Cycloastragenol crystal form D and preparation method thereof
A technology of cycloastragenol and crystal form, which is applied in the field of organic chemical drug preparation, and can solve problems such as complex process, unstable three-membered ring structure, and multiple by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
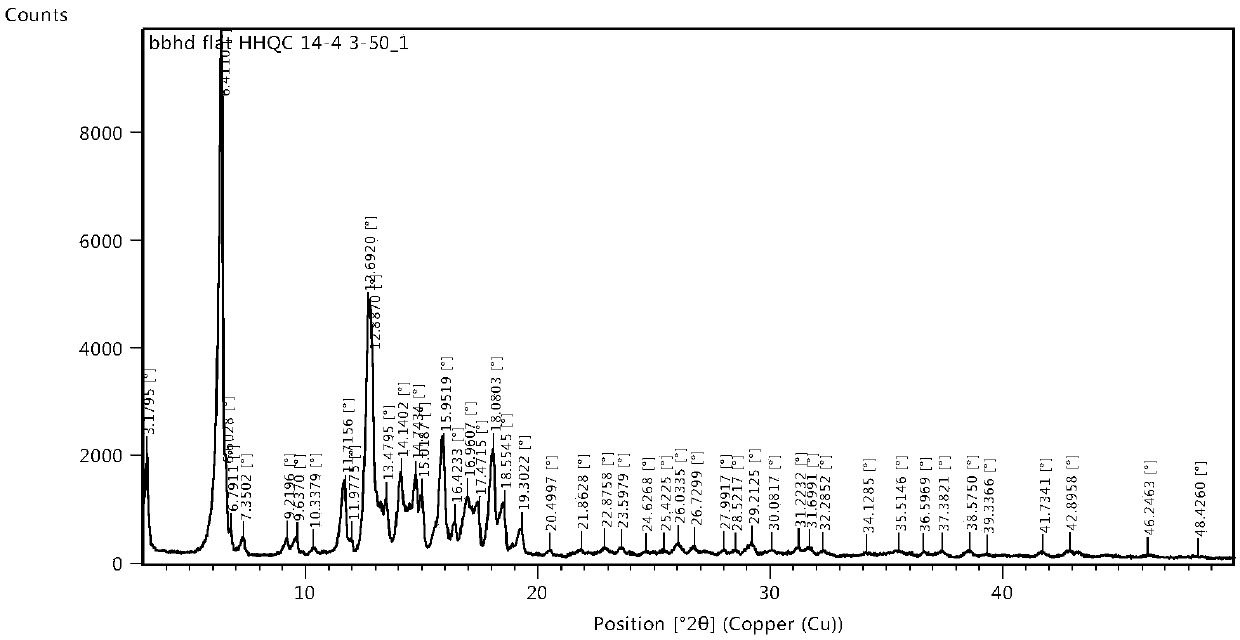
Image
Examples
Embodiment 1
[0037] Add 250mg of cycloastragenol into 1.ml of methanol and 5ml of acetonitrile mixed solvent to prepare a 41.67mg / ml solution of cycloastragenol in methanol and acetonitrile, heat to 50°C and stir for 2-4 hours to completely dissolve cycloastragenol. Heat filtration, seal with a parafilm, put in an explosion-proof refrigerator for crystallization for 2 to 4 days, filter, collect the solid, and dry under reduced pressure at 50°C. Yield: 98.1%, HPLC purity: 99.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com