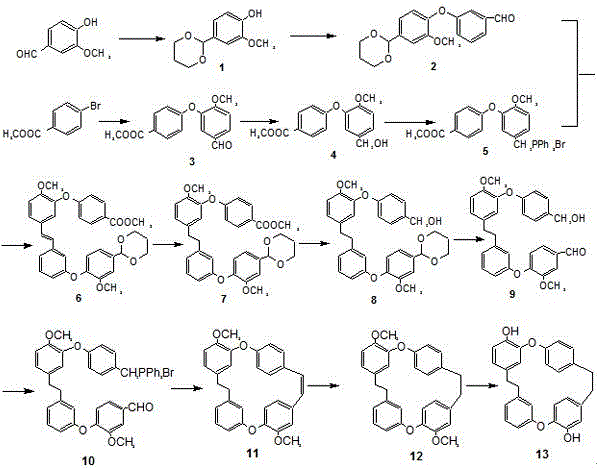
Artificial synthesis method of neomarchantin A
A technology of artificial synthesis and diqin, applied in the direction of organic chemistry, etc., can solve the problems of low availability of new diqin A, hindering research and application, etc., and achieve the effect of strong inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Synthesis of compound 1:
[0045] 1) A mixed solution of 3.9 mL of N,N-dimethylformamide and 4.7 mL of dimethyl sulfate was heated at 50-60 °C for 3-5 h to form a DMF-DMS adduct;
[0046] 2) Dissolve 5g of 4-hydroxy-3-methoxybenzaldehyde in 30mL of dichloromethane, then add 7.2mL of propylene glycol, then add the DMF-DMS adduct prepared above, and react at 20-30°C for 20-26h After the reaction, the system was placed under an ice bath, 6-8 mL of triethylamine was added to the reaction system, extracted three times with 20-25 mL of diethyl ether, the organic layers were combined, and the mixture was mixed with 5% sodium bisulfite and saturated sodium acetate. The solution was washed three times, and then washed three times with a mixture of saturated sodium chloride and saturated sodium acetate in equal proportions. The obtained organic phase was dried with anhydrous sodium sulfate, rotary evaporated, and recrystallized to obtain brown crystals, which was compound 1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com