Method for synthesizing quinazolinone compound through visible light induction
A quinazolinone and compound technology, which is applied in the field of visible light-induced synthesis of quinazolinone compounds, can solve the problems of cumbersome separation and purification steps, product metal residues, etc., and achieve wide substrate adaptability, high reaction efficiency, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A method for visible light-induced synthesis of the following quinazolinone compounds, the specific steps are as follows:
[0038]
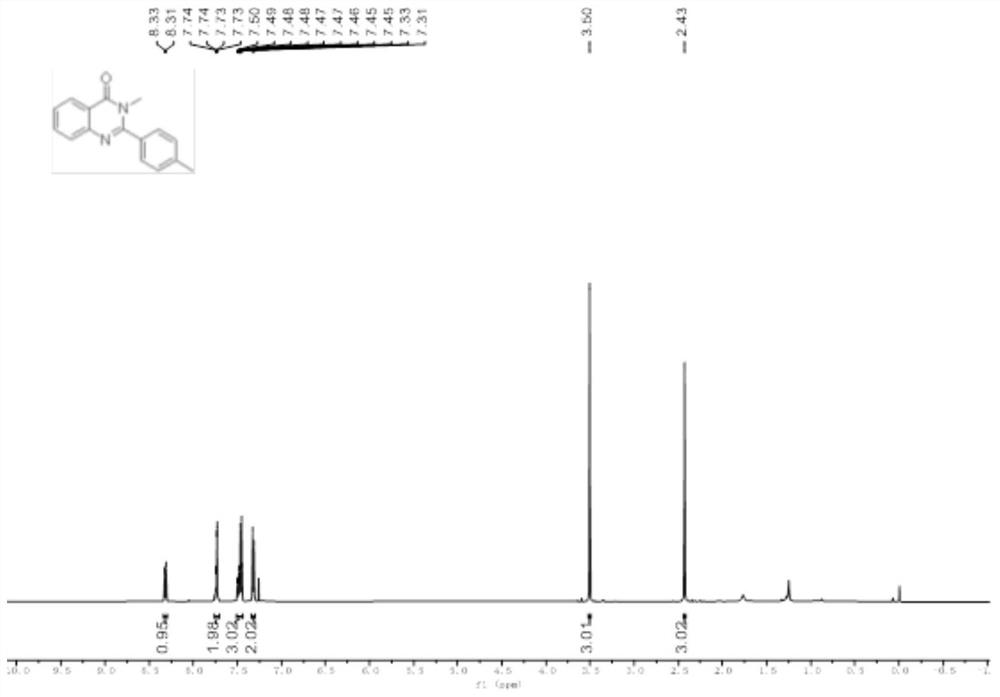
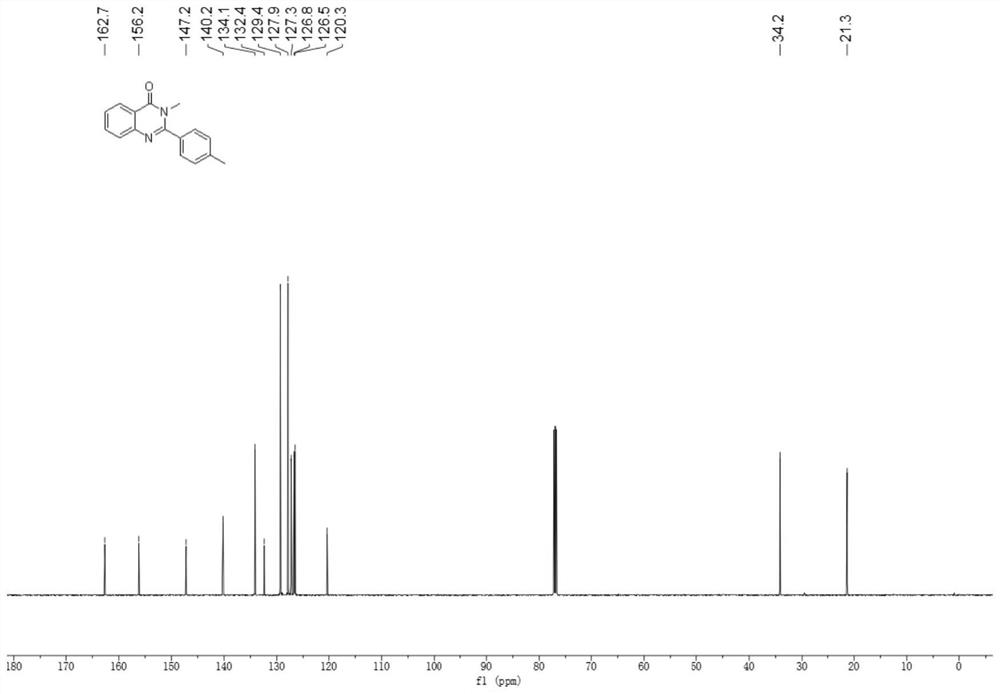
[0039] Add 2-amino-N-methylbenzamide (0.2mmol), p-tolualdehyde (0.22mmol), 2mL glacial acetic acid in the 10mL reaction bottle, stir well, the gained reaction solution is in the purple light LED lamp of 5W (purple light Wavelength 390 ~ 400nm) irradiation, stirring reaction at room temperature for 16 hours, application of TLC to detect the completion of the reaction, add 10mL of distilled water to the reaction solution, stir at room temperature for 10 minutes, filter, wash the obtained solid with water three times, each 3mL, and then in 40 ℃ Vacuum drying for 6 hours gave a white solid which was the target product with a yield of 78%.
[0040] The hydrogen spectrogram and the carbon spectrogram of the obtained product are respectively as follows figure 1 and figure 2 As shown, nuclear magnetic resonance shows no impurities, and the p...
Embodiment 2
[0042] A method for visible light-induced synthesis of the following quinazolinone compounds, the specific steps are as follows:
[0043]
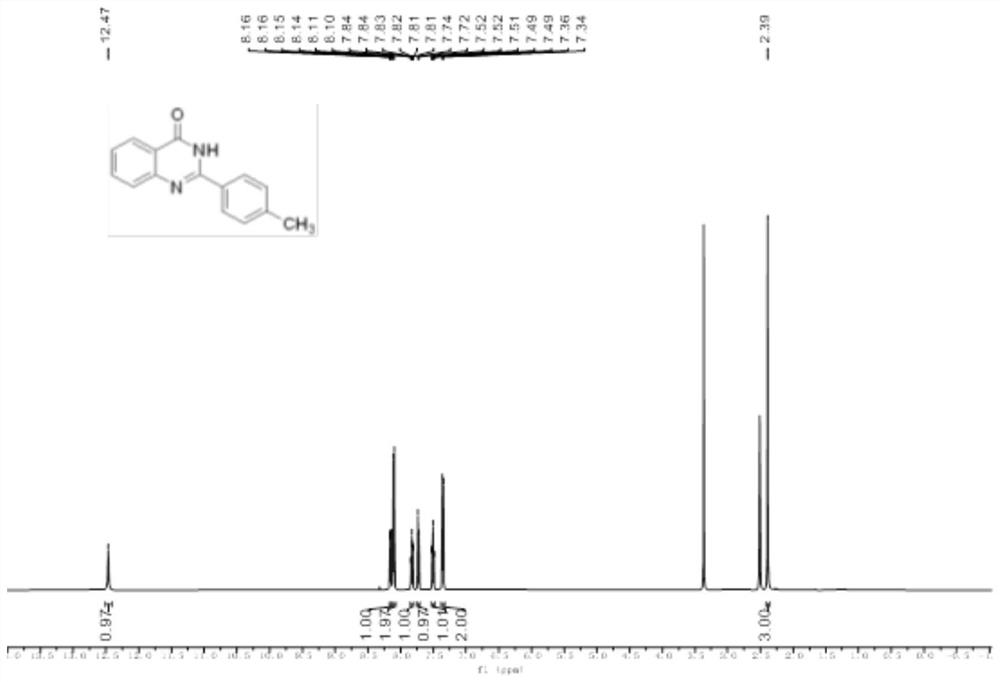
[0044] Add 2-aminobenzamide (0.2mmol), p-tolualdehyde (0.22mmol), and 2mL glacial acetic acid into a 10mL reaction bottle, stir well, and the resulting reaction solution is irradiated by a 5W purple LED lamp and stirred at room temperature for reaction After 16 hours, use TLC to detect that the reaction is complete, add 10 mL of distilled water to the reaction solution, stir at room temperature for 10 minutes, filter, wash the obtained solid with water three times, 3 mL each time, and then vacuum-dry at 40 ° C for 6 hours to obtain a white solid that is the target product , yield 78%.
[0045] The hydrogen spectrogram and the carbon spectrogram of the obtained product are respectively as follows image 3 and Figure 4 As shown, the structural characterization data are as follows:
[0046] 1 H NMR (500MHz, DMSO-d 6 )δ12.47(s,1H),8.1...
Embodiment 3
[0054]A method for visible light-induced synthesis of the following quinazolinone compounds, the specific steps are as follows:
[0055]
[0056] Add 2-aminobenzamide (0.2mmol), p-hydroxybenzaldehyde (0.22mmol), 2mL glacial acetic acid in a 10mL reaction flask, stir well, and the resulting reaction solution is irradiated by a 5W purple LED lamp and stirred at room temperature for 16 hour, after using TLC to detect that the reaction is complete, add 10 mL of distilled water to the reaction solution, stir at room temperature for 10 minutes, filter, wash the resulting solid with water three times, 3 mL each time, and then vacuum-dry at 40 ° C for 6 hours to obtain a white solid that is the target product. Yield 85%.
[0057] The hydrogen spectrogram and the carbon spectrogram of the obtained product are respectively as follows Figure 5 and Image 6 As shown, the structural characterization data are as follows:
[0058] 1 H NMR (500MHz, DMSO-d 6 )δ12.29(s,1H),10.14(s,1H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com