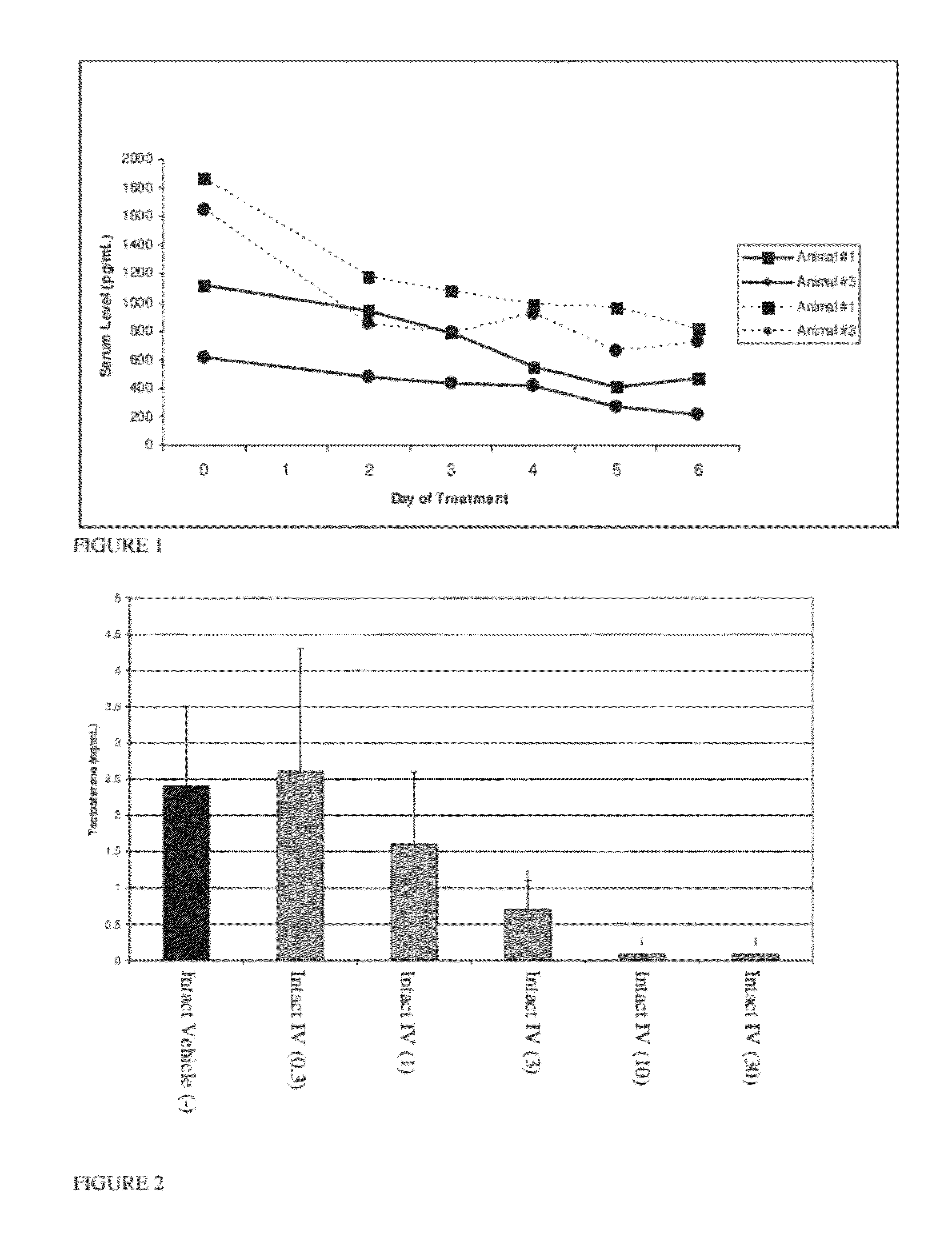
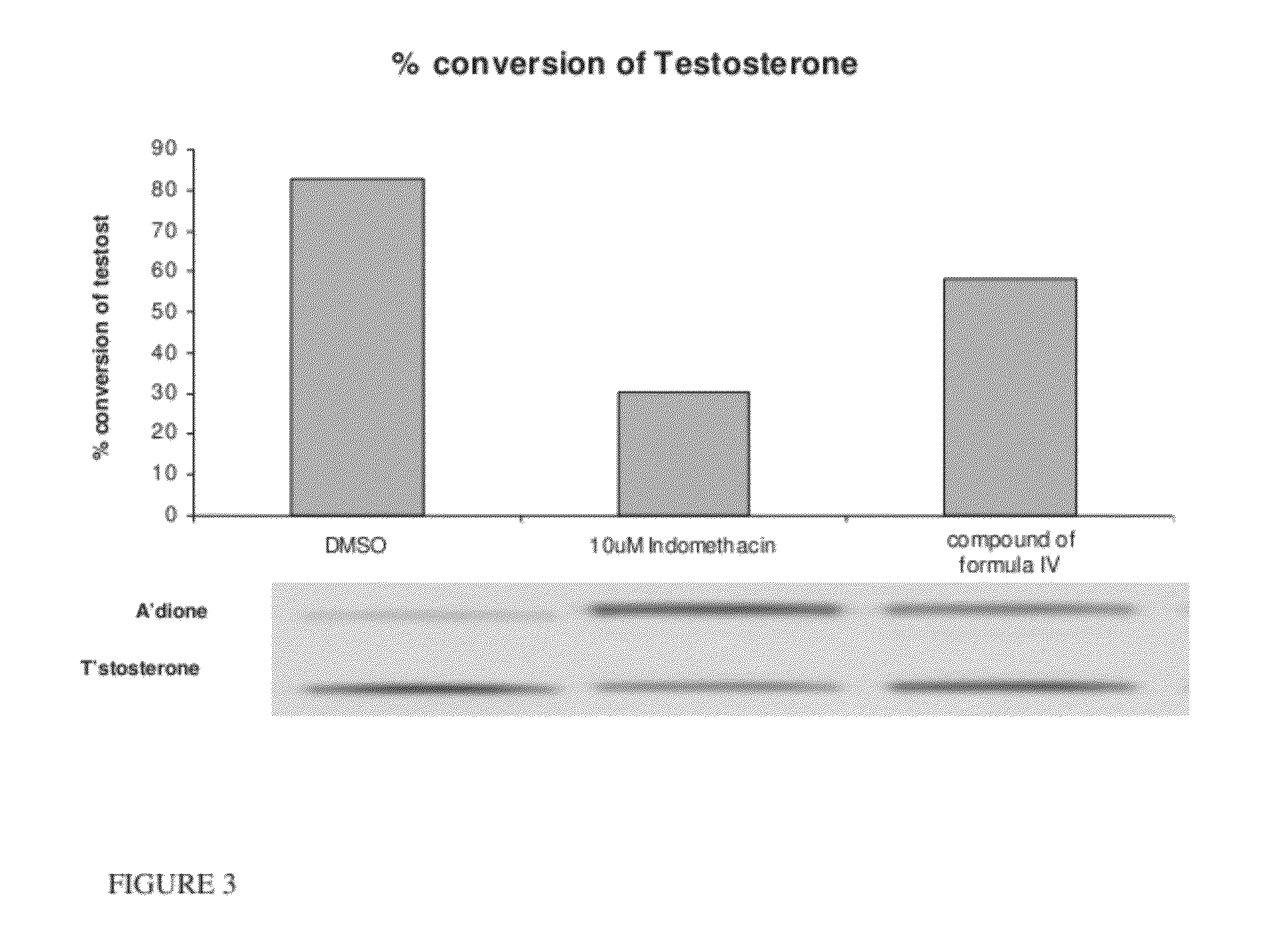
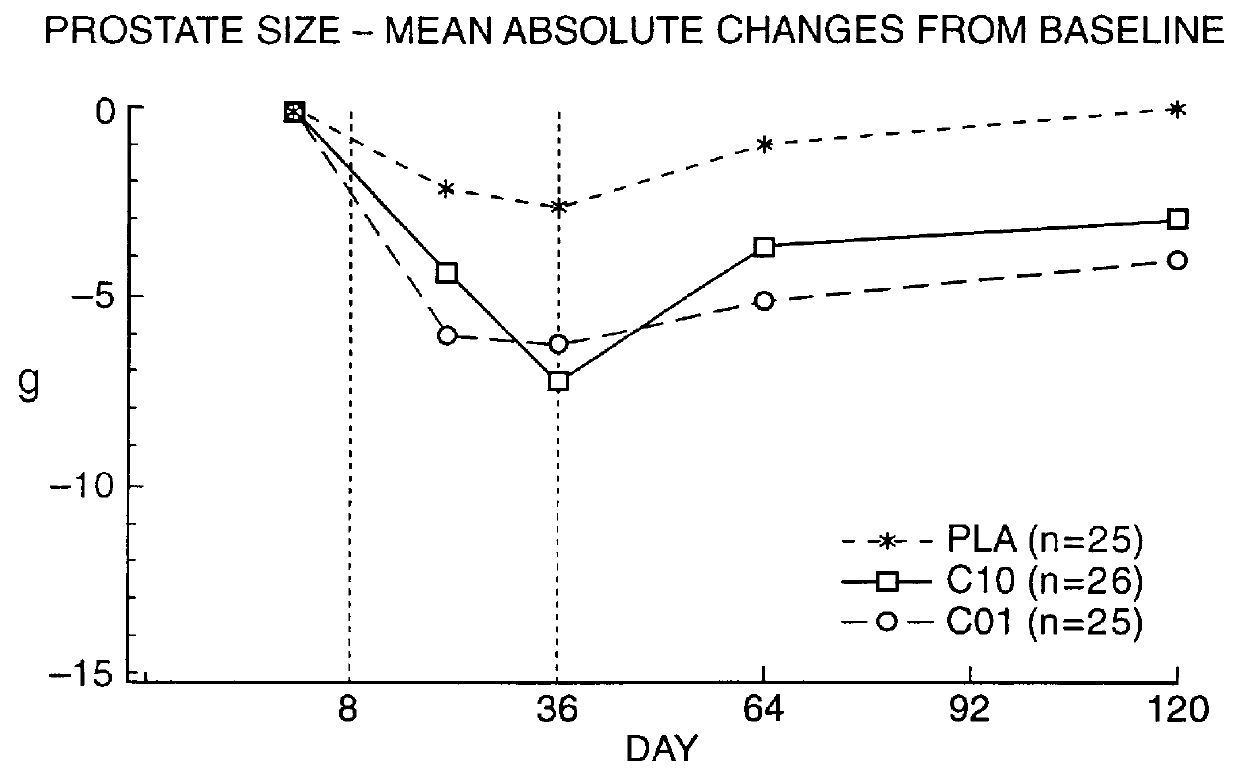
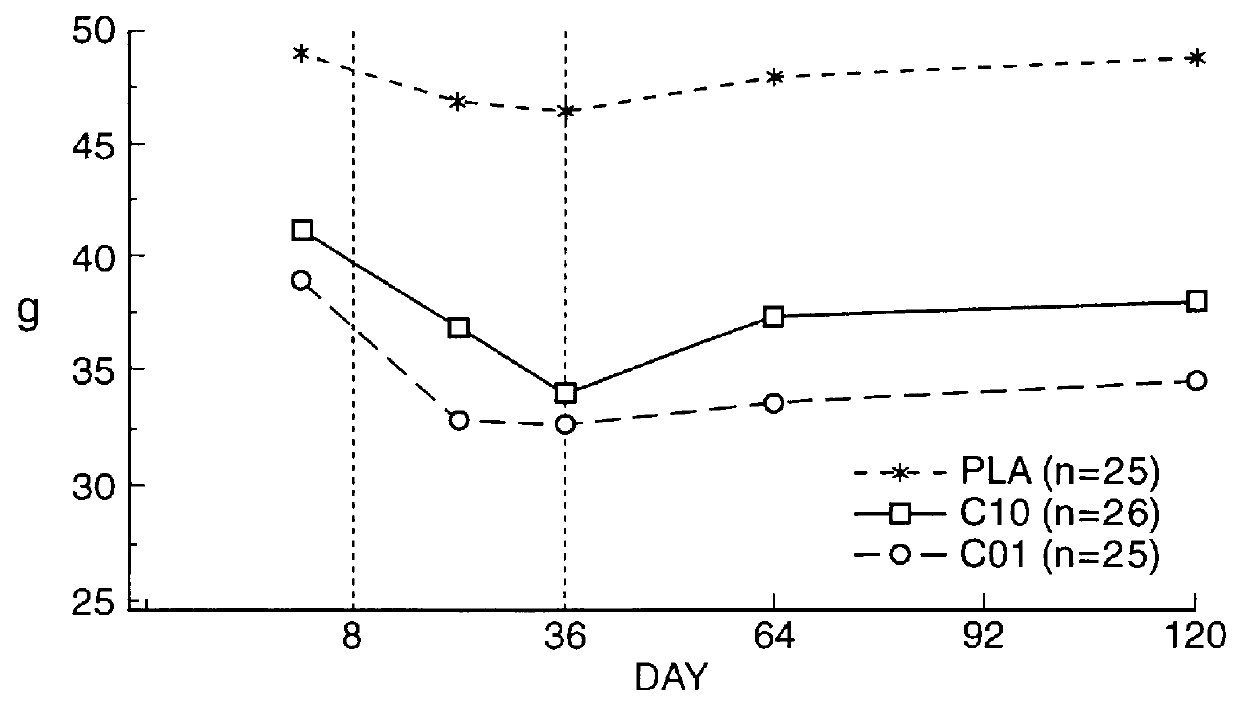
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
90 results about "Testosterone level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
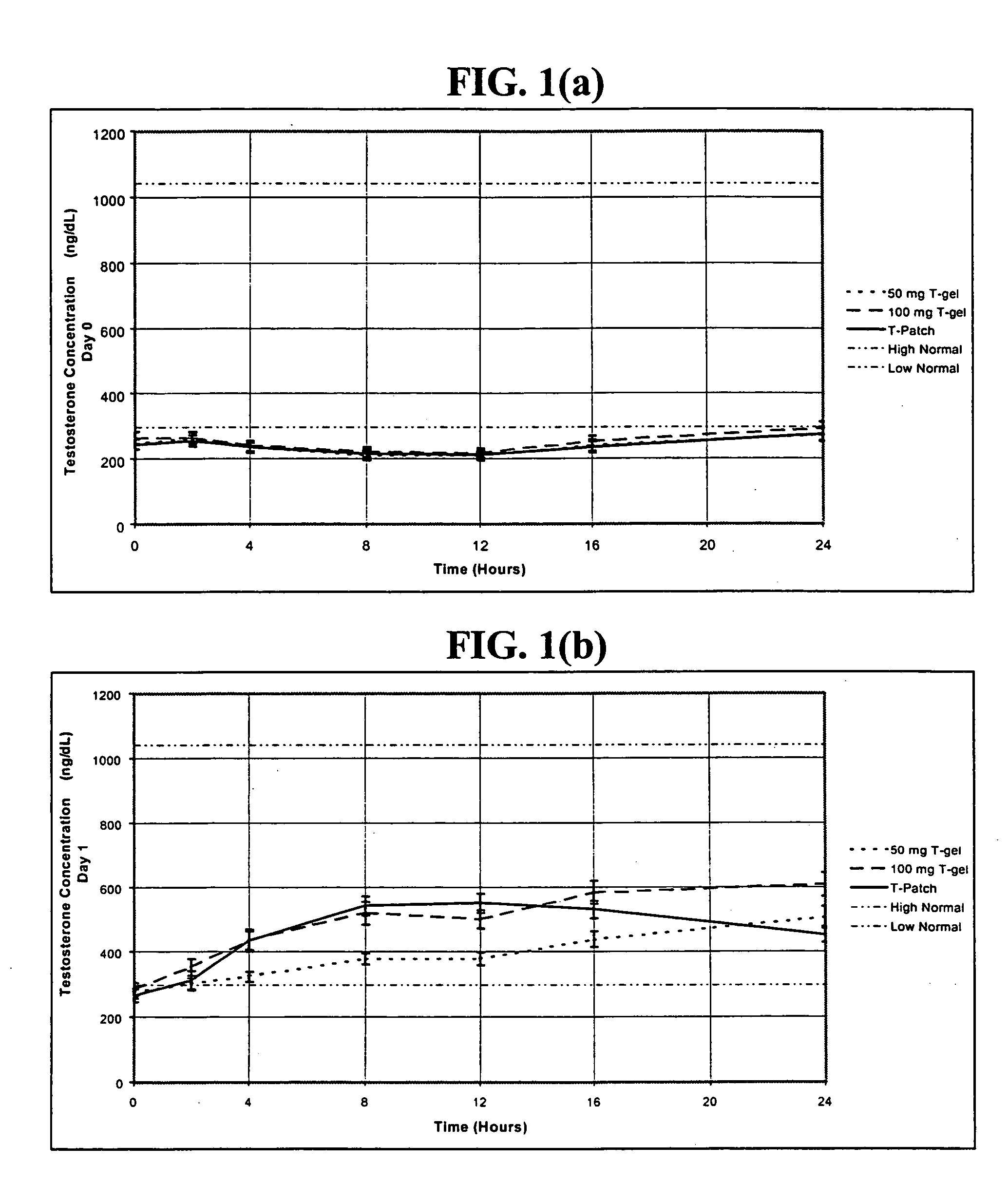
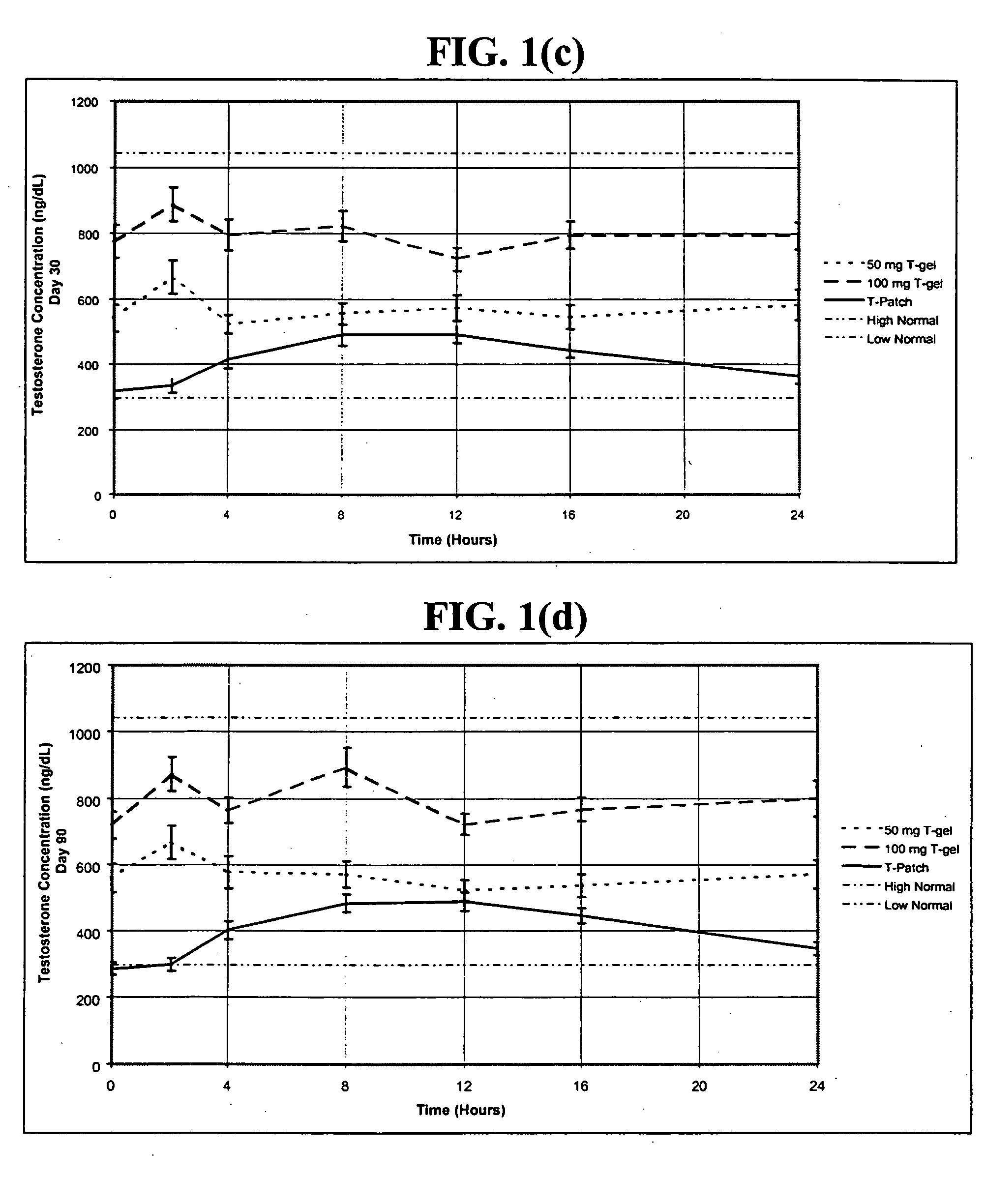
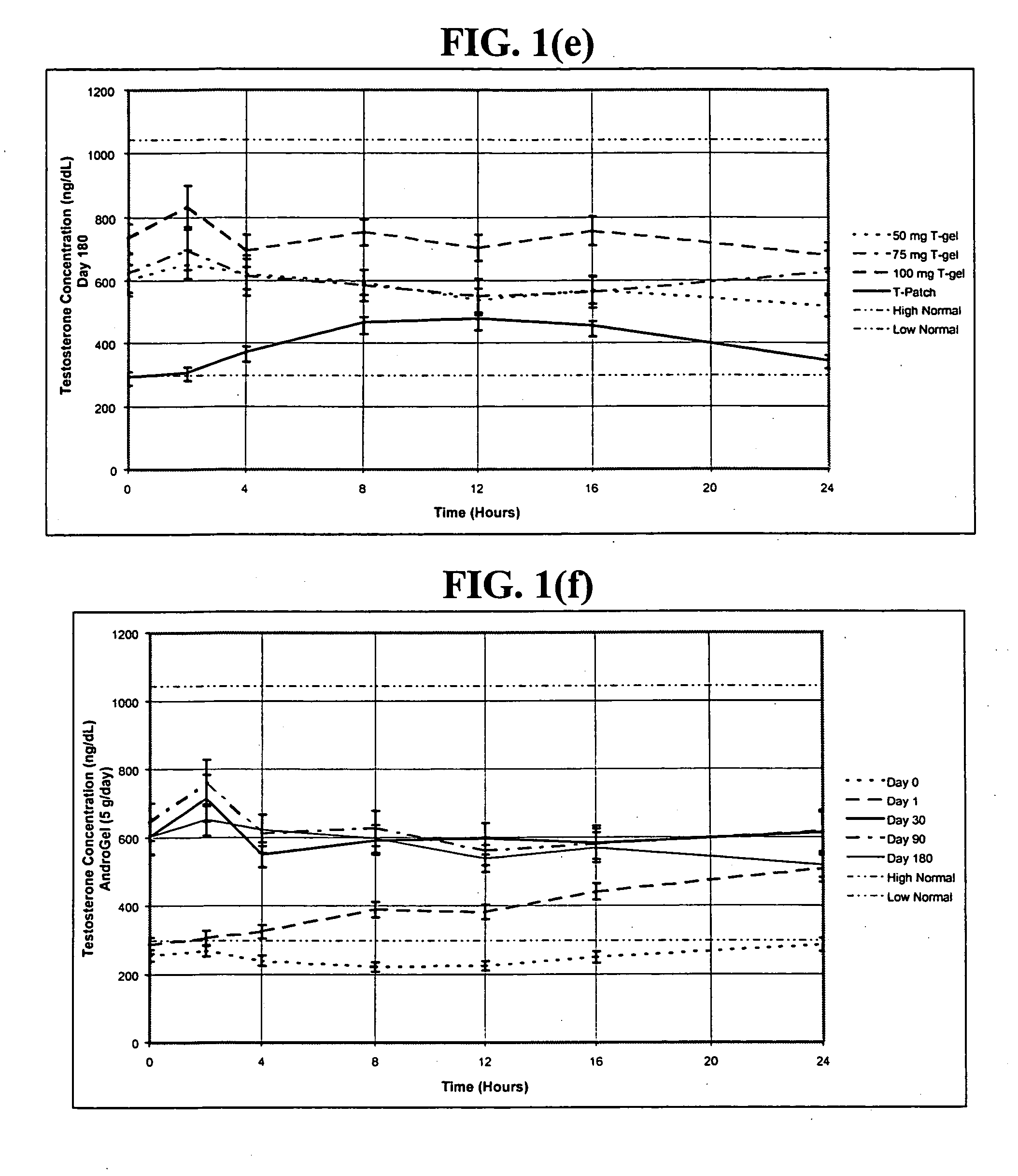
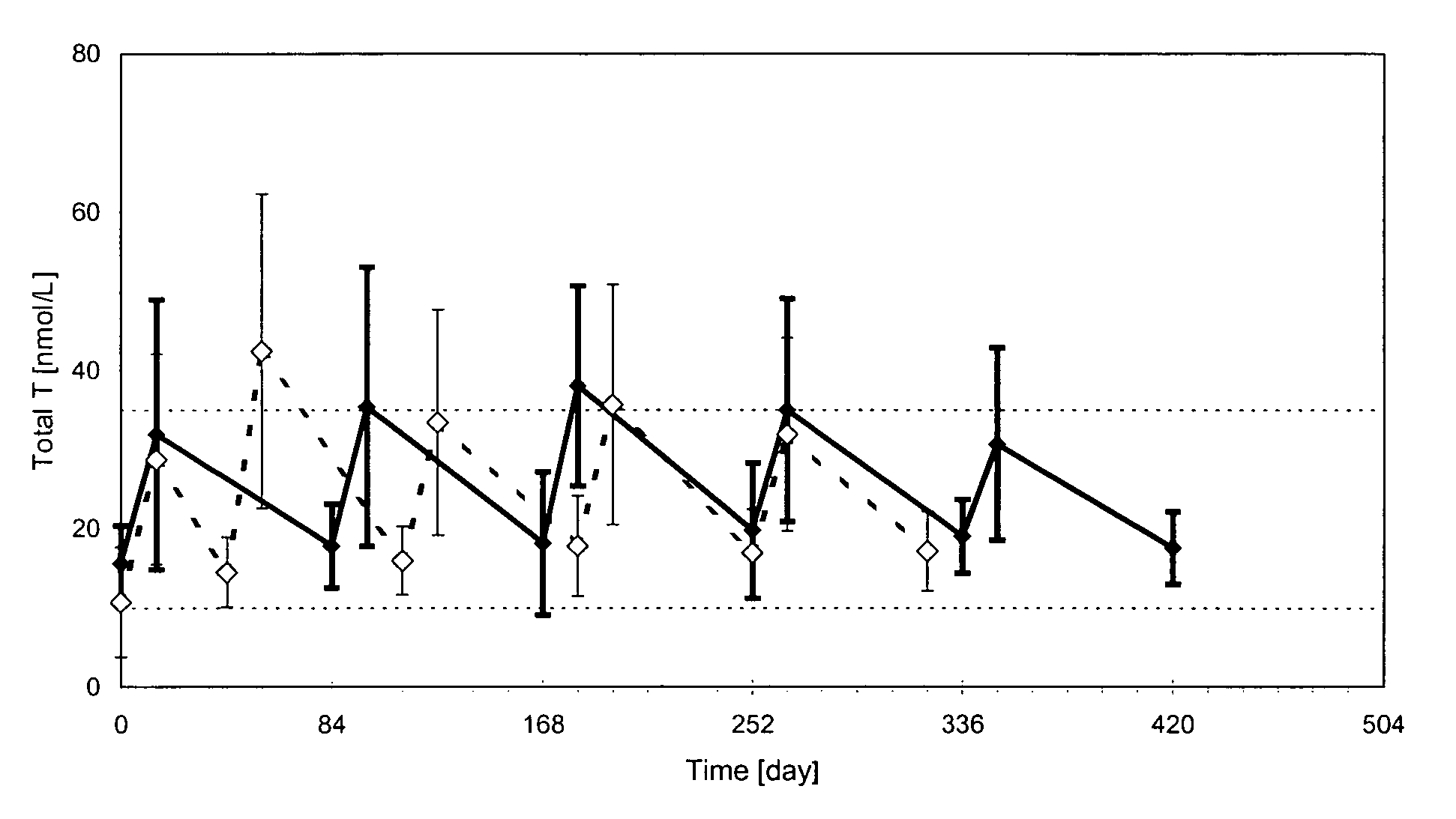
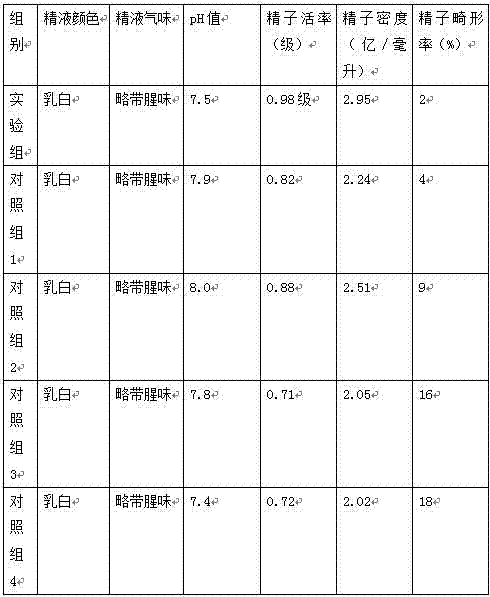
In general, the normal range in males is about 270 to 1070 ng/dL with an average level of 679 ng/dL. A normal male testosterone level peaks at about age 20, and then it slowly declines.
Method for treating erectile dysfunction and increasing libido in men
InactiveUS20050054623A1Little and no skin irritationImprove sexual performanceSenses disorderNervous disorderSexual impotenceCombined use
The present invention relates to a transdermal hydroalcoholic testosterone gel formulation that overcomes the problems associated with other testosterone delivery mechanisms by providing, among other things, a desirable pharmacokinetic hormone profile with little or no skin irritation. The gel may be used as a method of improving sexual performance, including treating erectile dysfunction, and increasing libido by increasing testosterone levels in men. In addition, the gel may be used in conjunction with pharmaceuticals aimed at treating erectile dysfunction, such as VIAGRA®, to enhance their effectiveness.
Owner:UNIMED PHARMA LLC
Methods and pharmaceutical compositions for reliable achievement of acceptable serum testosterone levels
The present invention relates to pharmaceutical compositions, formulated for injectable administration, which comprises a testosterone ester, in particularly testosterone undecanoate, in a vehicle comprising castor oil and a co-solvent. Upon injecting the compositions according to a particular administration scheme, reliable levels of testosterone in serum in the normal physiological range is achieved for a long period. This allows for the use of the compositions in hormone replacement therapy and male contraception without concomitant monitoring of testosterone levels in serum by a physician.
Owner:SCHERING AG +1
Methods and pharmaceutical compositions for reliable achievement of acceptable serum testosterone levels
ActiveUS20050032762A1Maintain serum levelMaintain levelBiocideOrganic active ingredientsDosing regimenPhysiology
The present invention relates to pharmaceutical compositions, formulated for injectable administration, which comprises a testosterone ester, in particularly testosterone undecanoate, in a vehicle comprising castor oil and a co-solvent. Upon injecting the compositions according to a particular administration scheme, reliable levels of testosterone in serum in the normal physiological range is achieved for a long period. This allows for the use of the compositions in hormone replacement therapy and male contraception without concomitant monitoring of testosterone levels in serum by a physician.
Owner:SCHERING AG +1
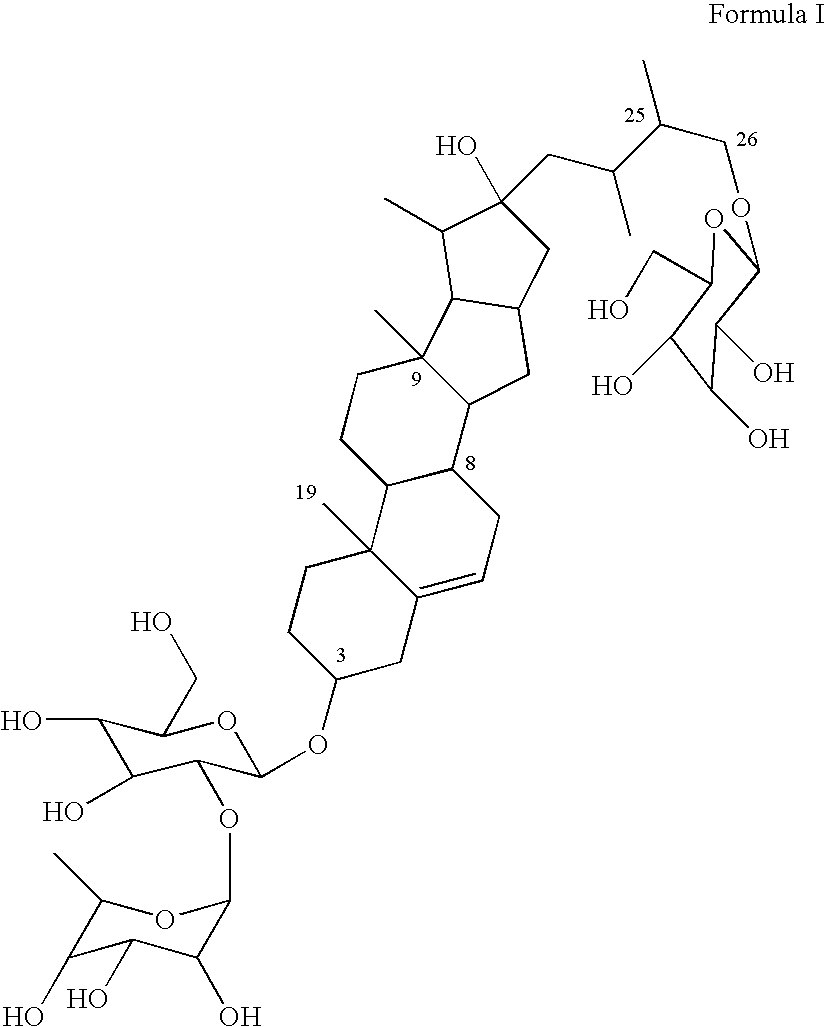
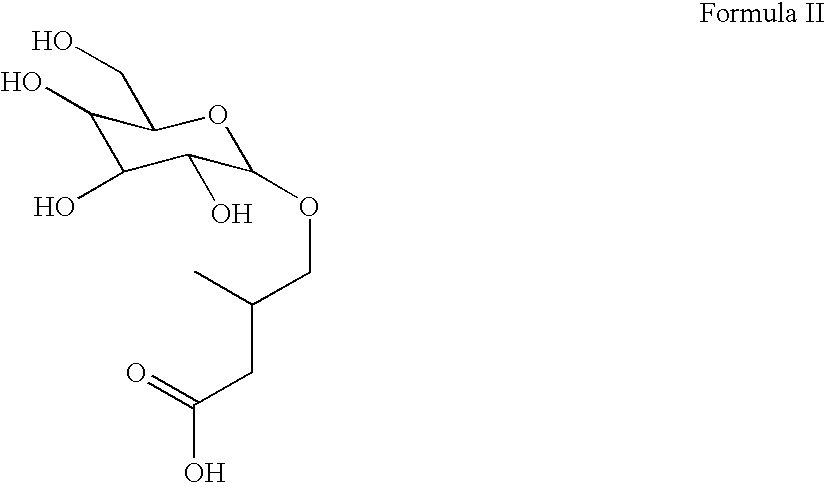
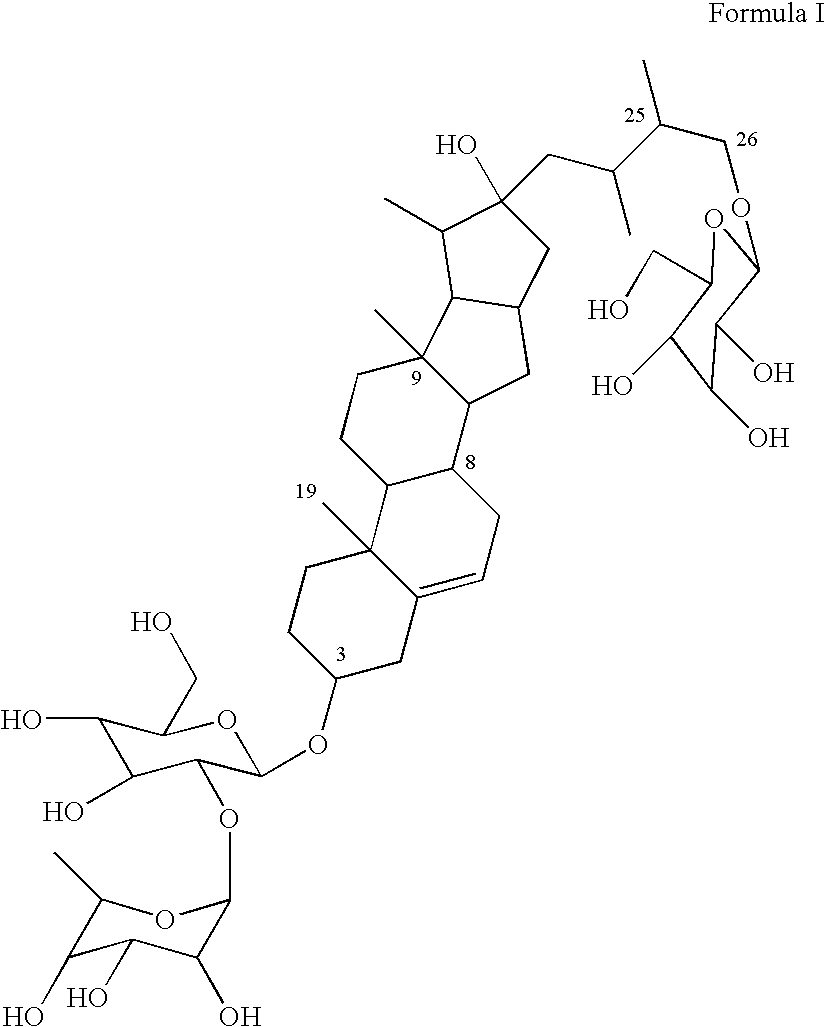
Eurycoma longifolia extract product and application thereof
InactiveCN102948569AHigh purityBoost testosterone levelsPre-extraction tea treatmentCoffee extractionSeparation technologyDistillation
The invention discloses a eurycoma longifolia extract product and an application thereof. The invention is characterized in that a eurycoma longifolia active component is extracted with an ultrasonic distillation technology or a membrane separation technology; and the active component is applied in the field of beverages, such as that the active component can be prepared into coffee, tea, and the like. The extracted active component provided by the invention has higher purity, and the prepared beverage can more efficiently improve human testosterone level. The beverage also has the functions of kidney clearing, diuresis, sterilizing, inflammation eliminating, body blood circulation enhancing, metabolism enhancing, endocrine system improving, and body immune function enhancing. The beverages are suitable for daily applications.
Owner:顾立峰
Combination of testosterone and ornithine decarboxylase (ODC) inhibitors
InactiveUS20130178454A1Avoid side effectsPromotes beneficial effectOrganic active ingredientsBiocideSide effectVirilization
The present invention is generally directed to a method of increasing testosterone levels in a subject comprising; administration of testosterone or analogue thereof along with an ornithine decarboxylase (ODC) inhibitor. The method selectively promotes beneficial effects of testosterone, while preventing side effects associated with testosterone administration. The present invention also directed to pharmaceutical composition and kits comprising testosterone or analogue thereof; ornithine decarboxylase inhibitors. The composition selectively promotes beneficial effects of testosterone, while preventing side effects of testosterone administration. In some embodiments, the present invention relates to a method of treatment of low testosterone levels in a subject by administrating testosterone and ODC inhibitor while preventing side effects associated with testosterone administration. The method and composition of the invention particularly prevents potential adverse effects on the prostate in men and virilization in women.
Owner:FUNCTION PROMOTING THERAPIES
Oral Transmucosal Pharmaceutical Compositions Including Testosterone and a C-SERM
ActiveUS20160051564A1Improve the level ofRelieve symptomsOrganic active ingredientsBiocideSolid Dose FormMucoadhesion
Formulations for oral transmucosal compositions including a synergistic combination of low doses of testosterone with a clomiphene-like selective estrogen receptor modulator (C-SERM) that are combined with transmucosal absorption enhancers are disclosed. Oral transmucosal compositions can be for fast release or slow release, and can be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Oral transmucosal compositions include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms are: mucoadhesive liquids such as gel-forming liquids; gel-forming semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of testosterone and C-SERM. Oral transmucosal compositions will deliver testosterone and C-SERM directly into the patient's bloodstream, and provide high bioavailability of testosterone and C-SERM; therefore, the required doses are lower.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
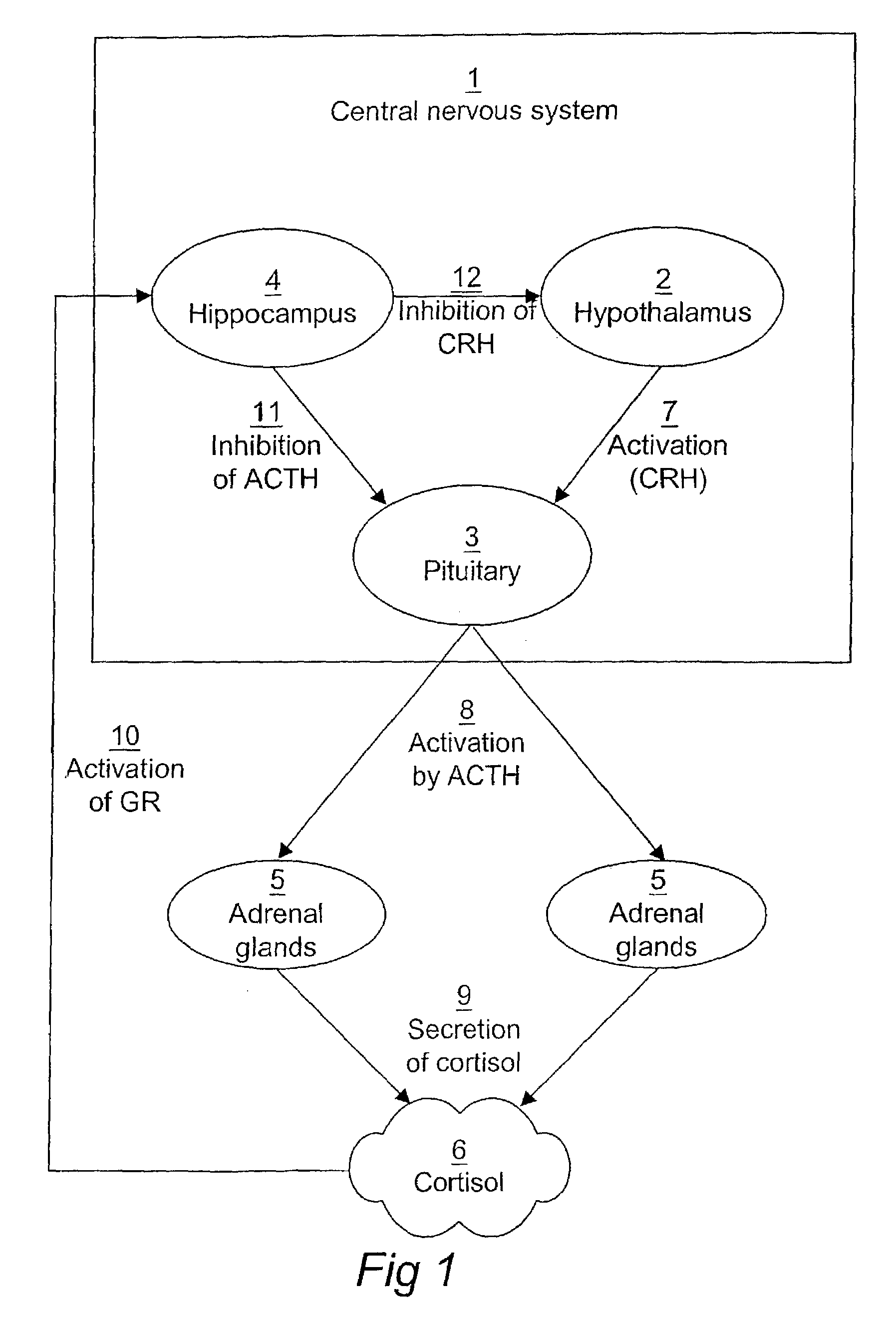
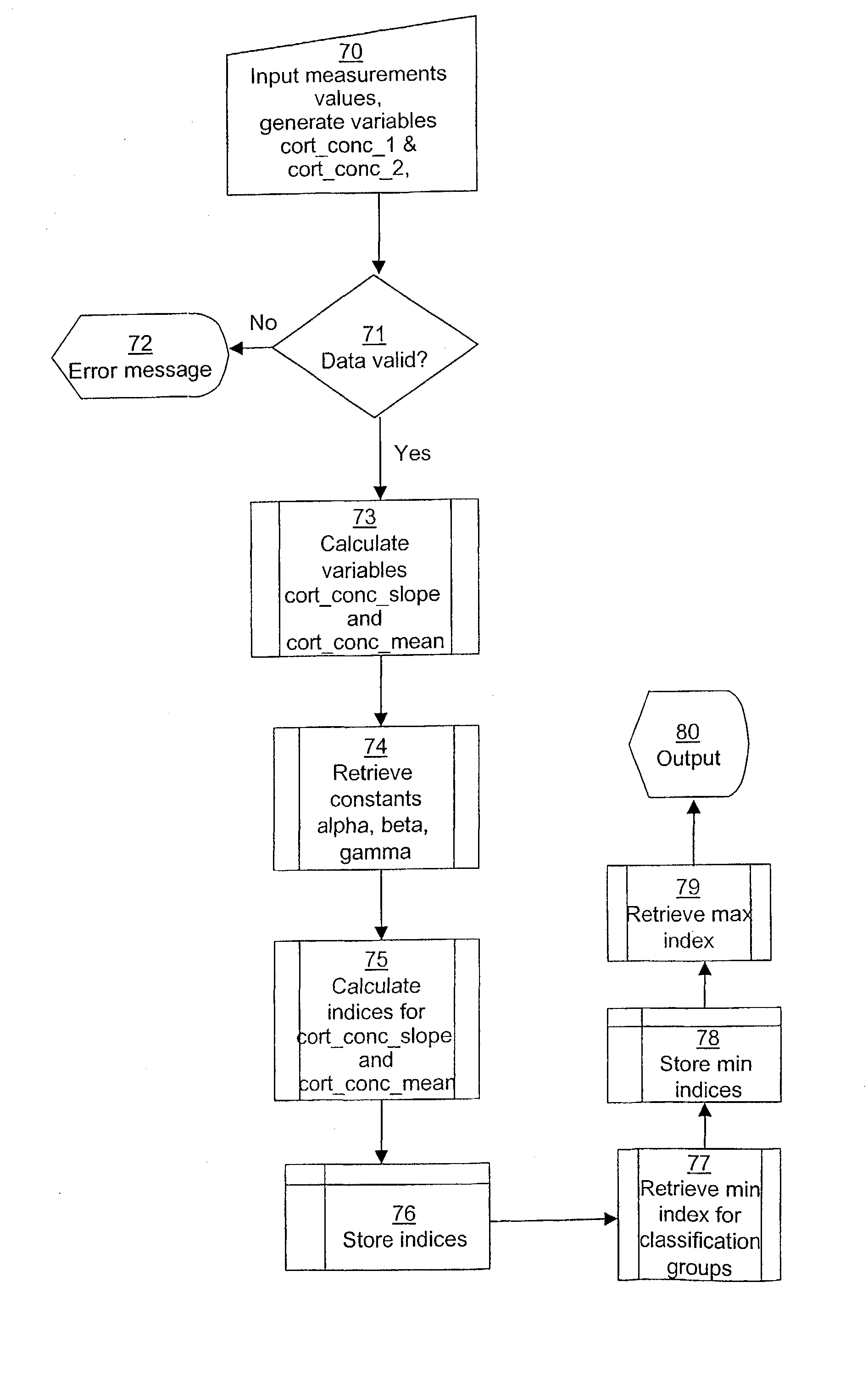
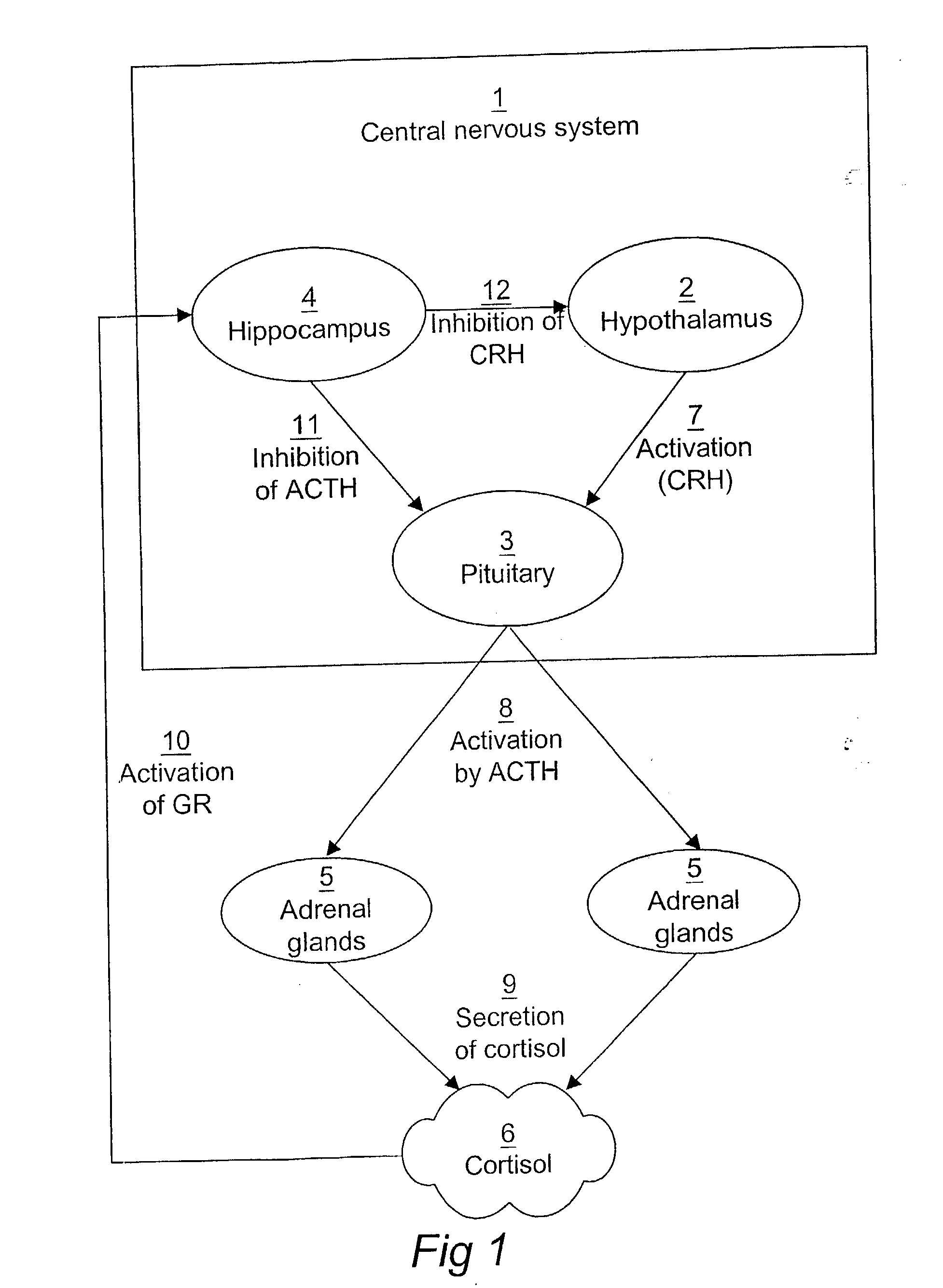
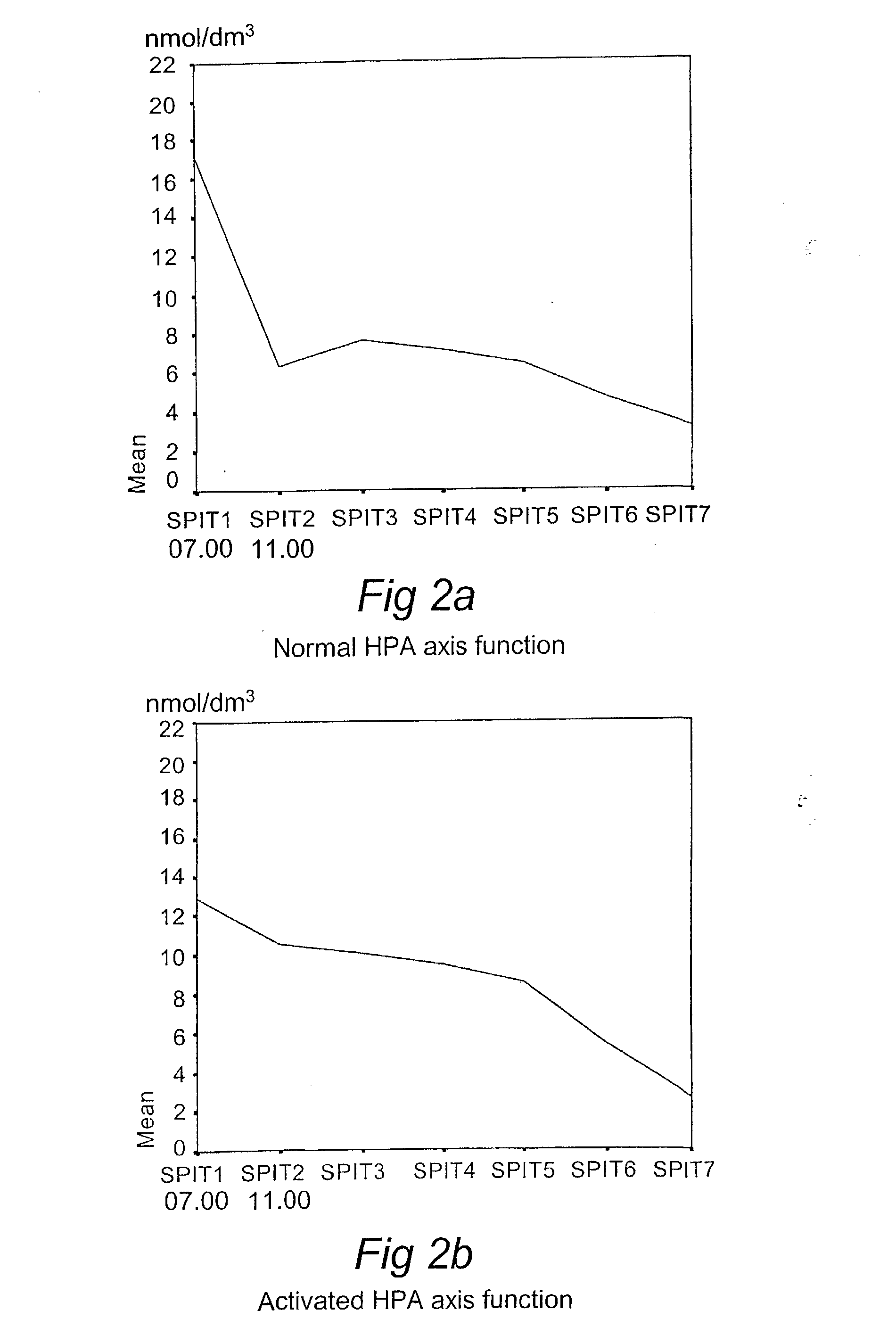
Computerized identification of normal and abnormal diurnal cortisol secretion patterns and levels of testosterone from human saliva samples
InactiveUS7805396B2Improve accuracyDigital computer detailsComputer-assisted medical data acquisitionSaliva samplePhysiology
Normal and abnormal diurnal cortisol secretion patterns are identified from human saliva samples by an in vitro method. First and second saliva samples are taken from one human individual at first and second predetermined times during the same day. A first cortisol concentration is determined in the first saliva sample, and a second cortisol concentration is determined in the second saliva sample. An abnormal secretion pattern is then compared to a normal secretion pattern with the help of a fuzzy logic algorithm. A function of the hypothalamic-pituitary-adrenal (HPA) axis is then determined for the human individual. Optionally, a testosterone level is determined from one of the samples and is used in combination with the cortisol concentrations to provide a redefined determination.
Owner:DIAGNO INT
Composition and a process thereof
InactiveUS20080199517A1Improving physiological factorImprove the level ofOrganic active ingredientsCosmetic preparationsSugar derivativesAdemetionine
The present invention relates to a novel herbal composition for improving exercise physiology factors comprising saponins and sugar derivatives optionally along with pharmaceutically acceptable excipients. It also relates to the process of preparing the composition for improving exercise physiology factors comprising the steps of flaking, defatting, solvent extraction of seeds of trigonella species followed by concentration of the extract to obtain a saponin and sugar derivative. It also relates to the use of the composition for improving exercise physiology factors comprising, enhanced anabolic activity, enhanced muscle building, enhance creatine delivery and reuptake, an increase in testosterone levels and an enhanced immunity.
Owner:INDUS BIOTECH PVT
Novel composition to increase testosterone levels
InactiveUS20100303937A1Increasing testosterone levelIncrease muscle strengthBiocideTripeptide ingredientsAromatase inhibitorFlavanone
The present invention relates to a composition for increasing testosterone physiological levels comprising: a) a sufficient amount of at least one aromatase inhibitor chosen from a flavone substituted with at least one methoxy group at position 3′,4′,5 or 7, any combinations thereof, and any di, tri or tetra combinations thereof; and a flavanone substituted with at least one methoxy group at position 3′,4′,5 or 7, any combinations thereof, and any di, tri or tetra combinations thereof; and b) a sufficient amount of at least one 5α-reductase inhibitor that inhibit testosterone conversion to DHT, such as beta-sitosterols; in association with a pharmaceutically acceptable carrier.
Owner:ORAL DELIVERY TECH
Estrogen receptor ligands and methods of use thereof
InactiveUS20120077845A1Decreased bone mineral densityIncreased riskBiocideAmine active ingredientsIncreased riskEstrogen receptor
The present invention relates to methods for reducing testosterone levels by reduction of luteinizing hormone (LH) or independent of LH levels in a male subject and methods of treating, suppressing, reducing the incidence, reducing the severity, or inhibiting prostate cancer, advanced prostate cancer, and castration-resistant prostate cancer (CRPC) and palliative treatment of prostate cancer, advanced prostate cancer and castration-resistant prostate cancer (CRPC). The compounds of this invention suppress free or total testosterone levels to castrate levels which may be used to treat prostate cancer, advanced prostate cancer, and CRPC without causing bone loss, decreased bone mineral density, increased risk of bone fractures, increased body fat, hot flashes and / or gynecomastia.
Owner:GTX INCORPORATED
Means for treating prostate hyperplasia and prostate cancer
A regime for therapeutic management of a benign prostatic hyperplasia and prostatic cancer employs Cetrorelix alone or in combination with alpha -reductase inhibitors or alpha -receptor blocking agents. The regimen reduces the volume of the prostate and avoids the side effects associated with testosterone levels being in a castration range. Cetrorelix is administered at dosages between 0.5 mg / day and 20 mg / week or about 0.014 mg / kg body weight per day to 0.30 mg / kg body weight per week or at levels of about 25 to 120 mg of Cetrorelix per month or 0.376 mg / kg to 1.71 mg / kg per month. Cetrorelix can be administered with alpha -reductase inhibitors or alpha -receptor blocking agents.
Owner:ZENTARIS GMBH
Use of total saponin extract of fenugreek
The invention provides application of a common fenugreek seed total saponin extract in preparing a medicine or a health care product for treating or preventing the reduction of the testosterone level and the exercise tolerance. The usage and the dosage of the medicine are determined according to the illness state of patients; the medicine can be taken for 2 to 3 times per day; and the daily dosage per person is between 200 and 4,000 milligrams and preferably between 600 and 1,200 milligrams or is between 0.0036 and 0.0145 gram per kilogram of the body weight. Common fenugreek seed total saponin can improve the testosterone level of blood serum, so that the common fenugreek seed total saponin extract has important function of treating or preventing the reduction of the testosterone level and the exercise tolerance.
Owner:GENEHAM PHARMA CO LTD
Use of Sini decoction in preparing medicine for treating hypothyroidism sexual function reduction
InactiveCN101361953AReduced reproductive functionElevated serum T levelsEndocrine system disorderPlant ingredientsSexual functionSevere hypothyroidism
The invention relates to an application of a traditional Chinese drug composite, namely a sini decoction, in preparing a drug for treating hypothyroid sexual function reduction. When researching the effect of the sini decoction on rat models with hypothyroid sexual function reduction, the rats are found to experience obvious increase of testosterone level, obvious rise of T level in blood serum after being lavaged with the sini decoction; meanwhile, the level of thyroid hormone gradually recovers, thus indicating that the sini decoction can improve the generating function of rats with hypothyroid sexual function reduction and enhance sexual function by adjusting the secretion of sex hormone. The invention adds the new application of the sini decoction in treating hypothyrosis, especially hypothyroid sexual function reduction and extends indications for the traditional Chinese drug composite, namely the sini decoction.
Owner:SHANXI UNIV OF CHINESE MEDICINE
Repair effect of traditional Chinese medicine component-radix morindae officinalis polysaccharide for injury to male reproductive system
InactiveCN106668058AImprove the level ofPromote spermatogenesisPowder deliveryOrganic active ingredientsDiseaseLiver and kidney
The invention discloses use of radix morindae officinalis polysaccharide in preparation of medicines or health foods for preventing and treating male reproductive system diseases. The radix morindae officinalis polysaccharide has an average molecular weight of (1.0-1.5)*10<4>, and the dosage of the radix morindae officinalis polysaccharide is 200-300mg kg<-1>; after being directly used for preparing the medicines or the health foods for preventing and treating the male reproductive system diseases, the radix morindae officinalis polysaccharide can repair the injury to a male reproductive system, promote spermatogenic function, improve sexual behavior ability, enhance reproductive capacity and increase the testosterone level in a body, so that the technical problem that radix morindae officinalis has toxicity for liver and kidney when being directly eaten is avoided.
Owner:FUJIAN MEDICAL UNIV
Controlled release nasal testosterone gels, methods and pre-filled multi-dose applicator systems for pernasal administration
InactiveCN103813784AEasy to useImprove toleranceOrganic active ingredientsNervous disorderNasal cavityDisease
The present invention relates to intranasal testosterone gels for the controlled release of testosterone into the systemic circulation of males and females for providing constant effective testosterone blood levels, without inducing undesired testosterone spike in blood levels, following pernasal administration. The intranasal testosterone gels of the present invention are safe, convenient to use, well tolerated, stable and easily and reproducibly manufactured on scale up. Moreover, because supra- normal and sub-normal testosterone blood levels are believed to be essentially kept to a minimum or avoided and the testosterone serum levels are believed to remain essentially constant during dose life, i.e., the intranasal testosterone gels of the present invention mimic or restore testosterone blood levels to normal physiologic daily rhythmic testosterone levels, the novel intranasal testosterone gels of the present invention are uniquely suited for testosterone replacement or supplemental therapy and effective for treating males diagnosed with, for example, male testosterone deficiency, such as, low sexual libido, low sexual drive, low sexual activity, low fertility, low spermatogenesis, aspermatogenesis, depression and / or hypogonadism, and females who are diagnosed with, for example, female sexual dysfunction, such as, low sexual libido, low sexual drive, low sexual activity, low amygdala reactivity, low sexual stimulation, hypoactive sexual desire disease ("HSDD"), female sexual arousal disorder and / or anorgasmia. The present invention also relates to methods and pre- filled multi-dose airless applicator systems for pernasal administration of the nasal testosterone gels of the present invention.
Owner:ACERUS PHARMA SRL
Estrogen receptor ligands and methods of use thereof
InactiveUS20140187641A1Prolong progression-free survivalImprove survivalBiocideOrganic chemistryGynecomastiaEstrogen receptor
The present invention relates to methods for reducing testosterone levels in a male subject and methods of treating, suppressing, reducing the incidence, reducing the severity, or inhibiting prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC), metastatic castration resistant prostate cancer (mCRPC), and methods of reducing high or increasing PSA levels and / or increasing SHBG levels in a subject suffering from prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC) and metastatic castration resistant prostate cancer (mCRPC). The compounds of this invention suppress free or total testosterone levels despite castrate levels secondary to ADT and reduce high or increasing PSA levels. This reduction in testosterone levels may be used to treat prostate cancer, advanced prostate cancer, CRPC and mCRPC without causing bone loss, decreased bone mineral density, increased risk of bone fractures, increased body fat, hot flashes and / or gynecomastia.
Owner:GTX INCORPORATED
Peptide, immunogenic composition and vaccine or medical preparation, a method to immunize animals against the hormone LHRH, and analogs of the LHRH tandem repeat peptide and their use as vaccine
InactiveUS7361349B2Adequate and efficient useImprove efficacyPeptide/protein ingredientsLuteinising hormone-releasing hormoneVaccinationHormones regulation
A peptide that comprises a modified tandem GnRH decapeptide sequence which allows for a testosterone level that is essentially nondetectable after vaccination with the peptide in a suitable dosage and / or allows for an immunogenic response that allows for the effective discrimination between GnRH-I and GnRH-II and a method for the immunocastration of pigs.
Owner:PEPSCAN SYST
Computerized identification of normal and abnormal diurnal cortisol secretion patterns and levels of testosterone from human saliva samples
InactiveUS20100241606A9Improve accuracyComputer-assisted medical data acquisitionBiological testingSaliva sampleSecreted substance
Normal and abnormal diurnal cortisol secretion patterns are identified from human saliva samples by an in vitro method. First and second saliva samples are taken from one human individual at first and second predetermined times during the same day. A first cortisol concentration is determined in the first saliva sample, and a second cortisol concentration is determined in the second saliva sample. An abnormal secretion pattern is then compared to a normal secretion pattern with the help of a fuzzy logic algorithm. A function of the hypothalamic-pituitary-adrenal (HPA) axis is then determined for the human individual. Optionally, a testosterone level is determined from one of the samples and is used in combination with the cortisol concentrations to provide a redefined determination.
Owner:DIAGNO INT
Testosterone Solutions for the Treatment of Testosterone Deficiency
ActiveUS20130143851A1Lower testosterone levelsIncreased blood serum levelOrganic active ingredientsNervous disorderSerum testosterone levelTestosterone deficiency
Solutions of testosterone for oromucosal administration providing an increase in serum testosterone levels in subjects deficient in endogenous testosterone levels, and therapeutic methods for providing an increase in serum testosterone levels and methods for treating a disease or a symptom associated with deficient endogenous levels of testosterone.
Owner:INNOTESTO
Agent and method for increasing testosterone level in a body
A composition for regulating male sexual function includes at least one amino acid selected from a group consisting of L-Glutamic acid (glutamate), L-Arginine, glycine, L-aspartate, L-carnitine, L-tyrosine, L-glutamine and / or its pharmaceutically acceptable salt, such as any of monosodium-L-glutamate monogidrate, L-potassiumarginine, L,-ammoniumaspartate, L-arginine HCl, sodium-L-tyrasine, lithium aspartate, in the amount of 1-1000 mg, preferably 10-300 mg; at least one vitamin, in the amount of 1-500% of a daily recommended dose, preferably 10-200%; and at least one chelated compound of a formula: acid-Me-acid.nH2O, wherein Me is a metal chosen from any of Ca, Mg and Zn, wherein the acid is a dicarboxylic acid anion chosen from the group consisting of succinic acid, fumaric acid or aspartic acid, and wherein n=0-8. The composition increases testosterone level in a body of a male mammal.
Owner:AMBER IP LLC
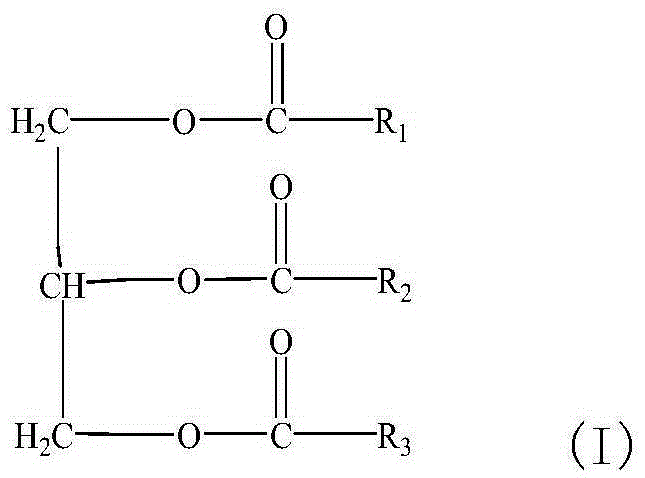
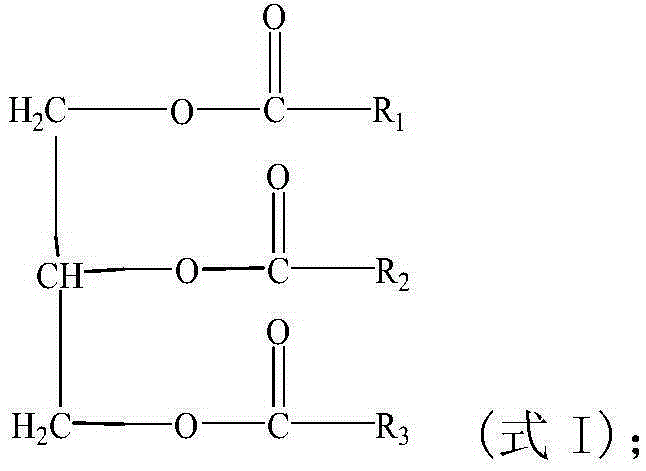
Application of triglyceride compound in preparation of medicine for treating polycystic ovarian syndrome
The invention discloses application of a triglyceride compound in the preparation of a medicine for treating polycystic ovarian syndrome. Specifically, the invention discloses application of a triglyceride compound and its pharmaceutically acceptable salt in the preparation of a composition or a preparation for treating polycystic ovarian syndrome, and / or a composition or a preparation for reducing testosterone level of mammals. The medicine of the invention can effectively treat polycystic ovarian syndrome, especially can remarkably reduce ovarian coefficient of mammals, and has little side effect.
Owner:ZHEJIANG XUCHEN PHARMA TECH CO LTD
Estrogen receptor ligands and methods of use thereof
The present invention relates to methods for reducing testosterone levels by reduction of luteinizing hormone (LH) or independent of LH levels in a male subject and methods of treating, suppressing, reducing the incidence, reducing the severity, or inhibiting prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC), metastatic castration resistant prostate cancer (mCRPC) and palliative treatment of prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC) and metastatic castration resistant prostate cancer (mCRPC), and methods of reducing high or increasing PSA levels and / or increasing SHBG levels in a subject suffering from prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC) and metastatic castration resistant prostate cancer (mCRPC). The compounds of this invention suppress free or total testosterone levels despite castrate levels secondary to ADT and reduce high or increasing PSA levels. This reduction in testosterone levels may be used to treat prostate cancer, advanced prostate cancer, CRPC and mCRPC without causing bone loss, decreased bone mineral density, increased risk of bone fractures, increased body fat, hot flashes and / or gynecomastia.
Owner:GTX INCORPORATED
Therapeutic treatment for increasing testosterone
The method of treatment for increasing testosterone levels of an adult male patient is disclosed. The treatment has the steps of activating an acoustic shock wave generator or source to emit acoustic shock waves; and subjecting a testicle through the scrotum to the acoustic shock waves stimulating said testicle wherein the testicle is positioned within a path of the emitted shock waves. The emitted shock waves can be convergent, divergent, planar or near planar.
Owner:SOFTWAVE TISSUE REGENERATION TECH LLC
Composition for increasing testosterone levels and enhancing libido
InactiveUS20160361257A1Increasing testosterone levelIncrease libidoOrganic active ingredientsPowder deliveryTestosterone levelBenzoflavones
The present invention features a compressible delivery formulation for buccal delivery, dosage forms and methods of using the same for increasing testosterone levels in a subject. The formulation, dosage forms and methods comprise effective amounts of 3,4-divanillyltetrahydrofuran, 7-methoxyflavone, 7,8-benzoflavone.
Owner:FARBER MICHAEL
Combination therapy for treating androgen deficiency
The present invention relates to a combination therapy for elevating testosterone levels in male mammals in which an antiestrogen or pharmaceutically acceptable salt thereof is co-administered to the mammal with an additional therapeutic agent selected from an androgen and an aromatase inhibitor. The invention is also directed to a combination therapy for treating males with hypogonadism and disorders related thereto, including reduction of muscle mass. limitation of body performance capacity, reduction of bone density, reduction of libido, reduction of potency, reduction of benign prostatic hyperplasia and infertility.
Owner:REPROS THERAPEUTICS
Oral Transmucosal Compositions including Aromatase Inhibitors for Low Testosterone Levels in Men
InactiveUS20160022643A1Efficient skinEfficient tissue penetrationBiocideOintment deliveryOral mucous membraneImmediate release
Formulations for oral transmucosal compositions that include aromatase inhibitors (AIs) in combination with transmucosal absorption enhancers are disclosed. Disclosed oral transmucosal compositions may be for immediate release or slow release, and may be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Disclosed oral transmucosal compositions may include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms may include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms may be: mucoadhesive liquids such as gel-forming liquid; gel-forming; semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of AIs. Disclosed oral transmucosal compositions may allow the delivery of AIs directly into the patient's bloodstream, thus providing high bioavailability of AIs; therefore, required dose may be lower. Additionally, adjustments of AIs dosages may be achieved when using disclosed oral transmucosal compositions.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Feed additive for improving reproduction performance of male media duck
InactiveCN107183355AHas the effect of tonifying the kidney and strengthening yangImprove reproductive abilityFood processingAnimal feeding stuffFood additiveAnimal science
The invention relates to the technical field of feed additives, specifically to a feed additive for improving reproduction performance of a male media duck. The additive comprises the following components in parts by weight: 0.01-0.15 part of nandrolone phenylpropionate, 3-5 parts of maggot protein, 15-18 parts of soybean oligosaccharides, 3-4 parts of phospholipid, 1-1.2 parts of vitamin B, 1-1.8 parts of vinasse extract, and 2-5 parts of orange peel concentrated powder. The feed additive is capable of obviously improving the reproduction performance of the male media duck, that is, the quality of seminal fluid, the activity and the in-vivo testosterone level of the male media duck can be improved; in addition, the feeding method is simple, and the feeding cost is low, so that the feed additive has a positive significance on realizing the quick propagation of the media duck.
Owner:安徽祥飞枞阳媒鸭养殖有限公司
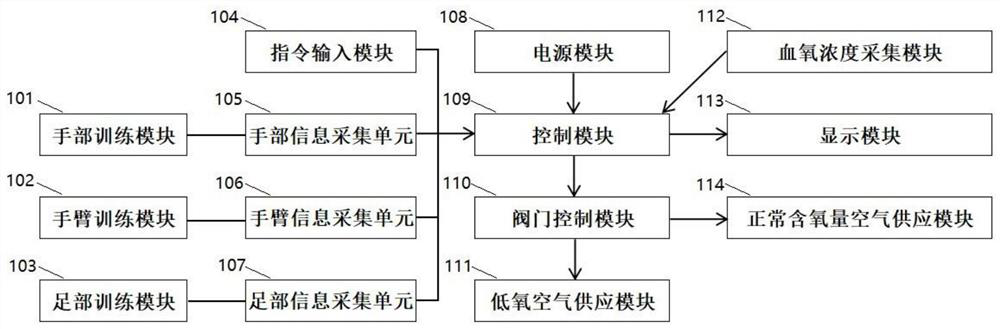
Intermittent hypoxia training device and control method thereof
ActiveCN112245876AQuality improvementImprove tensile propertiesGymnastic exercisingTherapy exerciseEngineeringRespiratory disease
The invention provides an intermittent hypoxia training device. The intermittent hypoxia training device comprises a hypoxia training system, a muscle stretching training instrument and a control module, the hypoxia training system is used for providing hypoxia air and normal oxygen content air required by intermittent hypoxia training, a valve control module arranged in the hypoxia training system is connected with the control module, and the muscle stretching training instrument is used for enabling a trainee to keep a training posture to carry out intermittent hypoxia training. An instruction input module arranged on the muscle stretching training instrument is connected with the control module. The invention further provides a control method of the intermittent hypoxia training device.By combining muscle static stretching training and intermittent hypoxia training, the auxiliary treatment of allergic respiratory diseases, the improvement of cardiovascular functions, the preventionof sudden cardiac death, the improvement of endogenous testosterone level and the improvement of whole-body microcirculation ability are facilitated, the training intensity is low, and the device andmethod are suitable for common trainees to use.
Owner:张良
Use of an aromatase inhibitor for the treatment of hypogonadism and related diseases
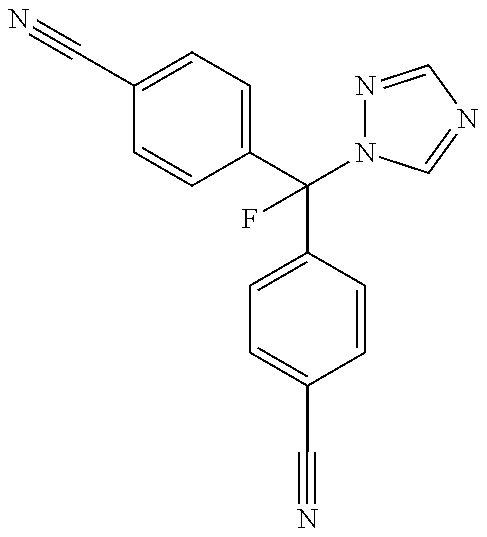
The aromatase inhibitor 4,4′-[fluoro-(1-H-1,2,4-triazol-1-yl)methylene]bisbenzonitrile increases testosterone levels and treats hypogonadism and related diseases. A particular dosing regimen is disclosed as well as pharmaceutical compositions comprising said of 4,4′-[fluoro-(1-H-1,2,4-triazol-1-yl)methylene]bisbenzonitrile, optionally in combination with other active ingredients and kits comprising the pharmaceutical compositions.
Owner:MEREO BIOPHARMA 2
Seafood seasoning and preparation method thereof
The invention discloses a seafood condiment and a preparation method thereof, and belongs to the field of condiment preparation. The product comprises the following raw materials in parts by weight: 20-40 parts of oyster protein peptide powder, 25-45 parts of fishbone leftovers, 15-45 parts of compound protease, 30-50 parts of mushroom fermentation liquor, 15-35 parts of a cymbidium wenyujin base material, 18-46 parts of an artemisia selengensis base material, 10-30 parts of milk, 20-30 parts of red wine, 10-30 parts of table salt, 5-15 parts of white sugar and 10-30 parts of table vinegar. Through the use of oyster protein peptide powder, oysters are rich in taurine, zinc, selenium and other characteristics, low in fat and cholesterol, contains a certain amount of highly unsaturated fatty acid and inorganic salt, is also rich in vitamins, has the functions of improving the testosterone level of male serum, regulating blood fat, inhibiting platelet aggregation, improving hyperglycemia symptoms, improving human immunity, promoting metabolism and the like, and also has certain effects of resisting cancers and preventing cancer cell diffusion, the delicious taste of food materials can also be enhanced in the aspect of mouth feel, fishbone leftovers are reasonably utilized, and nutrient substances of aquatic products are fully utilized.
Owner:PENGLAI JINGLU FISHERY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com