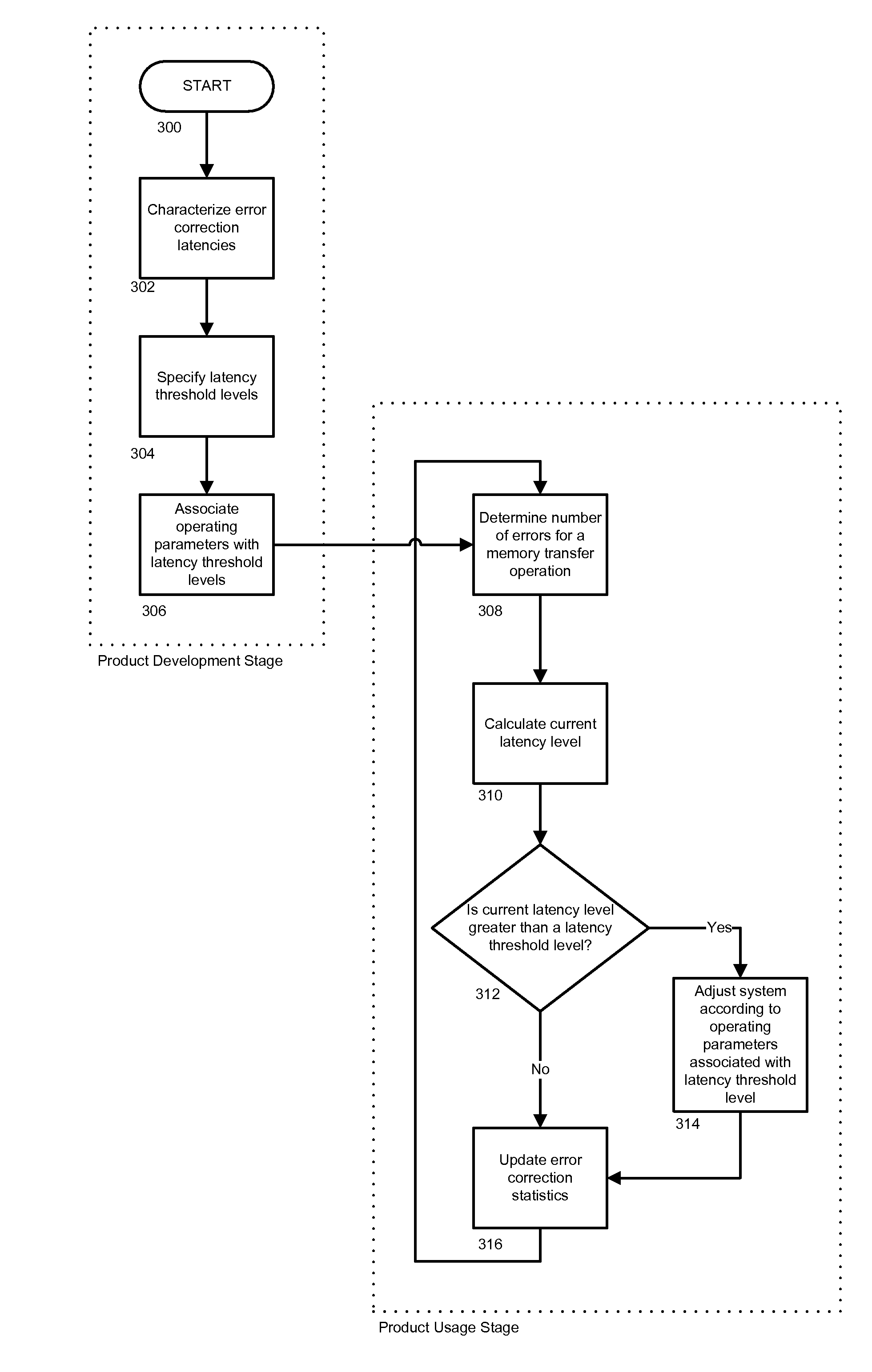
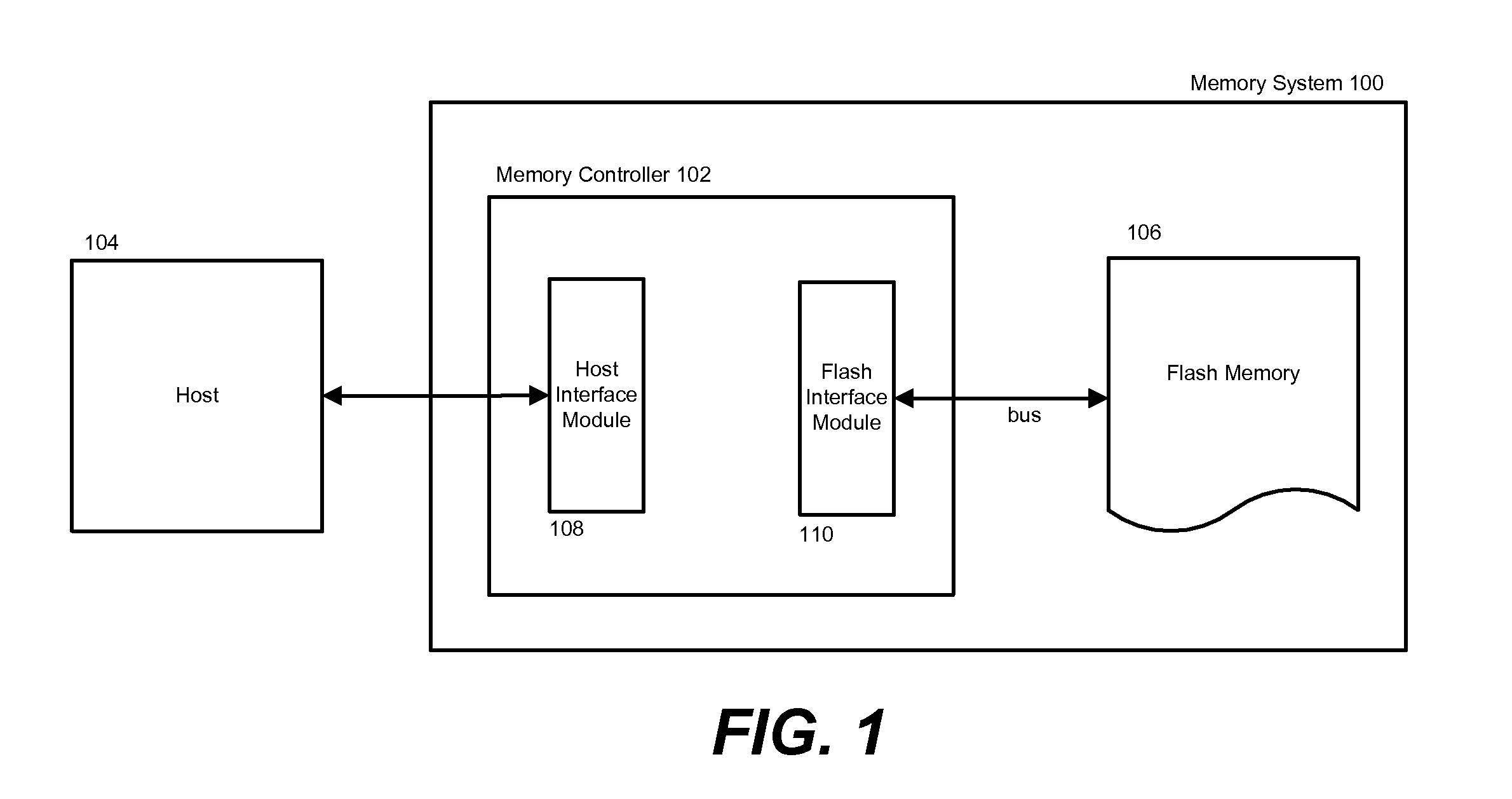
ActiveUS20090276570A1Quick correctionFast speedError detection/correctionMemory systemsReal-time computingMemory cards
In order to maintain a memory system's performance levels to its end-of-life, latency threshold level(s) are specified and associated with different memory system operating parameters. In one embodiment, the memory system monitors and gathers performance statistics in real time, and in accordance with specific memory transfer sizes. A current latency level can be dynamically calculated using the performance statistics and compared to previously established latency threshold levels. If the current latency level is greater than or equal to a specific latency threshold level, the memory system's configuration setting can be adjusted according to the operating parameters associated with the latency threshold level to offset the increased latency.
Owner:SANDISK TECH LLC
Controlled release formulations using intelligent polymers
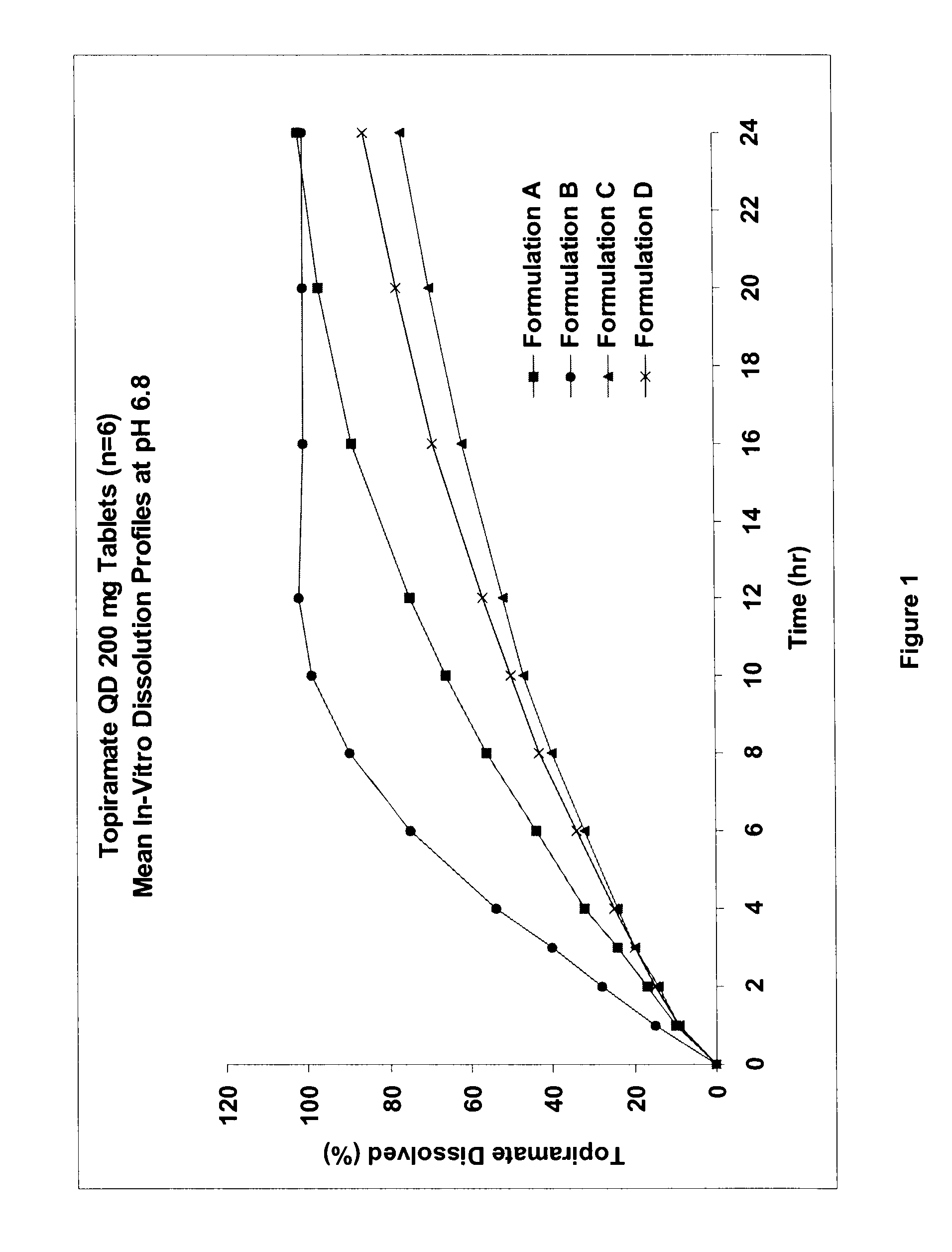
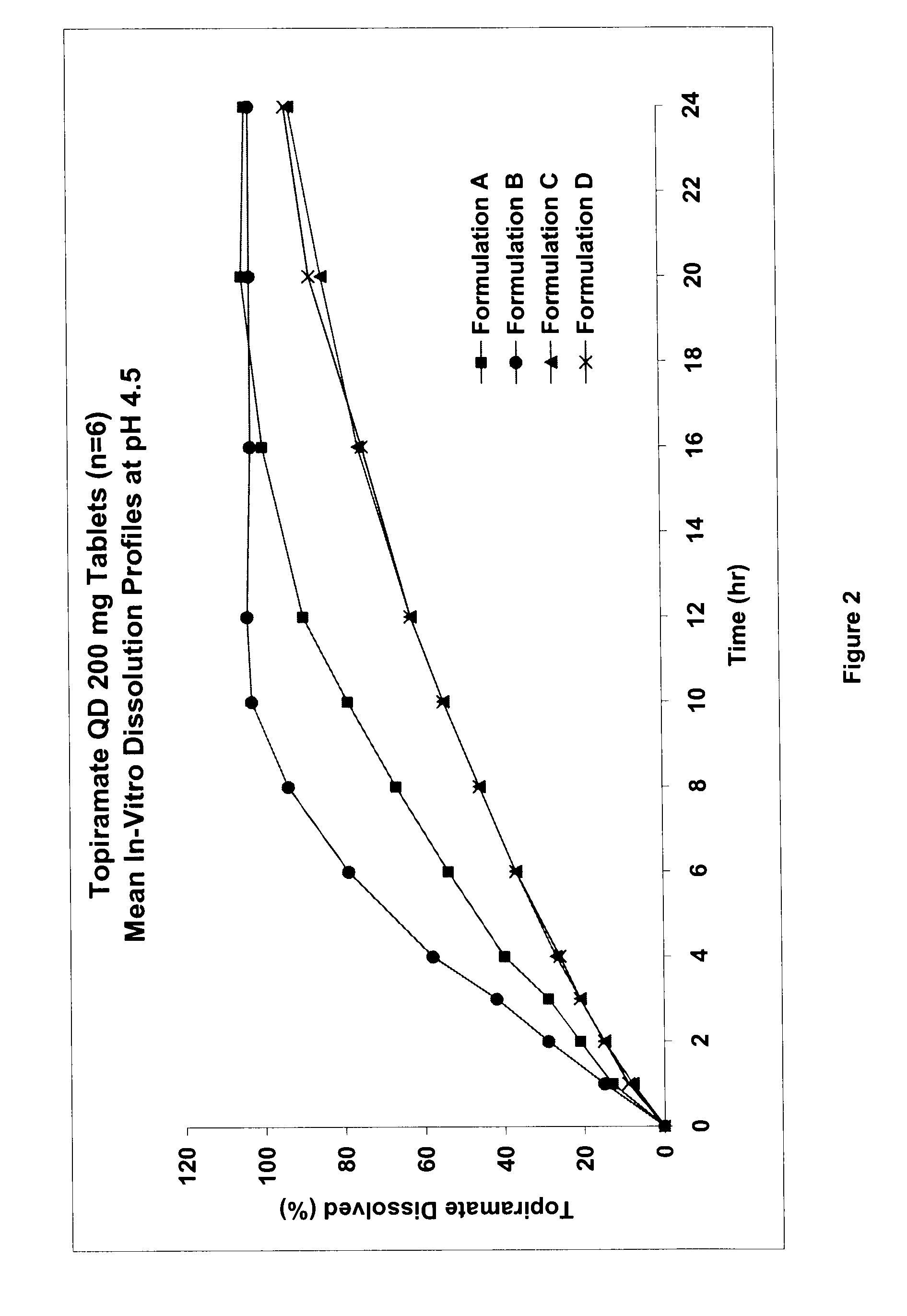
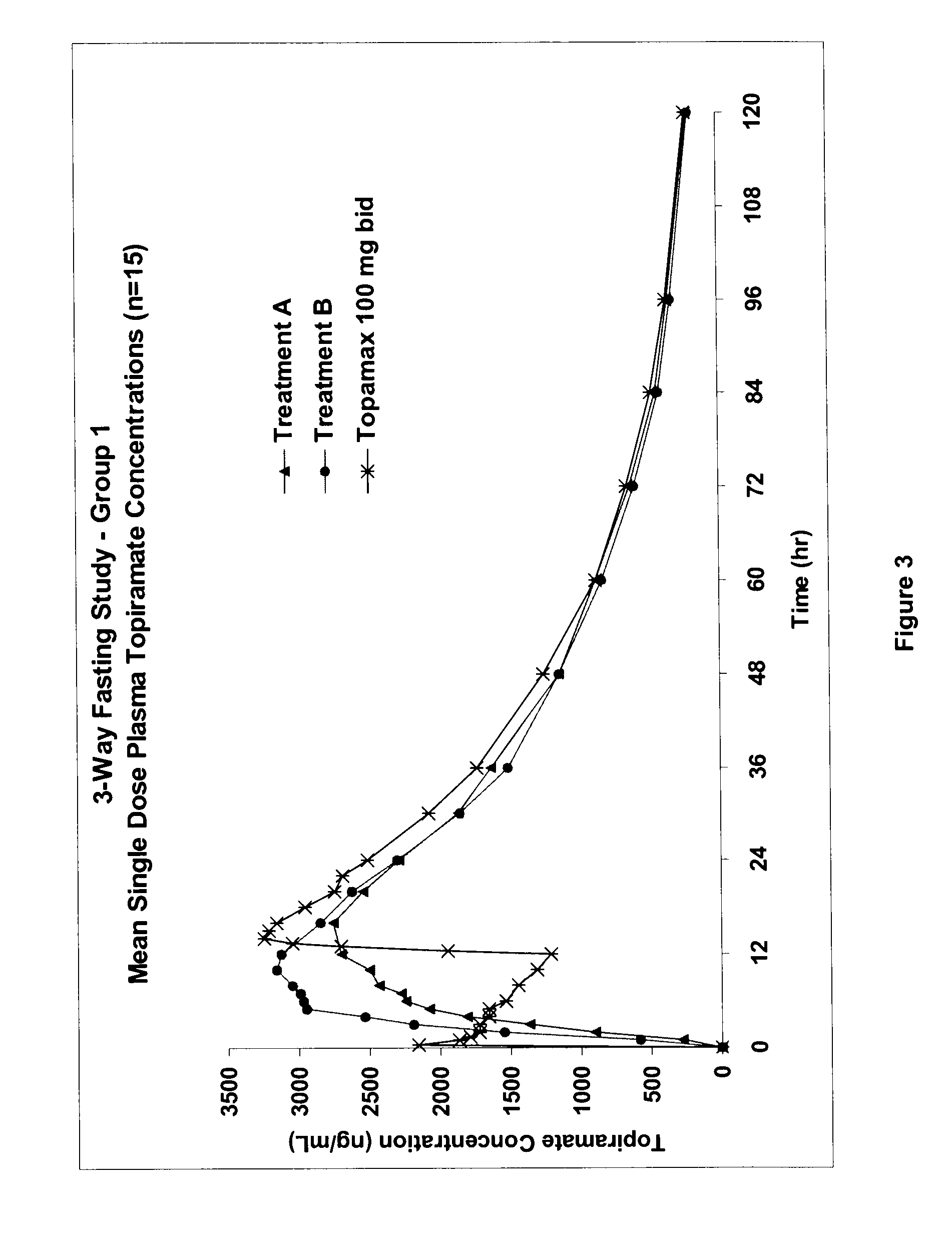
InactiveUS20080292700A1Prevent burstImparts gastrointestinal stealth characteristicNervous disorderMetabolism disorderSmart polymerTopiramate
A controlled release pharmaceutical composition comprises (a) topiramate or a pharmaceutically acceptable salt thereof, (b) a first intelligent polymer component; and (c) a second intelligent polymer component having opposite wettability characteristics to the first intelligent polymer component. The polymer components are effective for controlled release of the pharmaceutically active substance from the composition.
Owner:VALEANT INT BARBADOS
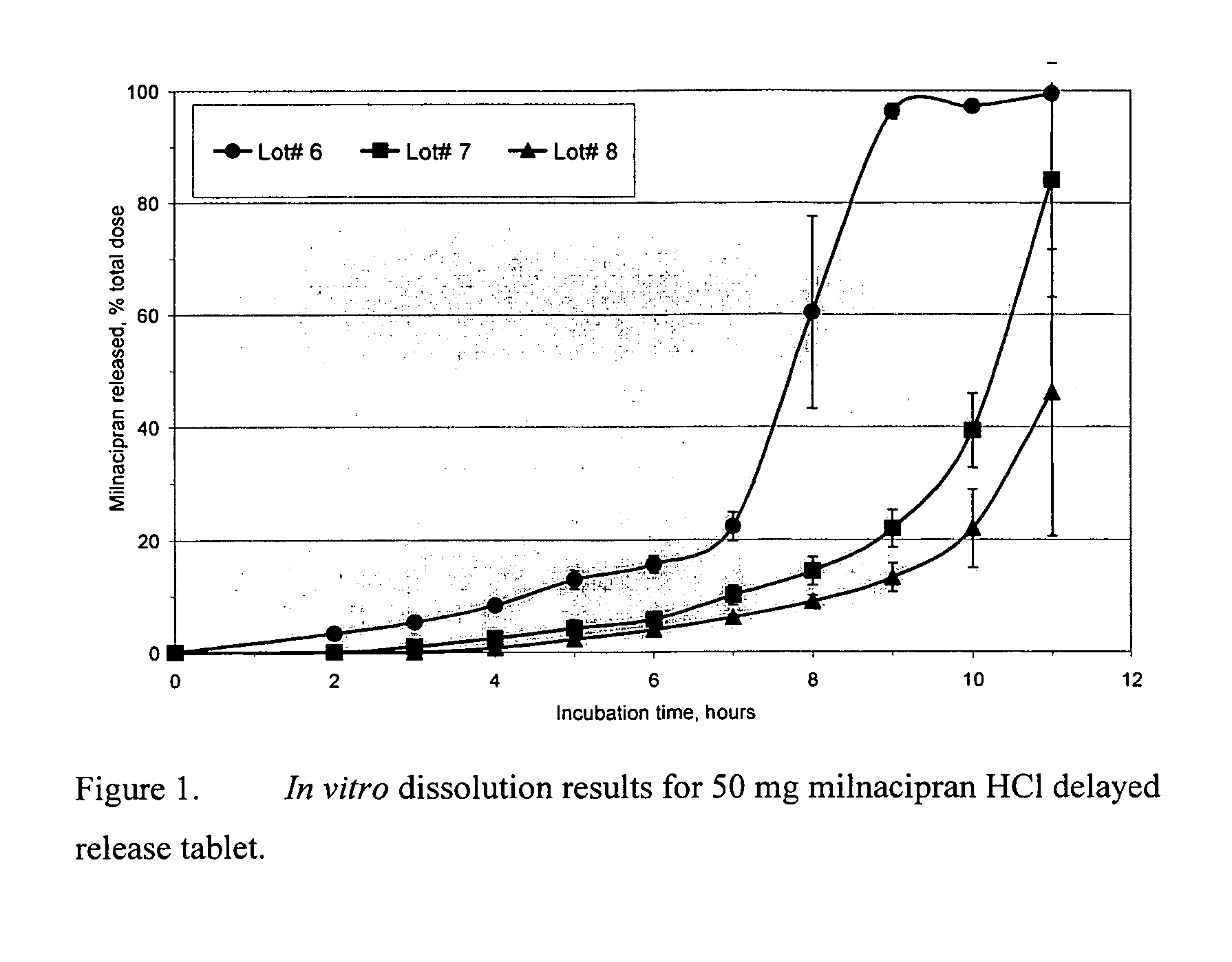
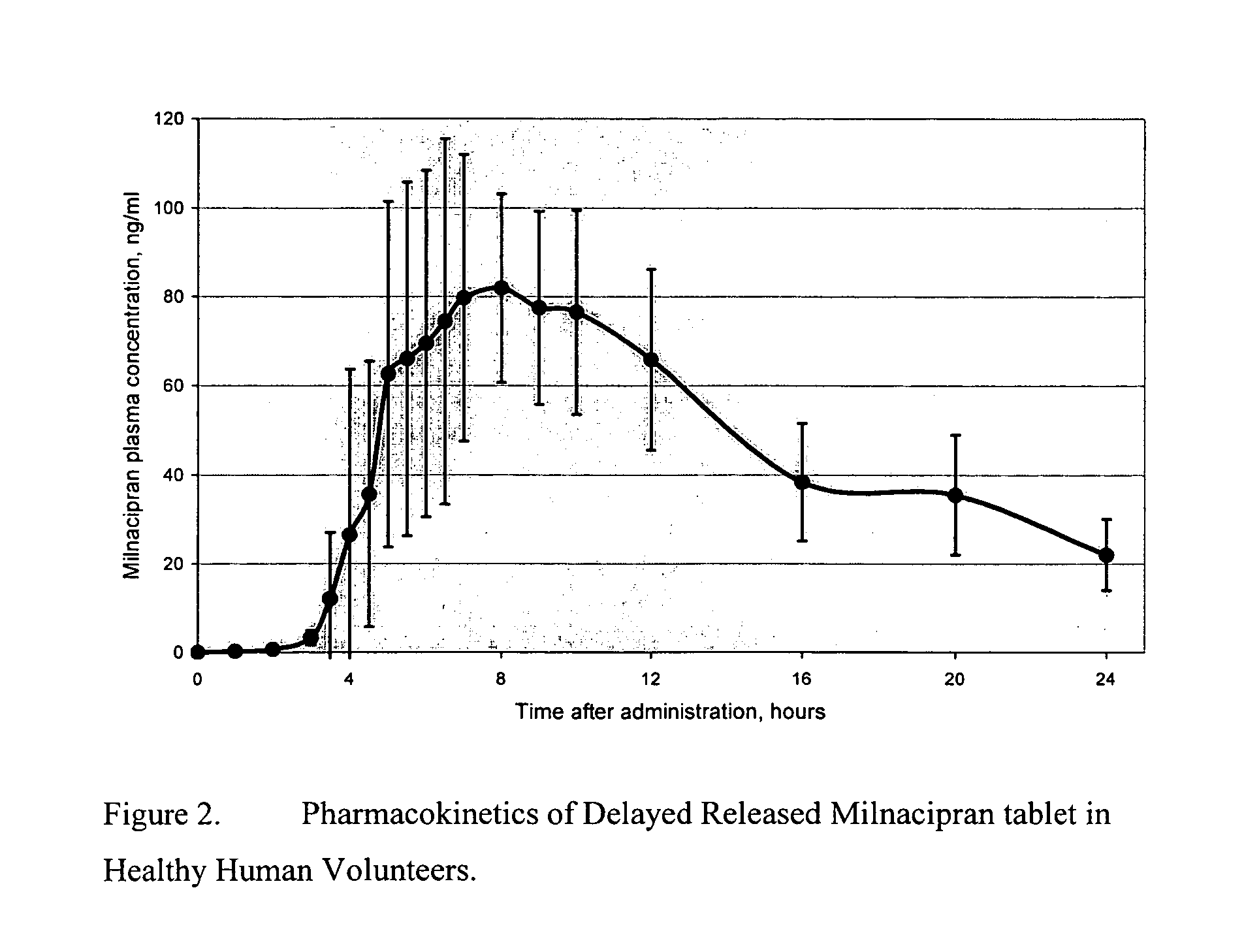
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
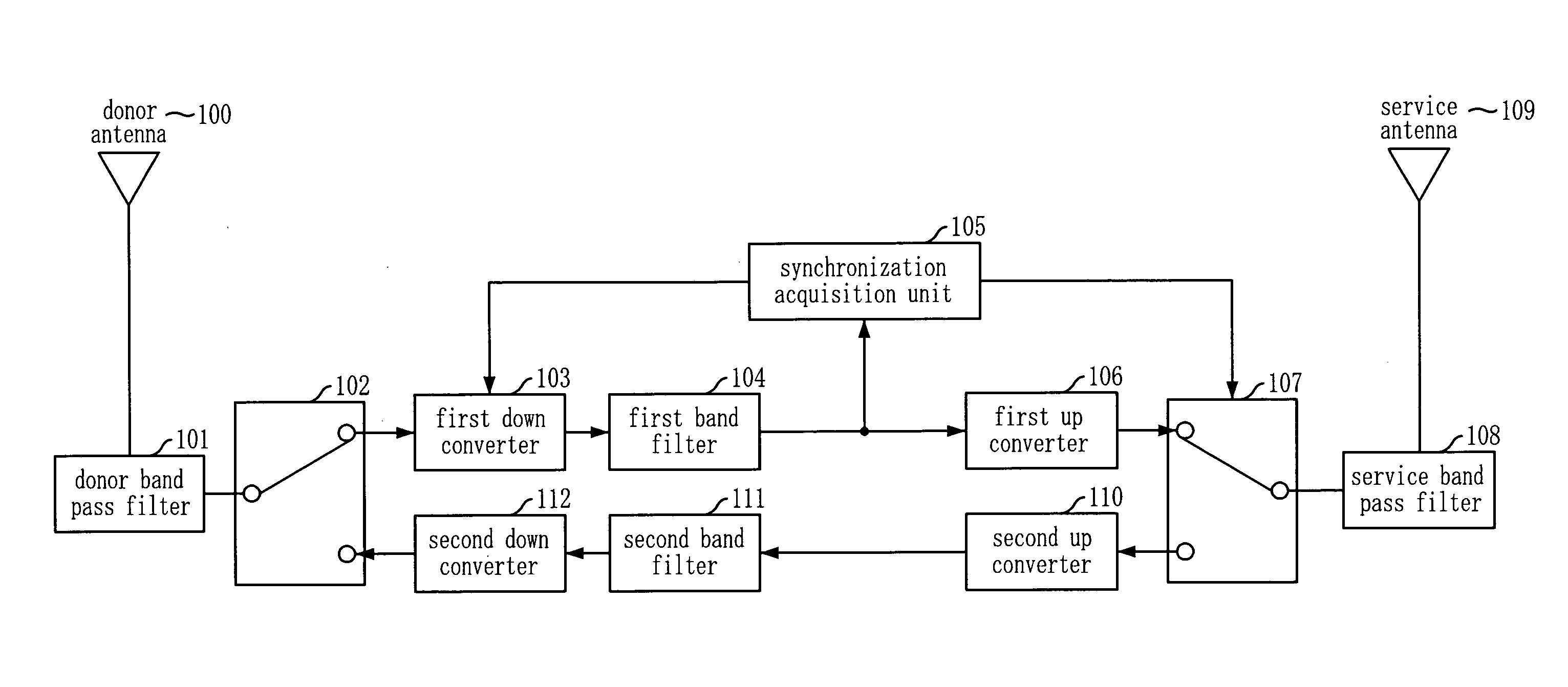
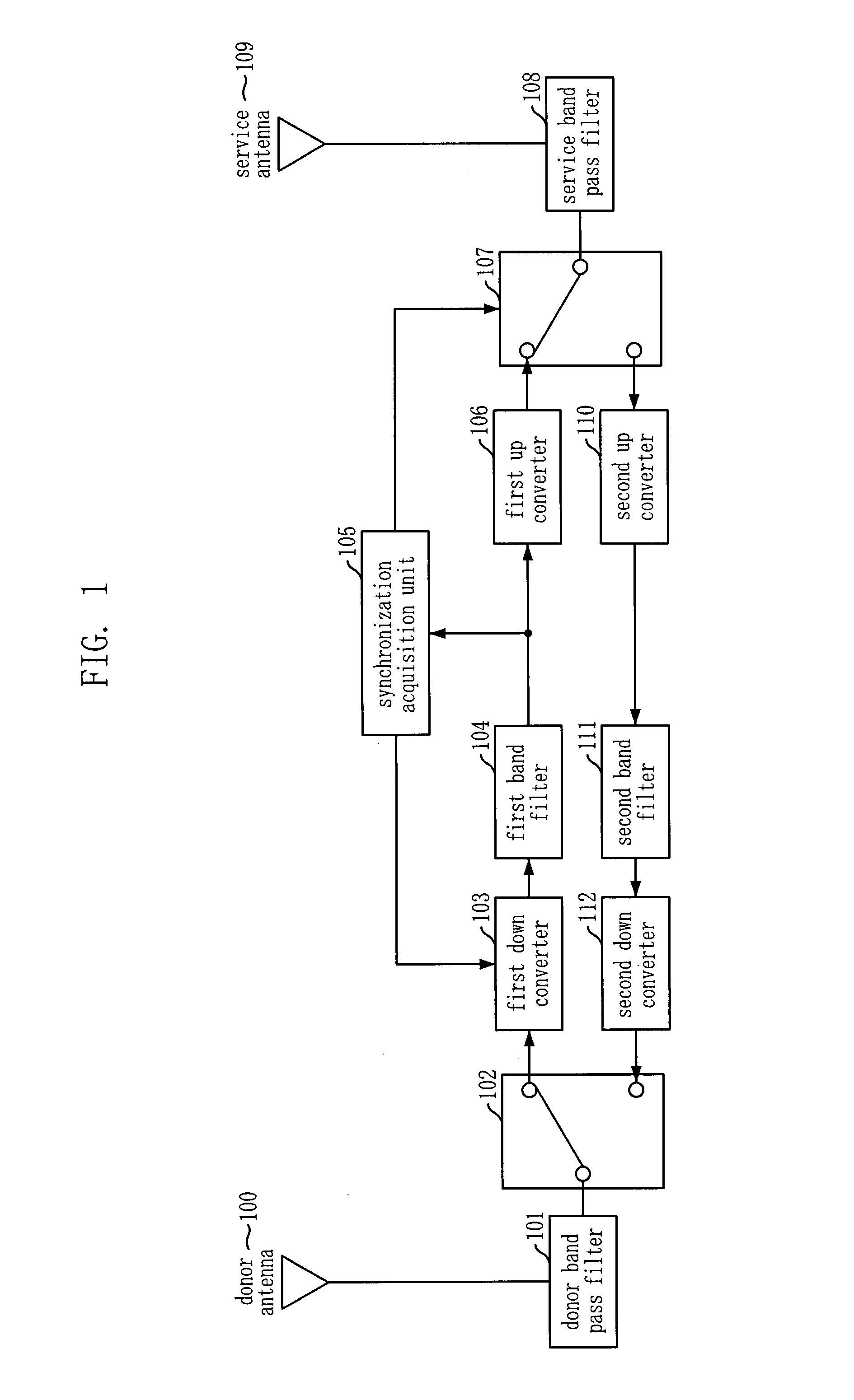
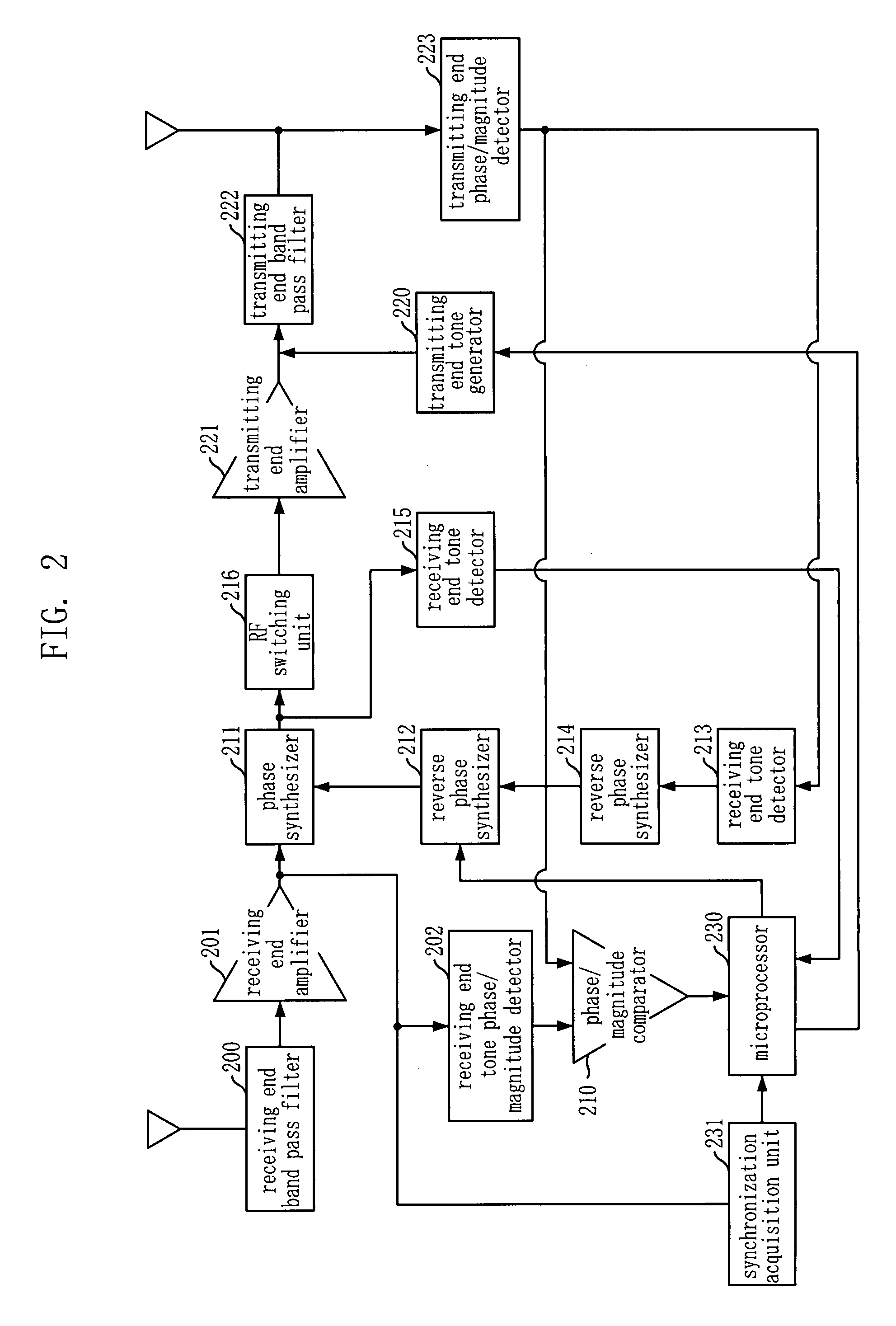
Adaptive Forward Error Corrector And Method Thereof, And TDD Radio Repeating Apparatus Using The Same
InactiveUS20090207776A1Efficient removalMinimizes installation costEar treatmentModulated-carrier systemsAutomatic controlSelf adaptive
An adaptive feedback estimation and cancellation (AFEC) apparatus includes: a controller for generating and outputting control information by using a synchronization signal from an external synchronization acquisition unit and base station information, in order to remove a feedback signal that exists in a forward / reverse repeater signal to be repeated and then send the forward / reverse repeater signal; a first feedback prediction canceller for adaptively removing a feedback signal that exists in the forward repeater signal based on the control information from the controller and automatically adjusting the gain of the forward repeater signal; and a second feedback prediction canceller for adaptively removing a feedback signal that exists in the reverse repeater signal based on the control information from the controller and automatically controlling the gain of the reverse repeater signal.
Owner:KT CORP +1
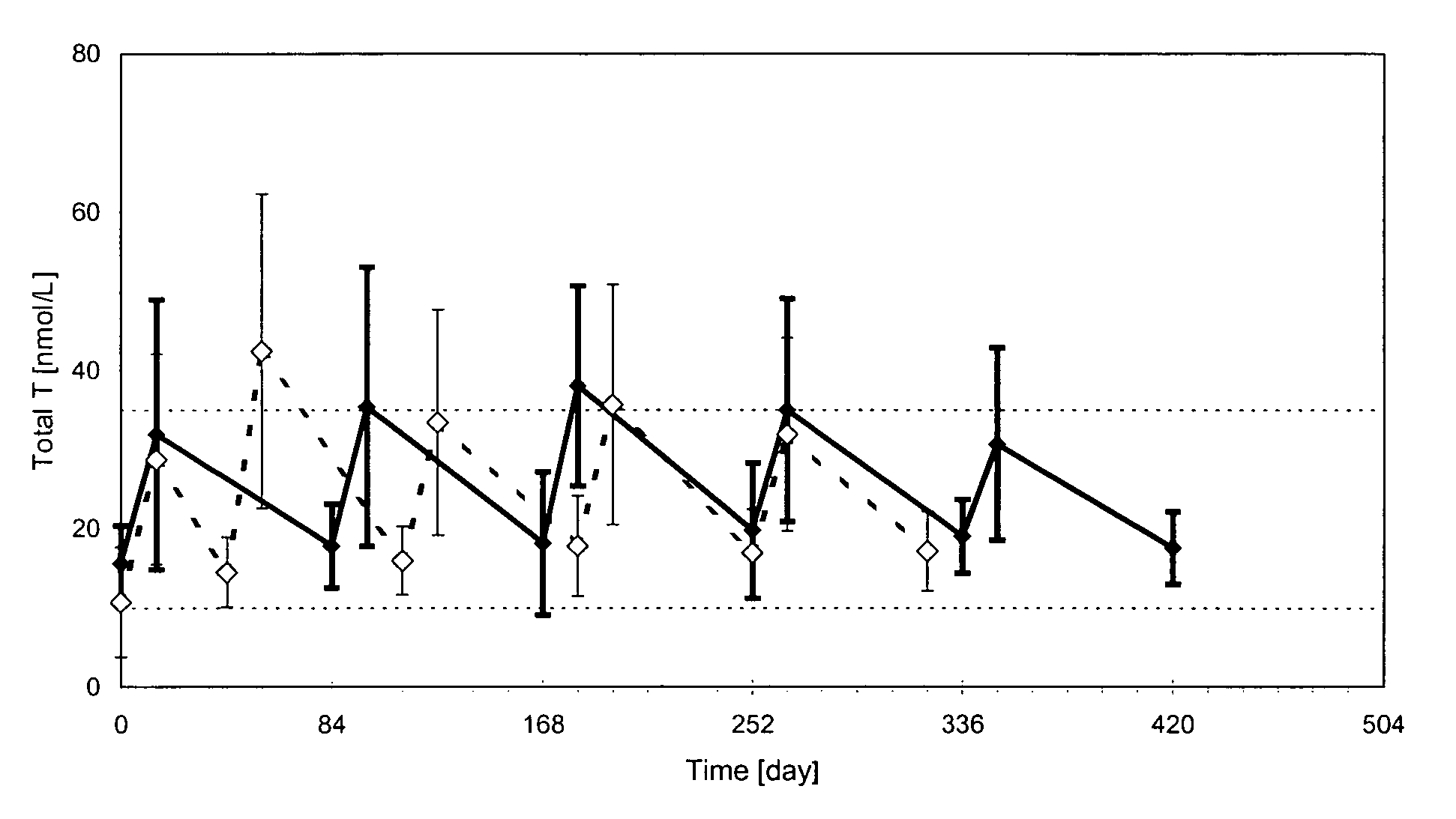
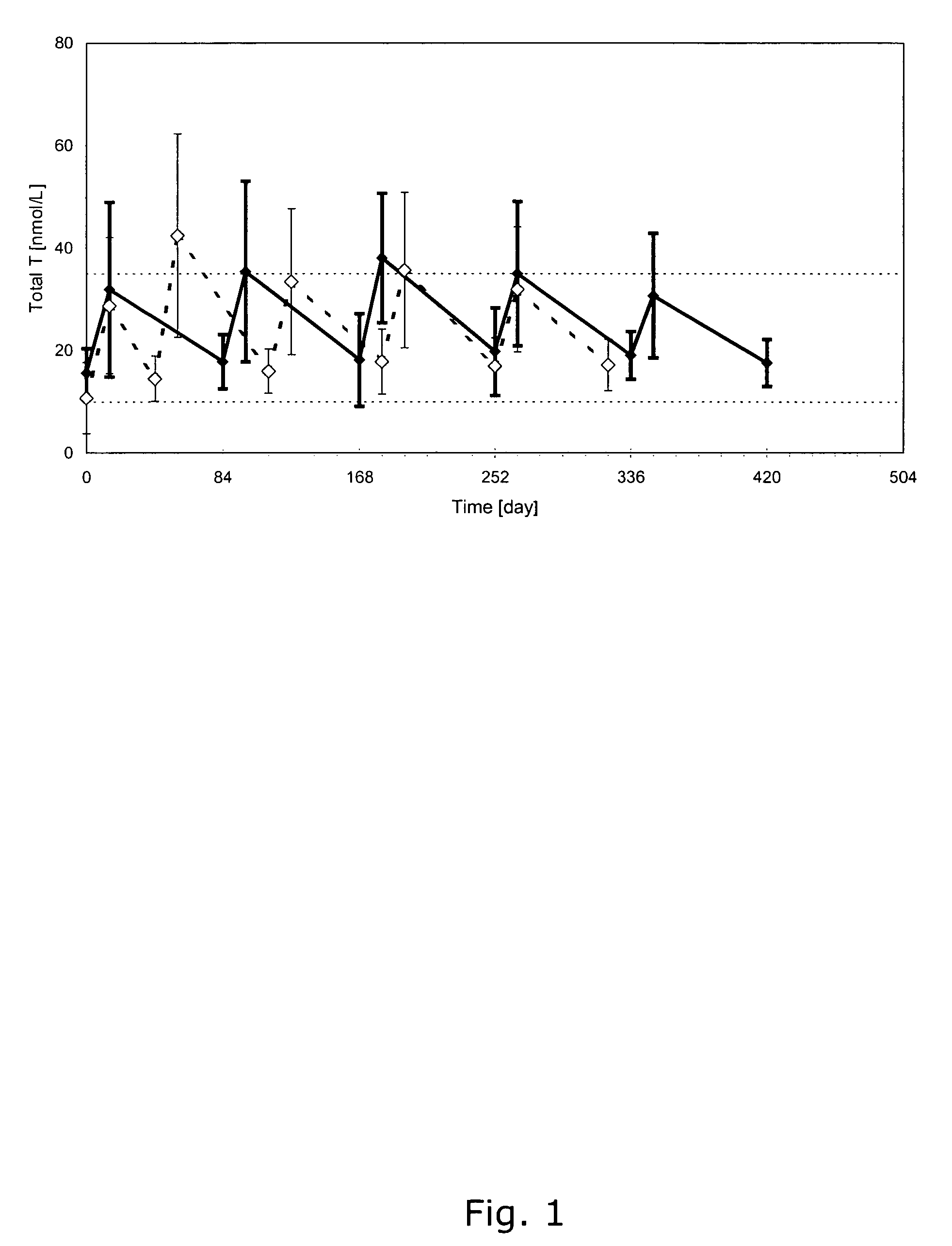
Methods and pharmaceutical compositions for reliable achievement of acceptable serum testosterone levels
The present invention relates to pharmaceutical compositions, formulated for injectable administration, which comprises a testosterone ester, in particularly testosterone undecanoate, in a vehicle comprising castor oil and a co-solvent. Upon injecting the compositions according to a particular administration scheme, reliable levels of testosterone in serum in the normal physiological range is achieved for a long period. This allows for the use of the compositions in hormone replacement therapy and male contraception without concomitant monitoring of testosterone levels in serum by a physician.
Owner:SCHERING AG +1
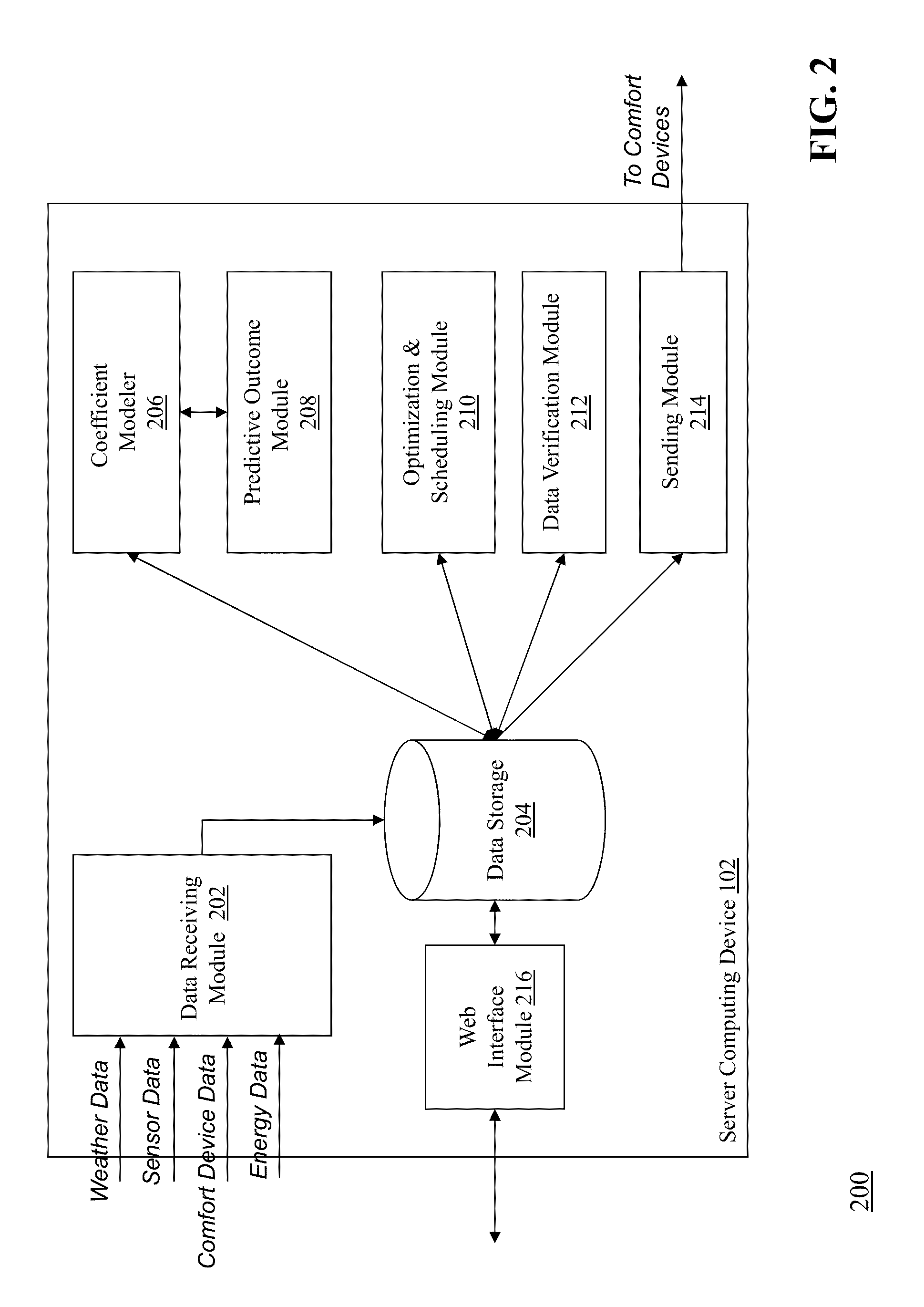
Optimizing and controlling the energy consumption of a building
ActiveUS9261863B2Optimize energy useLevel of comfortProgramme controlTemperature control using digital meansEnergy basedEngineering
Described herein are methods and systems, including computer program products, for optimizing and controlling the energy consumption of a building. A first computing device generates a set of thermal response coefficients for the building based on energy characteristics of the building and weather data associated with the location of the building. The first computing device predicts an energy response of the building based on the set of thermal response coefficients and forecasted weather associated with the location of the building. The first computing device selects minimal energy requirements of the building based on an energy consumption cost associated with the building. The first computing device determines one or more temperature set points for the building based on the energy response and the minimal energy requirements. The first computing device transmits the one or more temperature set points to a thermostat of the building.
Owner:UNIV OF MARYLAND +1
Measurement Patch Device
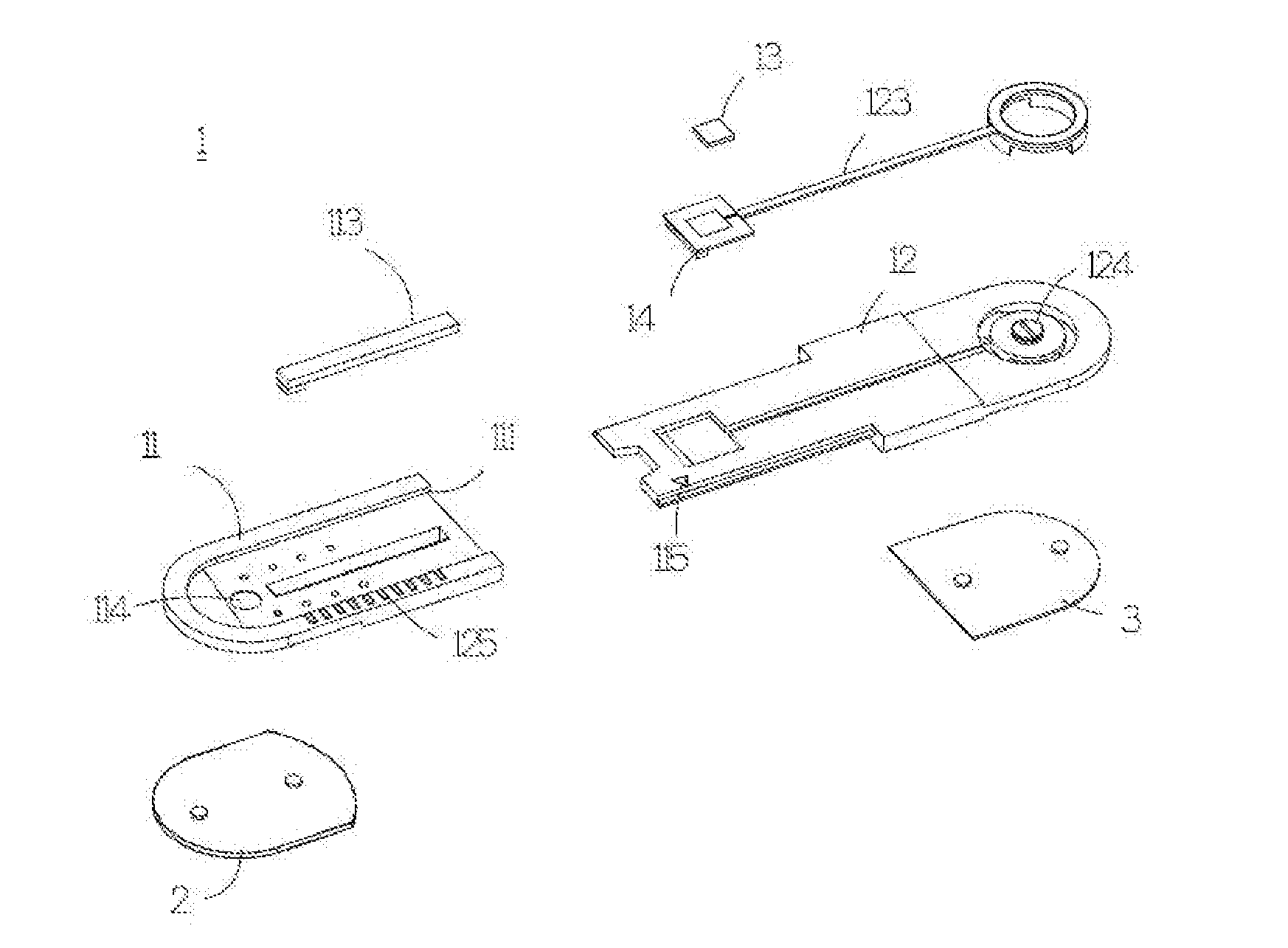
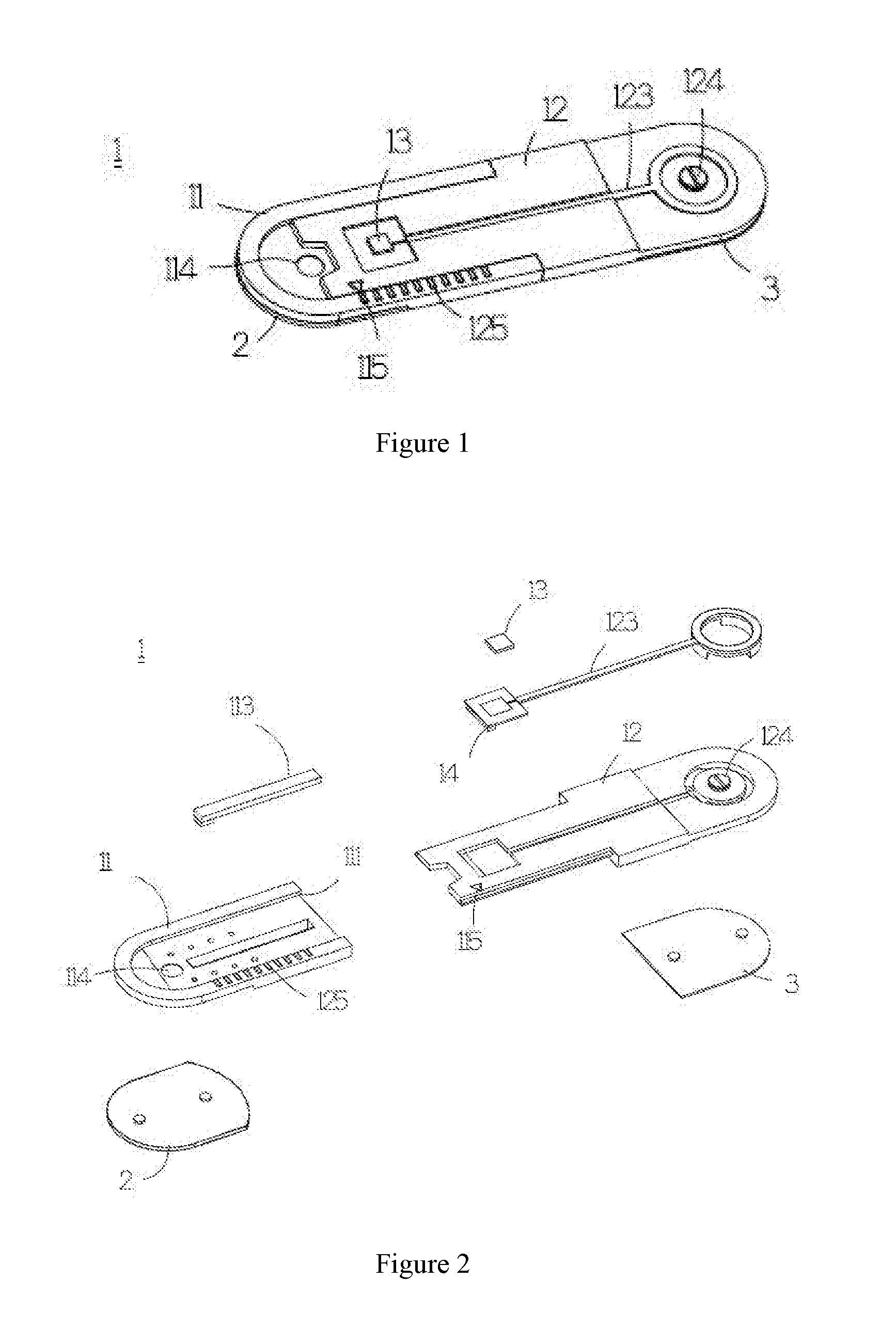
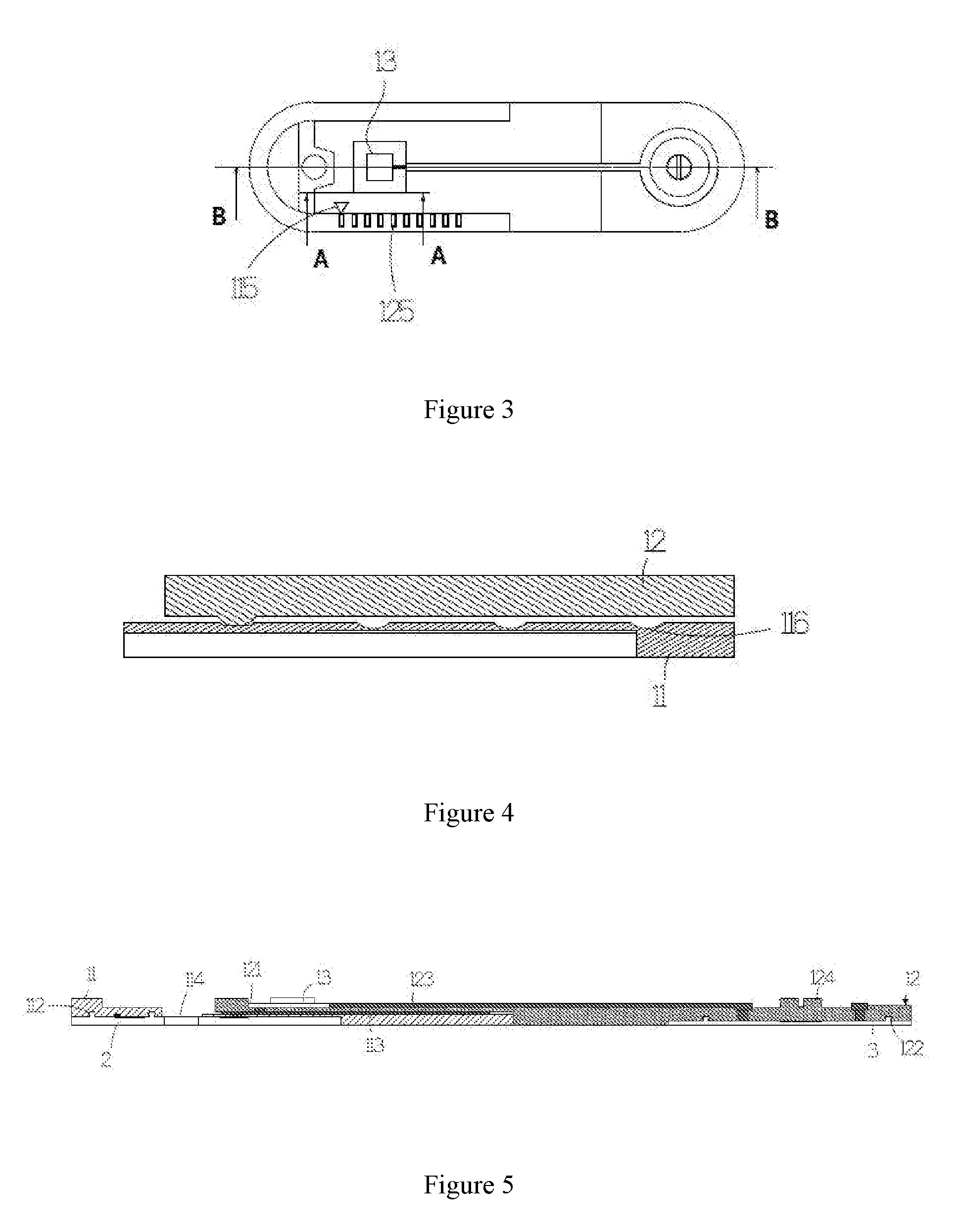
ActiveUS20160198972A1Maintain levelSave measurement timeElectrocardiographyElectro-oculographyEngineeringSomatotypes
A measurement patch device may change length according to somatotype of a user, and may be joined with another measurement patch device integrally according to the need of different physiological lead measurement. Moreover, the two joined measurement patch devices may rotate with respect to each other, such that multi-channel or multi-lead physiological signal may be measured to save measurement time of physiological signal.
Owner:LEE SHUENN YUH
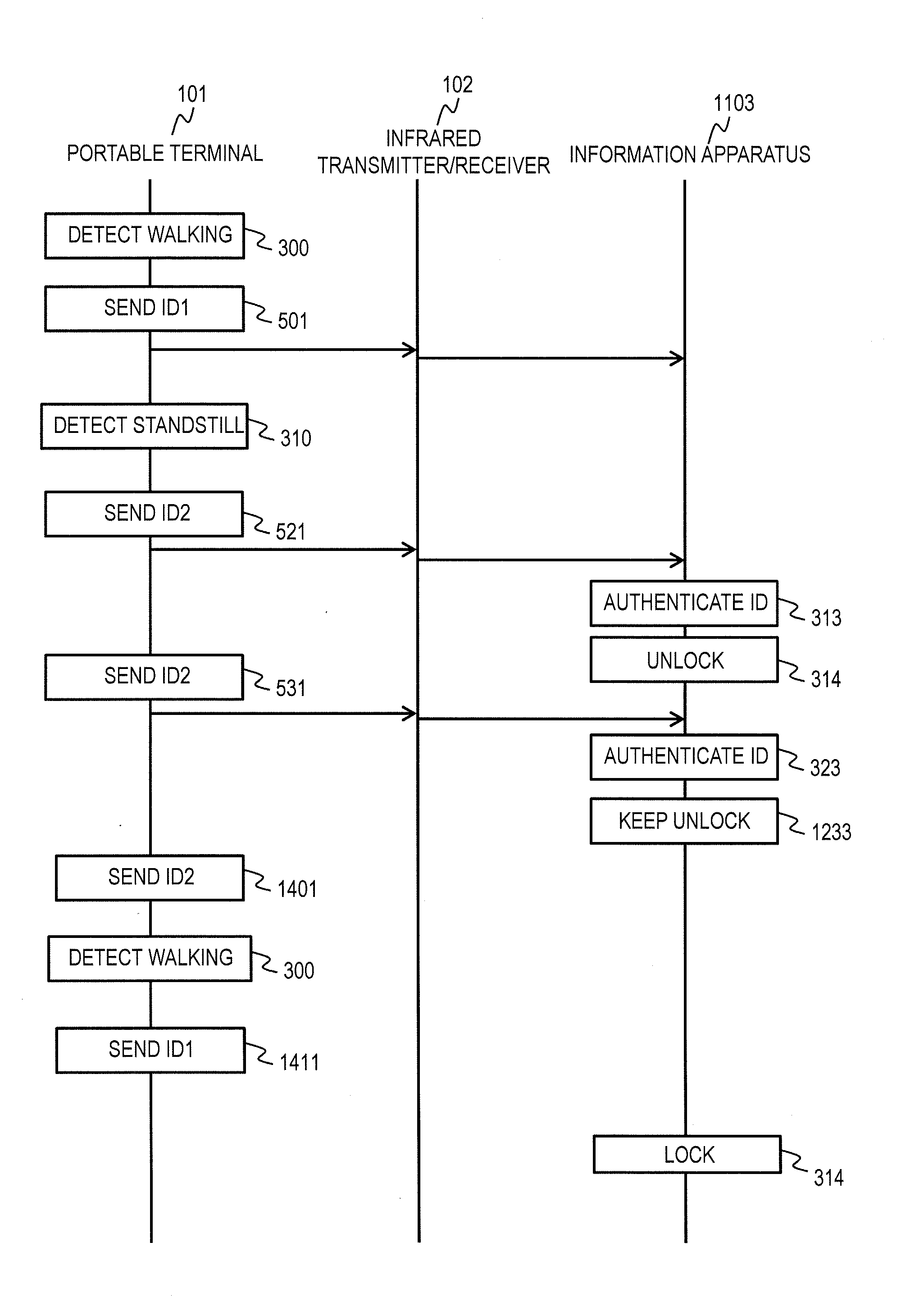
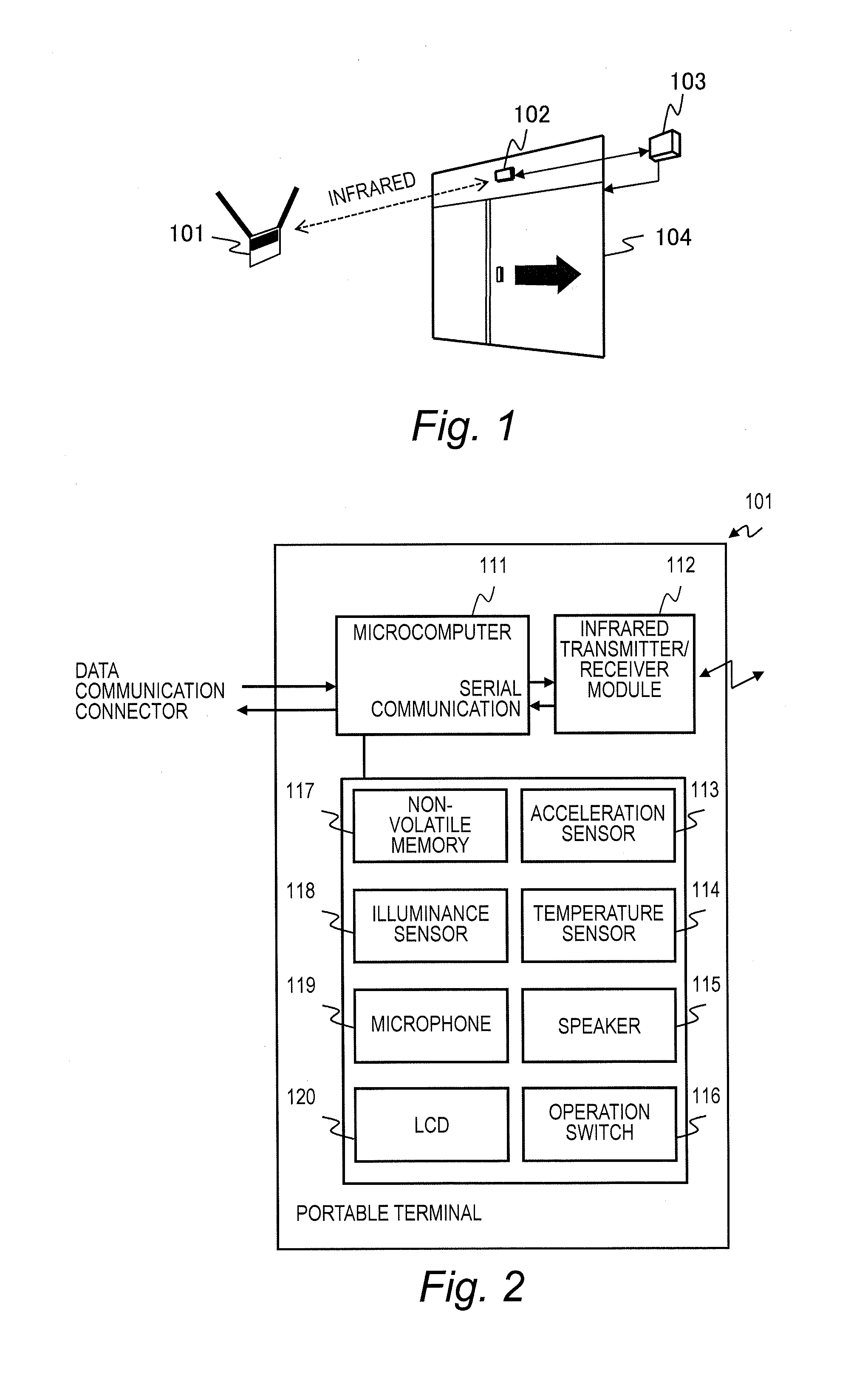
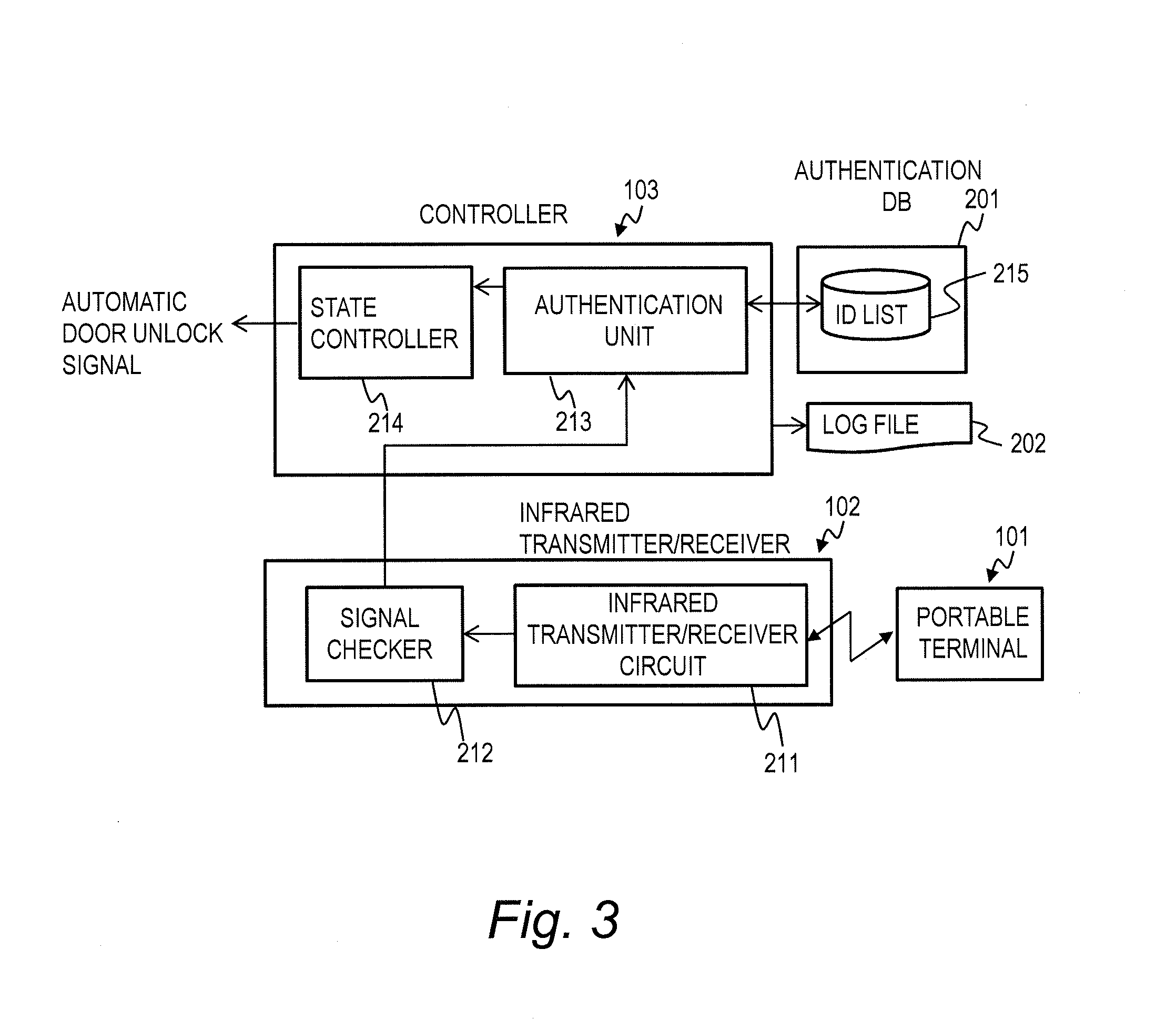
State Control System and State Control Method
InactiveUS20130272714A1Save power consumptionMaintain levelElectric signal transmission systemsNon-electrical signal transmission systemsComputer hardwareControl system
A state control system comprises a portable terminal and a control apparatus. The portable terminal sends a signal including identification information to an external. The control apparatus controls a controlled object in either one of a locked state and an unlocked state based on the signal sent from the portable terminal. The portable terminal sends a first signal, in a case where a event detector has not detected the event, and sends a second signal having a data length longer than a data length of the first signal, in a case where the event detector has detected the event. The control apparatus controls a controlled object to the unlocked state in a case where the controlled object is in the locked state and the controlled apparatus receives the second signal.
Owner:HITACHI LTD
Optimizing and controlling the energy consumption of a building
ActiveUS20150192911A1Optimize energy useEncouraging efficient energy usageMechanical apparatusLevel controlOperating energyEnergy control
Described herein are methods and systems, including computer program products, for optimizing and controlling a building's energy consumption and comfort. A computing device receives measurements from a plurality of sensors, at least some of which are located inside the building, where the measurements include temperature readings and comfort characteristics. The computing device generates a set of thermal response coefficients based on energy characteristics of the building, the measurements from the sensors, and weather data associated with the building's location. The computing device predicts an energy response of the building based on the set of thermal response coefficients and forecasted weather. The computing device selects minimal energy requirements of the building based on an energy consumption cost associated with the building and determines energy control points based on the energy response and the minimal energy requirements. The computing device transmits the energy control points to comfort devices in the building.
Owner:UNIV OF MARYLAND +1
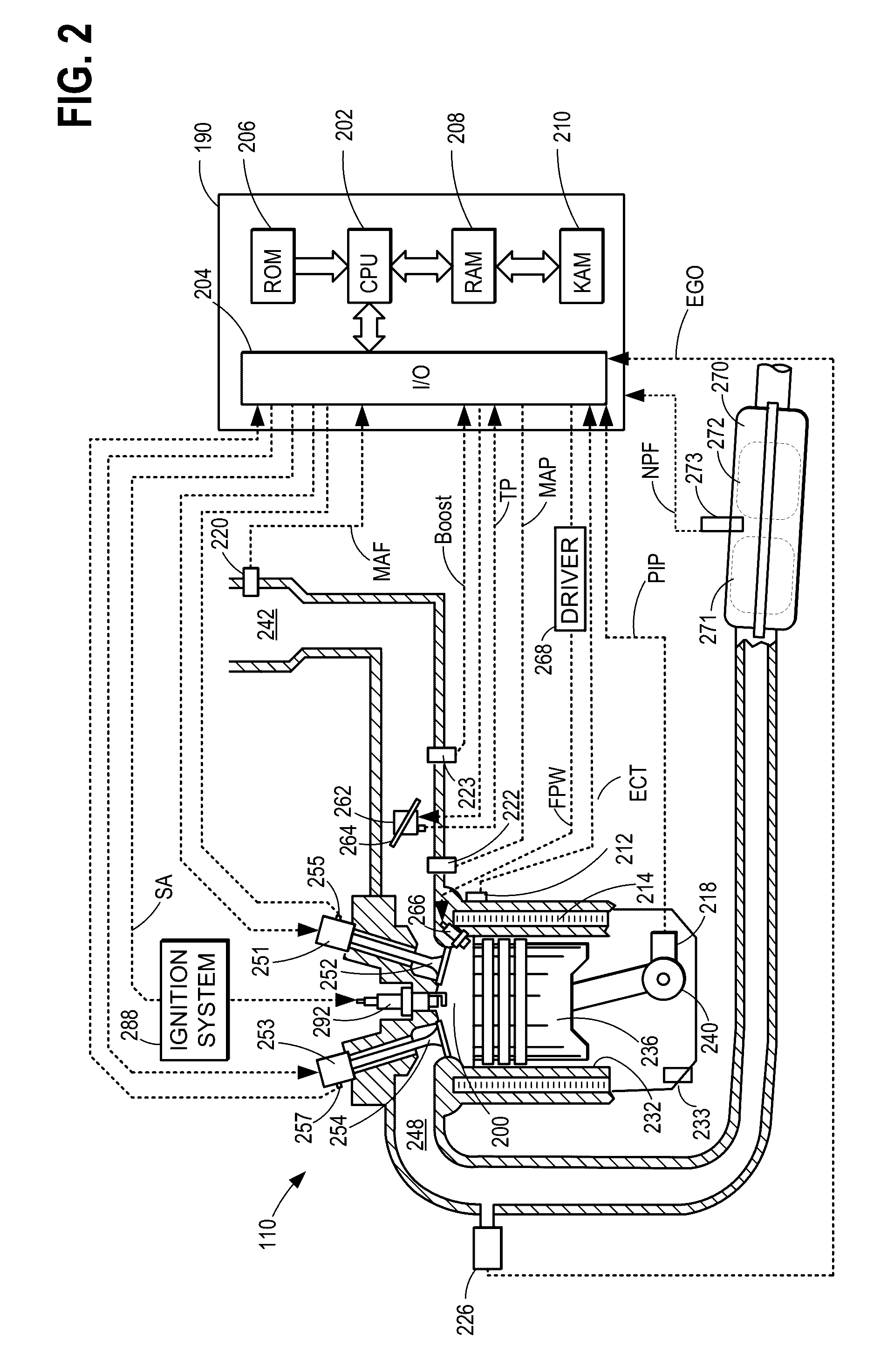
System and methods for reducing particulate matter emissions
ActiveUS20160363019A1Manufacturing cost is increasedImprove filtration efficiencyNon-fuel substance addition to fuelExhaust apparatusAutomotive engineeringEngineering
A method for a vehicle comprises responsive to installation of a new exhaust particulate filter, doping fuel with an ash-producing additive, and combusting the doped fuel to produce ash, wherein the ash deposits as an ash coating on the new exhaust particulate filter. In this way, a filtration efficiency of an exhaust particulate filter can be increased quickly as compared to a filter with no deposited ash coating, inexpensively as compared to conventional methods using membranes, and with a lower back pressure drop as compared to conventional methods.
Owner:FORD GLOBAL TECH LLC
Controlled release formulations using intelligent polymers
InactiveUS20050214368A1Promote absorptionMaintain levelOrganic active ingredientsBiocideSmart polymerWater contact
A controlled release pharmaceutical composition comprises (a) at least one pharmaceutically active substance having a water contact angle (θ) such that cos θ is between +0.9848 and −0.9848, (b) a first intelligent polymer component; and (c) a second intelligent polymer component having opposite wettability characteristics to the first intelligent polymer component, the first and second polymer components being present in a ratio in the range of about 1:100 to about 100:1 by weight. The polymer components are effective for controlled release of the pharmaceutically active substance from the composition.
Owner:BIOVAIL LAB INT SRL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com