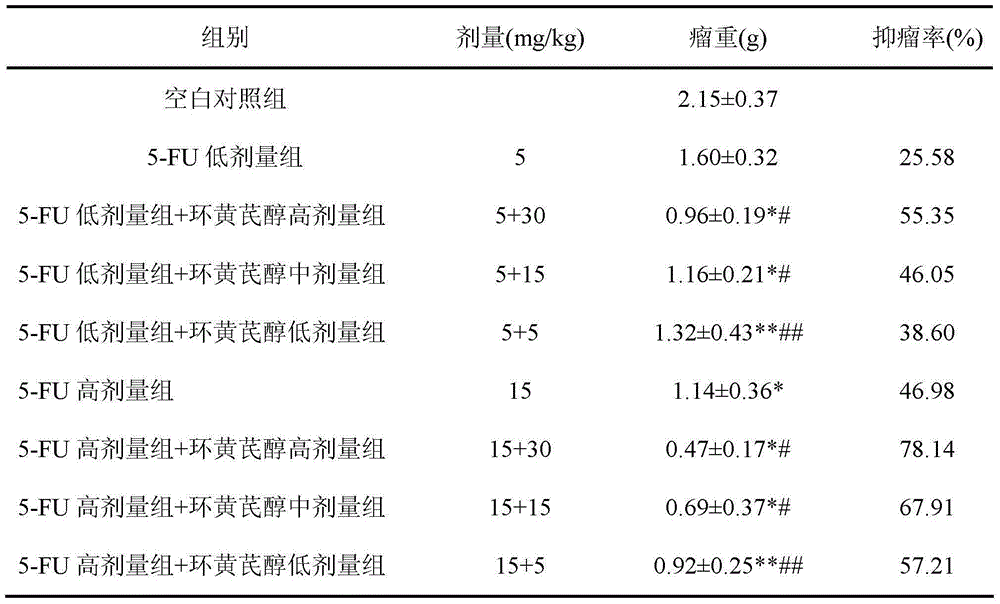
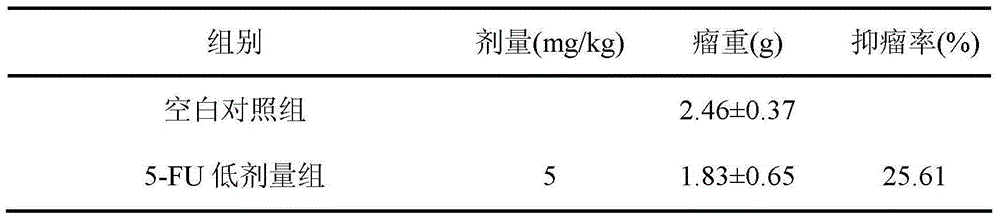
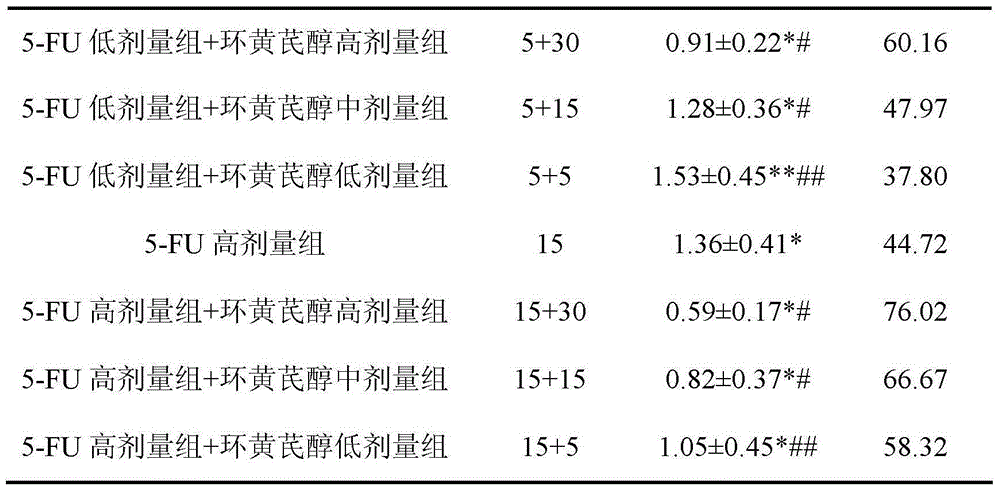
A kind of preparation method and application of cycloastragenol
A technology for cycloastragaloside and astragaloside IV is applied in the field of preparation of cycloastragaloside, which can solve the problems of insufficient relative bioavailability, limited drug development and promotion, etc., and achieves remarkable effect of anticancer adjuvant therapy, simple operation, and technological conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A. Oxidation
[0052]Take 780 mg (about 1 mmol) of astragaloside IV as a raw material, add water to it to form a suspension with a content of astragaloside IV of 0.01mol / L, add hydrochloric acid to adjust the pH of the suspension to 3-4, and add to the suspension Add oxidant—sodium periodate and react in the dark at 20°C for 48 hours, then add ethylene glycol to terminate the oxidation, wherein the molar ratio of astragaloside IV to oxidant is 1:10; then add Na 2 CO 3 Adjust the pH to 7-8, concentrate under reduced pressure until there is no alcohol smell, add an equal volume of n-butanol to extract 3 times, combine the n-butanol extraction part, wash twice with water, take the n-butanol part, evaporate the n-butanol to obtain the oxidized product.
[0053] B. Restore
[0054] Add the oxidation product obtained in step A to 30% aqueous methanol to disperse into a suspension, and the mass ratio of the added 30% aqueous methanol to astragaloside IV in step A is 1:0.7; ...
Embodiment 2
[0063] A. Oxidation
[0064] Get 1.5g (about 2mmol) astragaloside IV as a raw material, add water to it to configure a suspension with a content of astragaloside IV of 0.001mol / L, add sulfuric acid to adjust the pH of the suspension to 4-5, and add to the suspension Add an oxidizing agent——periodic acid to react in the dark at 5°C for 96 hours, then add ethylene glycol to terminate the oxidation, wherein the molar ratio of astragaloside IV to the oxidizing agent is 1:2; then add NaOH to adjust the pH to 8-9, Filter the reaction solution to obtain a filter cake, and rinse the filter cake with water several times to obtain the oxidation product
[0065] B. Restore
[0066] Disperse the oxidation product obtained in step A with water to form a suspension, and the mass ratio of the added water to astragaloside IV in step A is 1:20; then add sodium borohydride as a reducing agent, and after the reduction reaction for 12 hours, add 50% acetic acid The reaction is terminated until ...
Embodiment 3
[0075] A. Oxidation
[0076] Take 800mg (1mmol) astragaloside IV as a raw material, add 20% methanol aqueous solution to it to form a suspension with a content of astragaloside IV of 0.05mol / L, add phosphoric acid to adjust the pH of the suspension to 5-6, and add to the suspension Add an oxidant to the solution—periodic acid reacts in the dark at 25°C for 48 hours, then adds ethylene glycol to terminate the oxidation, and the molar ratio of astragaloside IV to the oxidant is 1:15; then add KHCO 3 Adjust the pH to 7-8, concentrate under reduced pressure until there is no alcohol smell, add an equal volume of n-butanol to extract 3 times, combine the n-butanol extraction part, wash twice with water, take the n-butanol part, evaporate the n-butanol to obtain the oxidized product.
[0077] B. Restore
[0078] Add 50% methanol aqueous solution to the oxidation product obtained in step A to disperse into a solution, and the mass ratio of the added 50% methanol aqueous solution to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com