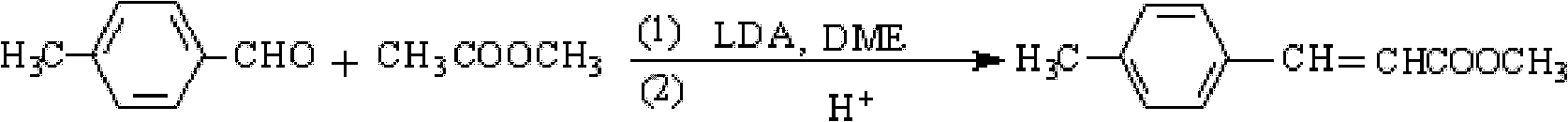Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Lithium diisopropylamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula [(CH₃)₂CH]₂NLi. It is used as a strong base and has been widely accepted due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group.
Parecoxib preparation method
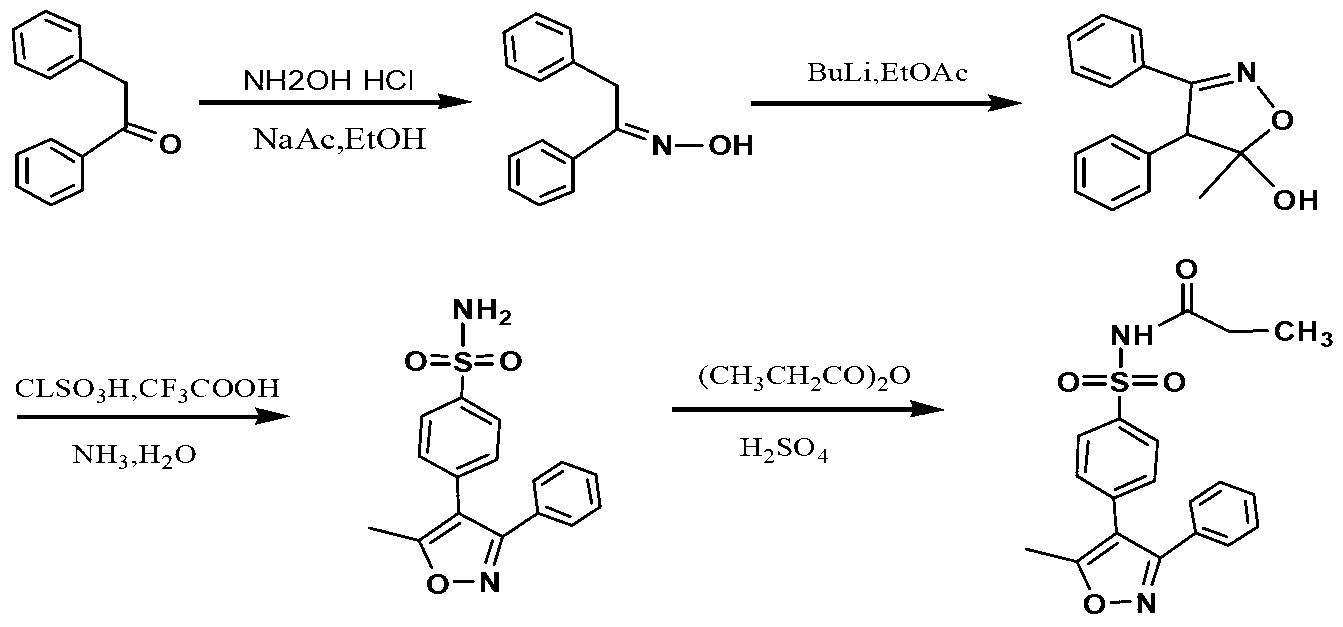
A parecoxib preparation method comprises the following steps: reacting diacetophenone oxime with ethyl acetate in a lithium diisopropylamide / tetrahydrofuran system to generate 4,5-dihydro-5-methyl-5-hydroxy-3,4-dibenzylisoxzzole; reacting 4,5-dihydro-5-methyl-5-hydroxy-3,4-dibenzylisoxzzole with halogenated sulfonic acid, and ammonolyzing to obtain valdecoxib; and reacting valdecoxib with propionic anhydride to obtain parecoxib. Compared with the prior art, the preparation method disclosed in the invention has the advantages of low initial raw material cost, simple and feasible technological process, low equipment requirement, and suitableness for the industrialized large-scale production.
Owner:深圳市资福药业有限公司
Compounding method for 3- ethyoxyl-4-ethoxycarbonyl phenylacetic acid
ActiveCN101891621AEasy to operateRealize industrial productionOrganic compound preparationCarboxylic acid esters preparationN dimethylformamidePhenylacetic acid
The invention discloses a compounding method for 3- ethyoxyl-4-ethoxycarbonyl phenylacetic acid, comprising the following steps: making 4-methyl salicylate and bromoethane which are used as starting raw materials to carry out double alkylation reaction in N,N-dimethylformamide to prepare 2-ethyoxyl-4-ethyl methylbenzoate; directly dissolving the unpurified 2-ethyoxyl-4-ethyl methylbenzoate into 2-methyl tetrahydrofuran, adding lithium diisopropylamide, and introducing carbon dioxide; acidizing the mixture by sulphuric acid to prepare the 3-ethyoxyl-4-ethoxycarbonyl phenylacetic acid. The product of the invention is white powders with a melting point of 78 to 80 degrees and the content of 99.4%. In the invention, the solvent of N, N-dimethylformamide and 2-methyl tetrahydrofuran can be recycled and repeatedly applied. The invention has the advantages of simple operation, environment friendliness, simple industrial operation, industrial production realization and low cost due to the adoption of the common industrial raw materials.
Owner:QIDONG HUDONG CHEM
Preparation method of methyl 4-methylcinnamate
InactiveCN102627559AIncrease exposureHigh yieldOrganic compound preparationCarboxylic acid esters preparationDistillationOrganic layer
The invention provides a preparation method of methyl 4-methylcinnamate. The preparation method comprises the following steps: stir-mixing glycol dimethyl ether which is used as a solvent, lithium diisopropylamide which is used as a catalyst, and methyl acetate, and slowly adding p-tolualdehyde in a dropwise manner under stirring at below 5DEG C; reacting for 3-6h at 0-10DEG C after ending the dropwise addition, and slowly adding sulfuric acid with the mass percent of 10% in a dropwise manner to regulate the pH of a reaction solution between 6.8-7.2; and separating the resulting organic layer, carrying out reduced pressure distillation to recover the solvent and excess p-tolualdehyde and obtain a white solid, washing the white solid with water, drying to obtain crude methyl 4-methylcinnamate, and recrystallizing the crude methyl 4-methylcinnamate to obtain methyl 4-methylcinnamate. The preparation method which has the advantages of low costs of raw materials, mild reaction condition, preparation of methyl 4-methylcinnamate through a one-step reaction, simplicity, easy operation, high yield, stable and reliable product quality, and no pollution to environment is a green synthetic method.
Owner:湖北远成赛创科技有限公司
Use of lithium diisopropyl amido in 1-methyl cyclopropene preparation
ActiveCN101486721AHigh yieldSolve the technical problems of high energy consumption, low content and high costPlant growth regulatorsLithium organic compoundsHigh energy1-Methylcyclopropene
The invention relates to the application of lithium diisopropylamide which is an organic alkali in preparing 1-methylcyclopropene; the application of the lithium diisopropylamide as a catalyst to prepare the 1-methylcyclopropene well solves the technical problem that the production course of the 1-methylcyclopropene has high energy consumption, low content and high cost, simplifies the production processes, reduces the production costs and realizes high yield of 1-methylcyclopropene.
Owner:LINHAI LIANSHENG CHEM
Preparation method of iodine atom-substituted bis-fluorophenyl heterocyclic conjugated monomer
ActiveCN104610178AExperimental operation safetyHigh synthetic yieldOrganic chemistryChemical compoundReaction system
The invention relates to a preparation method of an iodine atom-substituted bis-fluorophenyl heterocyclic conjugated monomer. The preparation method comprises the following steps: firstly enabling a chemical compound with a bis-fluorophenyl heterocyclic conjugated structure to be reacted with lithium diisopropylamide, then adding an iodine elementary substance into a reaction system and finally introducing iodine atoms onto a benzene ring of the bis-fluorophenyl heterocyclic conjugated monomer. According to the method, the use of fuming sulfuric acid and other hazardous reagents is avoided, the synthetic efficiency and the experimental operation safety are improved, the yield is much higher than that obtained by a traditional iodization method, and the scale preparation and production are facilitated.
Owner:SOUTH CHINA UNIV OF TECH
Beta-hydroxy imine titanium metal catalyst and method for polymerizing ethylene
InactiveCN101880338ANovel structureHigh catalytic activityTitanium organic compoundsImino compound preparationImideTitanium metal
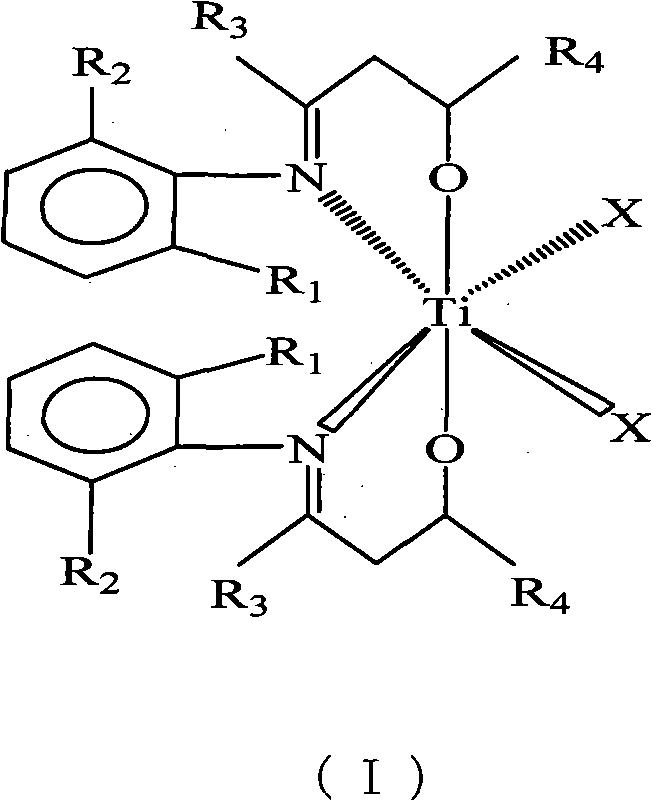
The invention discloses a beta-hydroxy imine titanium metal catalyst for polymerizing ethylene and a method for polymerizing ethylene. The catalyst can be prepared by the following steps: reacting an imide compound with lithium diisopropylamide; reacting the reacted imide compound with aldehyde or ketone to generate a beta-hydroxy imine ligand; and reacting the ligand with a titanium metal compound to generate the beta-hydroxy imine titanium metal catalyst. By adjusting the substituent group structure of the imide compound and the types of the aldehyde or ketone and the polymerization conditions, the controllability of the catalytic polymerization on the ethylene can be realized. The catalyst is a novel non-metallocene transition metal catalyst and has the characteristics of novel structure, high catalytic activity, easily-accessible raw materials, convenient preparation and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
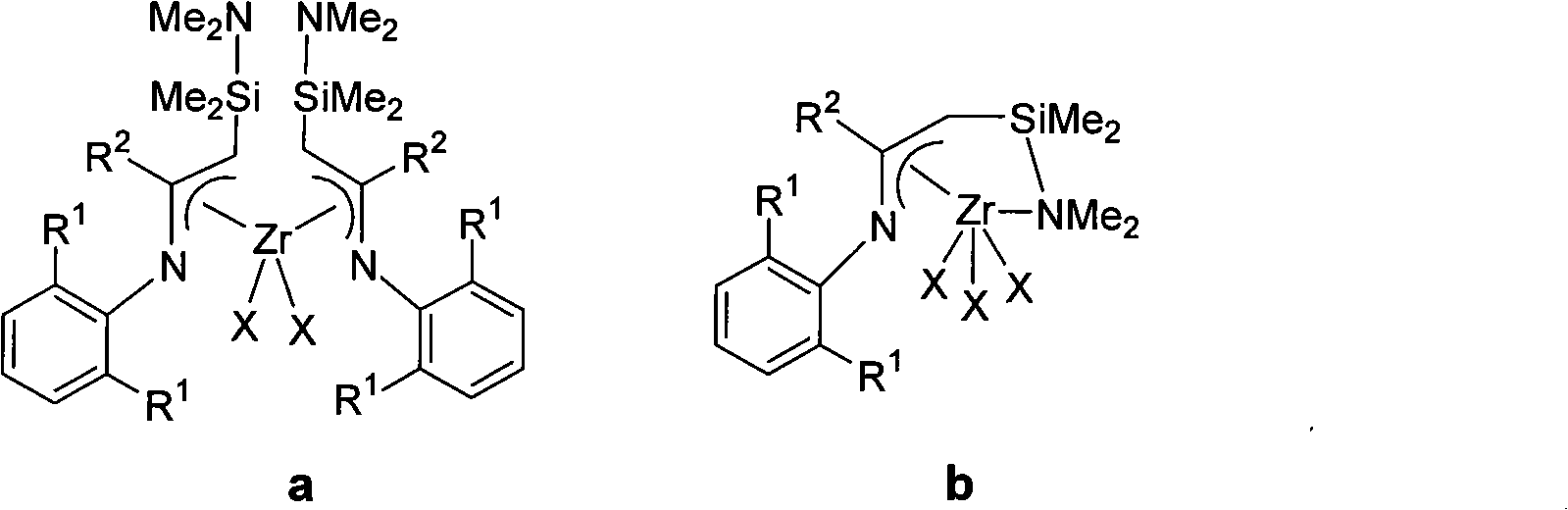
Amino-silicone bridge nitrogen-blending allyl zirconium catalyst and preparation method and application thereof
InactiveCN101786018AEasy to prepareRaw materials are easy to obtainOrganic-compounds/hydrides/coordination-complexes catalystsMagnesium saltNitrogen gas
The invention provides an amino-silicone bridge nitrogen-blending allyl zirconium catalyst, relating to a vinyl-polymerization IVB transition metal catalyst, in particular to a nitrogen-blending allyl ligand zirconium catalyst with an N-C-C-Si-N skeleton. The preparation method of the catalyst comprises the following steps of: dehydrogenating ketimine containing a substituent group in the protection of nitrogen by using lithium diisopropylamide (LDA); adding equimolar magnesium bromide to obtain magnesium salts A and then adding equimolar silane compounds to prepare a compound B; dehydrogenating once again by using the LDA to prepare amino-silicone bridge nitrogen-blending allyl lithium C; and finally reacting with ZrC14 to obtain the amino-silicone bridge nitrogen-blending allyl zirconium catalyst. The preparation method has the advantages of simplicity, simple and easy obtainment of materials, low cost and higher yield; and the catalyst displays very high catalytic activities when used for vinyl polymerization.
Owner:SHANXI UNIV
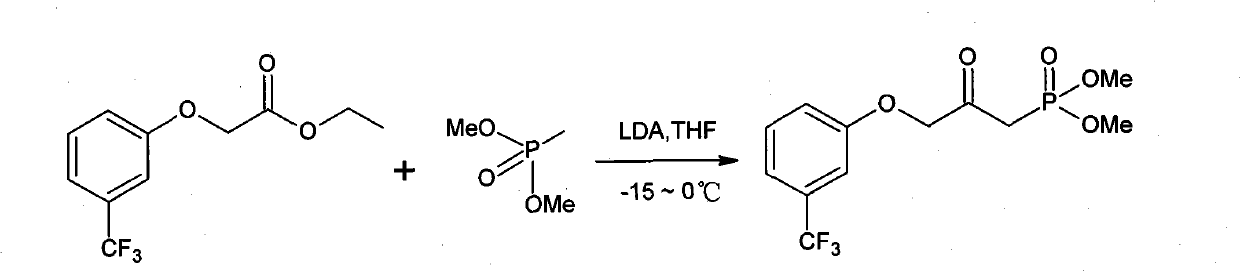
Method for preparing travoprost intermediate
ActiveCN101948484ASimple processSuitable for industrial productionGroup 5/15 element organic compoundsAcetic acidOrganic solvent
The invention relates to a method for preparing a travoprost intermediate, which is prepared by performing a reaction of dimethyl methyl phosphonate, (3-trifluoromethyl phenoxyl) ethyl acetate and lithium diisopropylamide in an organic solvent. The method for preparing the travoprost intermediate has the advantages of simple process, mild reaction conditions, high yield and suitability for industrialized production.
Owner:湖北远大天天明制药有限公司
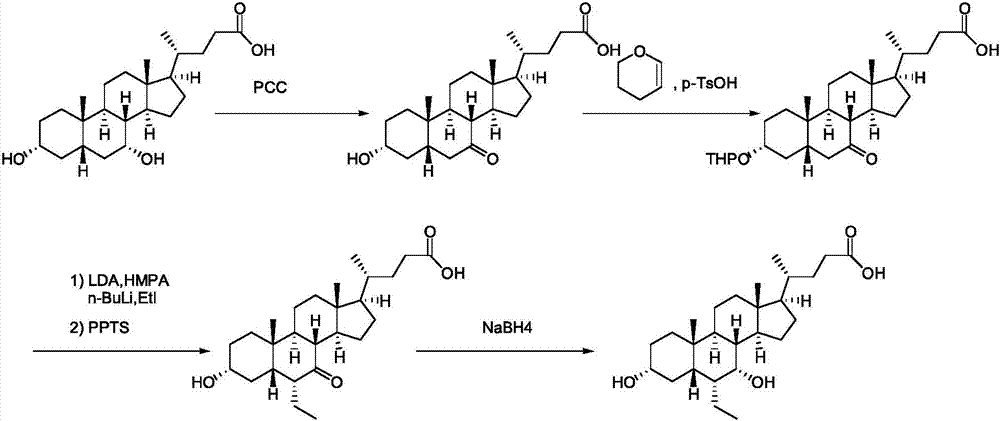
Preparation method for obeticholic acid
InactiveCN104926909AEasy to operateHigh yieldSteroidsBulk chemical productionEtherSodium borohydride
The invention provides a preparation method for obeticholic acid. The preparation method comprises the following steps of: adding 3alpha-hydroxyl-7-keto-5beta-cholanic acid and methanol into a reaction container and carrying out esterification reaction in an acidic environment to obtain 3alpha-hydroxyl-7-keto-5beta-methyl cholanate; adding 3,4-dihydro-2H-pyran and dioxane and protecting hydroxyl by tetrahydropyrane ether; adding bromoethane, lithium diisopropylamide and site alpha of diastereomeric methylated keto-carbonyl; then adding methanol, removing the protecting group which protects tetrahydropyrane ether and reducing hydroxyl in the acidic environment; adding sodium borohydride and reducing the mixture to 3alpha-hydroxyl-6beta-methyl-7-hydroxyl-5beta-methyl cholanate; and adding methanol and carrying out alkali hydrolysis to obtain 3alpha-hydroxyl-6beta-methyl-7-hydroxyl-5beta-cholanic acid, namely the target product obeticholic acid. According to the preparation method provided by the invention, the process line is mild in reaction, simple to operate and high in yield.
Owner:ZHEJIANG TIANSHUN BIOTECH
A seedling culture method for interplanting of paeonia suffruticosa for oil and garlic
InactiveCN105941057AImprove water absorptionPromote softeningSeed and root treatmentPlant cultivationPaeonia suffruticosaMoisture capacity
The invention provides a seedling culture method for interplanting of paeonia suffruticosa for oil and garlic and relates to the technical field of paeonia suffruticosa planting. The method comprises the steps of sowing seeds of paeonia suffruticosa for oil and garlic at the same time from the beginning of September to the beginning of October; selecting seeds by using a water selection method before sowing, soaking the seeds in a mixed liquid of potassium pyrophosphate and lithium diisopropylamide with a mass concentration of 1-5% and at a temperature of 40-50 DEG C for 20-40 hours, the mass ratio of the potassium pyrophosphate to the lithium diisopropylamide being 3-4:1, and performing disinfection treatment on the seeds for sowing when the seeds fully absorb water and expand; during seeding, maintaining a field moisture capacity of 65-75%, forming furrows 8-12cm deep and 4-5cm wide with a line spacing of 15-20cm in ready-made high ridges for sowing seeds, scattering the seeds in the furrows uniformly with a distance of 1-2cm, and covering the seeds with 3-5cm soil; performing field management. The method has the advantages of good germination accelerating effect, high germination rate, high survival rate, simple and convenient operation and low cost.
Owner:LANGXI TIANXIANG OIL PEONY CULTIVATION SPECIALIZED COOP
Obeticholic acid preparation method
The invention discloses an obeticholic acid preparation method, which comprises that (1) hyodeoxycholic acid II reacts with an alcohol compound III under the action of a catalyst to generate an ester compound IV; (2) the ester compound IV is subjected to PDC oxidation in dichloromethane to generate a compound V; (3) the compound V and trimethyl chlorosilane are subjected to a reaction at a temperature of -70 to -20 DEG C in tetrahydrofuran by using lithium diisopropylamide as an alkali to generate a silyl enol ether compound VI; (4) the silyl enol ether compound VI is subjected to m-chloroperoxybenzoic acid oxidation and deprotection in dichloromethane to generate a compound VII: (5) the compound VII and Yield generated from ethyltriphenylphosphonium bromide under the action of a strong alkali are subjected to a Wittig alkenylation reaction at a temperature of 0-70 DEG C to convert the ketone into the vinyl so as to generate a compound VIII; (6) the double bond of the compound VIII is subjected to catalytic hydrogenation reduction in a mixing solvent to generate a compound IX; and (7) the compound IX is hydrolyzed under an alkaline condition to generate the obeticholic acid.
Owner:XIAMEN HALOSYNTECH CO LTD
Ratio two-photon and near-infrared HClO detection fluorescence probe as well as preparation and application thereof
ActiveCN108947951ASimple structureSensitive highOrganic chemistryFluorescence/phosphorescenceInfraredStructural formula
The invention relates to a ratio two-photon and near-infrared HClO detection fluorescence probe as well as preparation and application thereof and belongs to the technical fields of synthesis and detection. The structural formula of the fluorescence probe is as shown in the specification. The fluorescence probe is prepared by the following steps: 1, reacting 4-(diethylamino) salicylaldehyde with ethyl acetoacetate and piperidine; 2, enabling 4-diethylaminobenzaldehyde to participate in the reaction; and 3, enabling cyclohexene to participate in the reaction to obtain a compound, respectively reacting the compound with lithium diisopropylamide and phenylselenenyl bromide, thereby obtaining the fluorescence probe. The probe can be reacted with the HClO to produce olefinic bonds, wavelength red shift is caused by the enlarged conjugated structure, and the ratio fluorescence change of which the short wavelength fluorescence intensity is reduced and long wavelength fluorescence intensity isenhanced is formed. Therefore, the design of the probe based on the Sharpless alkene synthesis reaction can be used for detecting the HClO, and the probe is simple in structure and has the advantagesof being high in selectivity, high in sensitivity, fast in transient response and the like.
Owner:SHANDONG NORMAL UNIV
Preparation process of aprepitant
The invention discloses a preparation process of aprepitant. The process comprises the steps as follows: Step 1, a compound A and a compound B are firstly added to a mixed solvent of N,N-dimethylformamide and triethylamine to be stirred and dissolved to obtain a mixed solution; Step2, adding lithium diisopropylamide to the mixed solution in a nitrogen atmosphere and performing uniform mixing; Step3, dropwise adding a diethyl sulfate xylene solution under conditions of nitrogen shielding and stirring; Step 4, after addition, conducting a stirring reaction at 30-35 DEG C for 12-15 h under shielding of nitrogen; Step 5, separating and purifying a reaction product in Step 4 to obtain a final aprepitant product. By process improvement and formula optimization, the aprepitant synthesis processhas the characteristics of mild reaction condition, low cost and the like and is suitable for large-scale industrial production; the synthesized aprepitant product has low impurity content and high purity, and the yield of aprepitant is greatly increased.
Owner:成都晶富医药科技有限公司
Method for preparing hydroxyl phosphonate
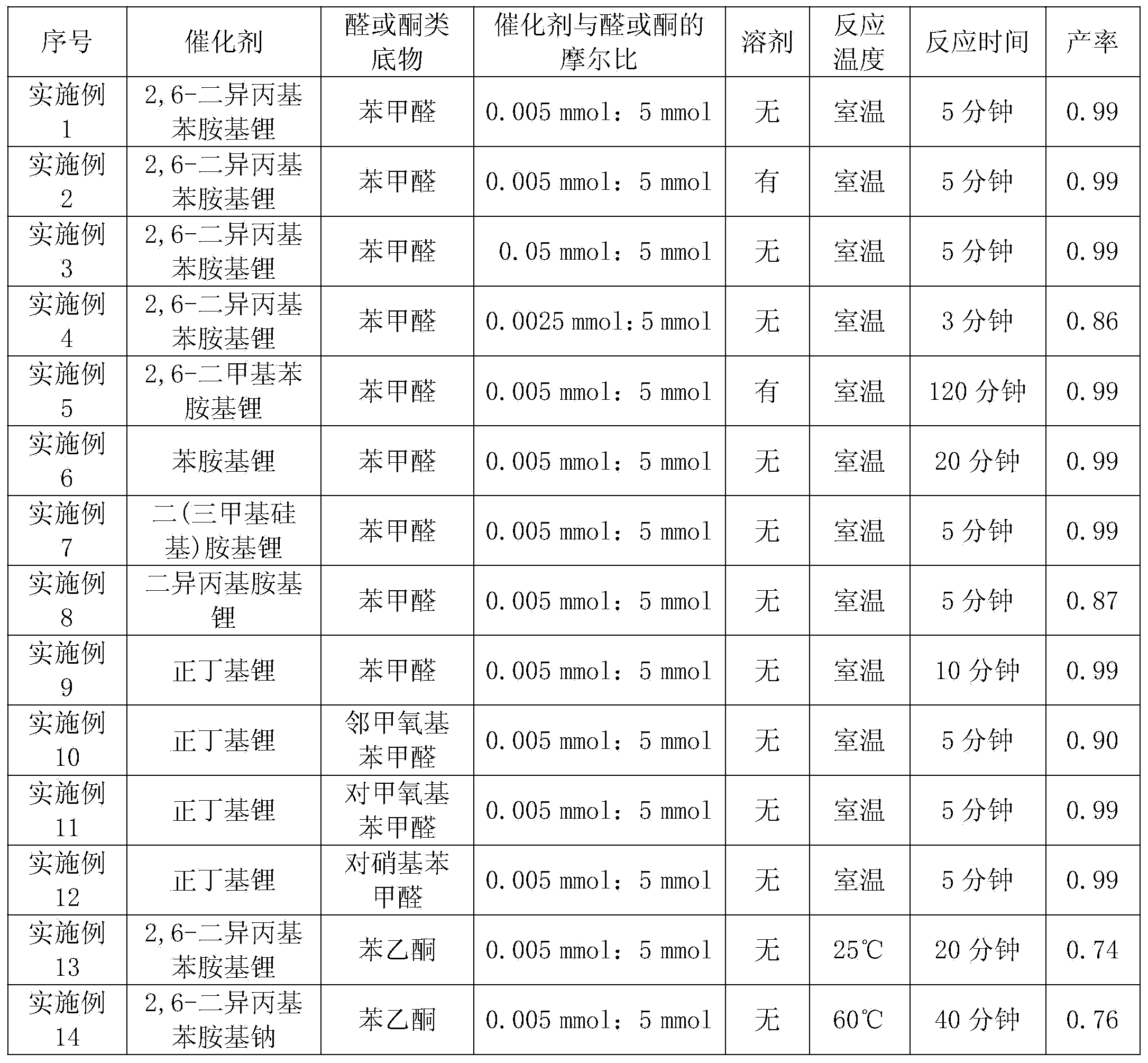
InactiveCN104045666AReduce dosageHigh catalytic activityGroup 5/15 element organic compoundsDimethylaniline N-oxidePotassium
The invention discloses a method for preparing hydroxyl phosphonate. According to the method, an alkali metal compound is used as a monocomponent catalyst to catalyze a hydrogen phosphonylation reaction of aldehyde or ketone so as to prepare hydroxyl phosphonate. The alkali metal compound is one compound selected from n-butyllithium, 2,6-lithium diisopropyl aniline, 2,6-sodium diisopropyl aniline, 2,6-potassium diisopropyl aniline, 2,6-lithium dimethylaniline, 2,6-sodium dimethylaniline, 2,6-potassium dimethylaniline, phenylamino lithium, phenylamino sodium, phenylamino potassium, bi(trimethylsilyl) amido lithium, bi(trimethylsilyl)amido sodium, bi(trimethylsilyl)amido potassium, lithium diisopropylamide, sodium diisopropylamide and potassium diisopropylamide. The method provided by the invention has advantages as follows: catalytic activity is high; reaction time is short; dosage of the catalyst can be reduced to one thousand; reaction conditions are mild; yield is high; and the method has good universality for aldehyde and ketone substrates.
Owner:ZHANGJIAGANG INST OF IND TECH SOOCHOW UNIV
Synthesis method of remdesivir intermediate
InactiveCN111187269AReduce usageLow costOrganic chemistryBulk chemical productionTetrahydrofuranolIsopropyl
The invention relates to the technical field of medical intermediates, in particular to a synthetic method of a remdesivir intermediate. The chemical name of the intermediate is (3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-di(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol. The preparation method comprises the following steps: performing amino protection on pyrrolo[2,1-F][1,2,4]triazin-4-amine with a protecting agent; reacting with lithium diisopropylamide; then adding 2,3,5-tribenzyloxy-D-ribonic acid 1,4-lactone, and finally removing the protective group to obtain the remdesivir intermediate that is the (3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-di(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol.
Owner:JIANGSU ALPHA PHARM CO LTD
Preparation method of voriconazole
InactiveCN104744441AEasy to prepareReduce manufacturing costOrganic chemistrySodium acetateFormamidine acetate
The invention relates to a preparation method of voriconazole, wherein the preparation method comprises the following specific steps: adding alpha-fluoropropionyl ethyl acetate and a methanol solution of formamidine acetate into a methanol solution of sodium methylate, carrying out cyclization to obtain 6-ethyl-5-fluoropyrimidine-4(3H)-one, chloridizing to obtain 4-chloro-6-ethyl-5-fluoropyrimidine, dissolving 4-chloro-6-ethyl-5-fluoropyrimidine in tetrahydrofuran, dropwise adding into a tetrahydrofuran solution of lithium diisopropylamide, adding a tetrahydrofuran solution of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole-1-yl)ethyl ketone, carrying out a reaction to obtain 3-(4-chloro-5-fluoropyrimidine-6-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazole-1-yl)butyl-2-ol, dissolving in ethanol, adding 10% palladium-carbon and sodium acetate, and then splitting to obtain voriconazole. According to the preparation method of voriconazole, the preparation method is simple, and the production cost is low.
Owner:李磊
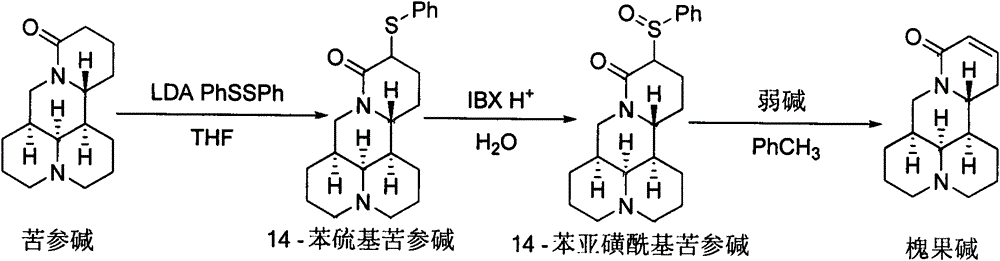
Method for synthesizing sophocarpine
The invention provides a new method for synthesizing sophocarpine. The method comprises the following steps: removing alpha-position hydrogen in amide in matrine by taking matrine as a raw material and taking lithium diisopropylamide (LDA) as alkali, then directly performing nucleophilic substitution reaction with diphenyl disulfide to obtain 14-thiophenyl matrine, oxidizing by IBX (o-iodyl-benzoic acid) in a water phase to obtain 14-phenylsulfinyl matrine and eliminating benzenesulfenic acid under the assistance of weak alkali to obtain sophocarpine. The method provided by the invention takes the step of oxidizing a nitrogen-containing thioether compound with IBX in a water phase to form sulfoxide as the key step for synthesizing sophocarpine. The method is characterized by high efficiency, environmental friendliness and easiness in operation. The synthesis route is as shown in the description.
Owner:NANKAI UNIV
O-methoxy phenylhydrazone dicaryon metal complex and preparation method and application thereof
InactiveCN105503939ASimple manufacturing methodRaw materials are cheap and easy to getTitanium organic compoundsDual coreBiological activation
The invention provides an o-methoxy phenylhydrazone dicaryon metal complex, a preparation method thereof. The complex is used as a catalyst for preparing high-molecular-weight polyethylene. The complex is obtained through the method including the steps that o-methoxy phenylhydrazone reacts with a tetrahydrofuran solution with a half of molar weight of TiX4(THF)2 after being subjected to the hydrogen abstraction together with lithium diisopropylamide (LDA). The prepared dual-core dicaryon complex serving as the catalyst is easy to prepare, applied raw materials are low in price and easy to obtain, and the activation of methylaluminoxane (MAO) or modified methylaluminoxane (MMAO) can be used for catalyzing vinyl polymerization. It is shown through experiment results that the catalyst system can be used for preparing high-molecular-weight linear polyethylene, and most of obtained polyethylene has the weight-average molecular weight above one million, the high melting point between 133.4 DEG C and 134.8 DEG C, the molecular weight between 1.90 and 3.33 and intermediate catalysis activity.
Owner:SHANXI UNIV
Synthesis method of 3-bromine-2-fluorobenzoic acid
ActiveCN108084013AAvoid problemsMeet quality requirementsSilicon organic compoundsOrganic compound preparationTrimethylsilyl chlorideOrganic synthesis
The invention discloses a synthesis method of 3-bromine-2-fluorobenzoic acid, and belongs to the field of organic synthesis. The synthesis method of the 3-bromine-2-fluorobenzoic acid comprises the following steps: taking fluorobenzene as a starting material, deprotonating lithium diisopropylamide, carrying out a reaction with trimethylchlorosilane to generate 2-fluorophenyl trimethylsilane, forming an intermediate 3-trimethyl silicon-2-fluorinated phenyl lithium salt, carrying out a reaction with carbon dioxide to generate 3-trimethyl silicon-2-fluorobenzoic acid, and carrying out a reactionwith a bromide reagent to obtain the 3-bromine-2-fluorobenzoic acid. The intermediate obtained by the method avoids separation of an isomer, the obtained product has high purity, and a concise approach is provided for synthesis of the compound.
Owner:烟台市蓬莱区融欣化工有限公司
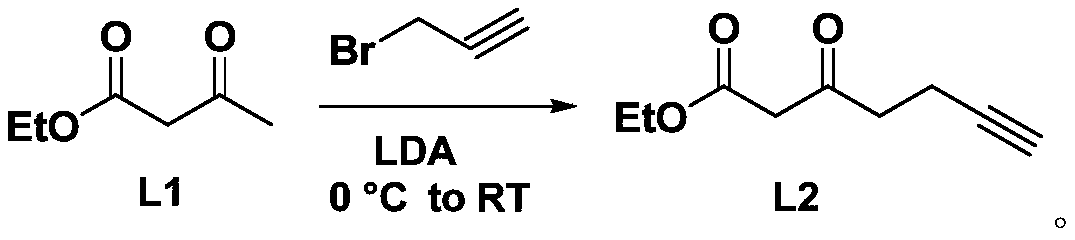
Amino acid compound containing diaziridine group and synthesis method thereof
The invention discloses an amino acid compound containing a diaziridine group and a synthesis method thereof. The method comprises the following steps: with ethyl acetoacetate as a raw material, performing a reaction under the action of lithium diisopropylamide; performing a reaction with propargyl bromide to generate 3-oxoheptyl-6-acetylenic acid ethyl ester; then hydrolyzing the reaction productto generate 3-oxoheptyl-6-acetylenic acid, so as to obtain 3-oxoheptyl-6-acetylenic acid; then performing a reaction with hydroxylamine-O-sulfonic acid in an ammonia gas methanol solution; performingiodine elementary substance oxidation to generate 2-(3-(butyl-3-acetylene-1-yl)-3H-diaziridine-3-yl)acetic acid; then carrying out a reaction on 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and N-hydroxysuccinimide to generate 2,5-dioxopyrrolidine-1-yl-2-(3-(butyl-3-yl-1-yl)-3H-diaziridine-3-yl)acetate, and then carrying out a reaction with amino acid molecules to generate the targetproducts. The compound synthesized by the invention contains diaziridine groups and amino acid molecules with biological activity, and can be used in biological activity test and analysis.
Owner:NANJING UNIV OF SCI & TECH
Temperature-control seedling raising method of rice in greenhouse
InactiveCN106171696AImprove water absorptionPromote softeningOrganic fertilisersRice cultivationTemperature controlHusk
The invention provides a temperature-control seedling raising method of rice in a greenhouse, and relates to the technical field of planting of rice. The method comprises the following steps of building the greenhouse; performing sowing in good time; treating seeds: firstly, selecting seeds by a water test method before sowing, soaking the seeds with mixed liquid of potassium pyrophosphate and lithium diisopropylamide, of which the mass concentration is 1-5% at 15-20 DEG C, for 2-3 days, wherein the proportion of the potassium pyrophosphate to the ithium diisopropylamide being (3-4) to 1, finding that rice husk is translucent, the rice bodies are white and clear, and the germs are expanded through observation, and performing disinfection treatment; then performing coating with a coating material, performing drying until sticking hands disappears, and then performing sowing; performing a sowing method; and performing field management. The germination accelerating effect is good, the germination percentage is high, the survival rate is high, the operation is simple and convenient and the cost is low.
Owner:全椒县西王镇发全家庭农场
Seedling growing method for oil peonies
InactiveCN105961002AImprove water absorptionPromote softeningPlant cultivationCultivating equipmentsLithium diisopropylamidePotassium Pyrophosphate
The invention discloses a seedling growing method for oil peonies, and relates to the technical field of peony planting. The seedling growing method is characterized by including the following steps that the seeding time of oil peonies is selected to be from early September to early October; before seeding, seed selection is carried out with the water selecting method, seed soaking is carried out for 20 hours to 40 hours with mixed liquid of potassium pyrophosphate with the mass concentration of 1%-5% at the temperature of 40 DEG C to 50 DEG C and lithium diisopropylamide, the mass ratio of the potassium pyrophosphate to the lithium diisopropylamide is (3-4):1, and after seeds fully absorb water, swell and are disinfected, seeds can be used for seeding; seeding is carried out with the seeding method, the field water capacity is 65% to 75%, ditching and seeding are carried out on made high ridges, ditching is carried out according to the row spacing of 15 cm to 20 cm, the ditch depth is 8 cm to 12 cm, the width is 4 cm to 5 cm, seeds are evenly scattered into ditches, the intervals between seeds is 1 cm to 2 cm, and then 3-5-cm earthing is carried out; field management is carried out. The seedling growing method is good in sprouting effect, high in germination rate and survival rate, easy and convenient to operate and low in cost.
Owner:LANGXI TIANXIANG OIL PEONY CULTIVATION SPECIALIZED COOP
Preparation method for 2-fluoropyridine-4-boric acid
InactiveCN104478913AHigh yieldRaw materials are cheap and easy to getGroup 3/13 element organic compoundsN-ButyllithiumIodine
The invention discloses a preparation method for 2-fluoropyridine-4-boric acid. The preparation method comprises the following steps: firstly, reacting 2-fluoropyridine with iodine in the presence of LDA (lithium diisopropylamide) to obtain an intermediate A; secondly, reacting the intermediate with water in the presence of LDA to obtain an intermediate B; thirdly, reacting the intermediate B with triisopropyl borate in the presence of n-butyl lithium to obtain the target product-2-fluoropyridine-4-boric acid. According to the preparation method, raw materials are simple and easily available, the process is safe and reliable, and the target product-2-fluoropyridine-4-boric acid is an important common intermediate in pharmaceutical chemistry.
Owner:DALIAN NETCHEM CHIRAL TECH
Synthetic method of bepidic acid
ActiveCN112479856AEasy to operateReduce manufacturing costElectrolysis componentsOrganic compound preparationPotassium hydroxideMalonate
The invention belongs to the field of medicine synthesis, and relates to a novel synthesis method of a compound bepidic acid. The preparation method comprises the following steps: (1) carrying out primary alkylation on ethyl isobutyrate and 1, 5-dibromopentane under the action of lithium diisopropylamide to generate a compound 1; (2) carrying out alkylation twice on dibenzyl malonate and the compound 1 under the action of sodium hydride to generate a compound 2; (3) removing benzyl from the compound 2 under the action of Pd / C and hydrogen to obtain a compound 3; (4) carrying out electrochemical reaction on the compound 3 in a methanol solution of methanol and ammonia to obtain a compound 4; (5) finally carrying out sodium borohydride hydrolysis and potassium hydroxide hydrolysis on the compound 4, and acidifying to obtain the final bepidic acid. The method is carried out under electrochemical conditions, is mild in conditions and high in efficiency, and is suitable for industrial production.
Owner:NANJING UNIV OF TECH
Synthesis and chiral resolution method for (R)-1-t-butyloxycarbonyl-3-benzyl-3-formate piperidine
InactiveCN106187865AImprove responseShort reaction timeOrganic chemistry methodsFormateTert-Butyloxycarbonyl protecting group
The invention belongs to the technical field of medical intermediate synthesis and chiral resolution, and particularly relates to a synthesis and chiral resolution method for (R)-1-t-butyloxycarbonyl-3-benzyl-3-formate piperidine. The method comprises the following steps that 3-piperidine-2-carboxylic acid is adopted as a raw material, 3-piperidinecarboxylicacid and thionyl chloride are subjected to an esterification reaction according to the molar ratio being 1:(1.2-1.5), the obtained product and di-tert-butyl dicarbonate ((Boc)2O) are firstly subjected to an addition reaction, then the obtained product and LDA (lithium diisopropylamide) are subjected to a hydrogen extracting reduction reaction, then the product obtained in the last step is subjected to the hydrolysis reaction of LiOH.H2O, and finally the product and R-(+)-alpha-methylbenzylamine are subjected to chiral resolution to obtain the target product. The method has the advantages of being cheap and available in raw material, simple in synthesis process, controllable in reaction, simple in step, low in cost, high in yield and the like, and has good application prospects.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
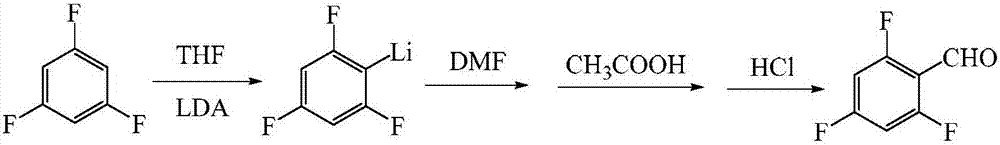
A method of preparing 2,4,6-trifluorobenzaldehyde
ActiveCN107043322ARaw materials are easy to obtainSimple production processLithium organic compoundsOrganic compound preparationAcetic acidAfter treatment
A method of preparing 2,4,6-trifluorobenzaldehyde is provided. The method includes (1) preparing a tetrahydrofuran solution of lithium diisopropylamide, and keeping the solution for later use; (2) cooling to a temperature ranging from -85 DEG C to -80 DEG C, adding dropwise the tetrahydrofuran solution of the lithium diisopropylamide into a tetrahydrofuran solution of 1,3,5-trifluorobenzene under nitrogen protection, and reacting at a maintained temperature for 3-5 h after the addition is finished; (3) adding dropwise dimethylformamide into the reaction solution, and maintaining the temperature at a temperature ranging from -80 DEG C to -75 DEG C for 50-70 min; (4) maintaining the temperature to be -5-0 DEG C, adding dropwise glacial acetic acid, water and diluted hydrochloric acid in order into the reaction solution, adjusting the pH to be 1-2, and stirring the solution for 10-20 min after addition is finished to obtain a crude product; and (5) subjecting the crude product to after-treatment to obtain a target product that is the 2,4,6-trifluorobenzaldehyde. The lithium diisopropylamide that is a non-nucleophilic organic base is applied, thus providing a novel route for preparing the 2,4,6-trifluorobenzaldehyde.
Owner:SHANGYU HUALUN CHEM
Method for preparing (4R-cis)-6-substituted-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate
ActiveCN102180862BHigh yieldLow costOrganic chemistryBulk chemical productionBlaise reactionAcetic acid
The invention relates to a method for preparing (4R-cis)-6-substituted-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate with a structural formula (VIII) shown in the specifications. In the formula, R is formaldehyde or methanol. In the preparation method, Blaise reaction is used for replacing complicated Claisen ester condensation reaction with a large number of side reactions, the aim of growing a carbon chain is fulfilled under the mild condition, and expensive lithium diisopropylamide (LDA) and a low-temperature environment is avoided; and a complicated route of oxidizing alcohol into aldehyde is avoided in the process of generating the compound VIII.
Owner:VALIANT CO LTD
Novel preparation method for butyrolactone derivative
The invention discloses a novel preparation method for a butyrolactone derivative. The method includes the steps of: (1) reacting pentanoic acid shown as formula (II) with ethyl bromoacetate under theaction of lithium diisopropylamide to obtain a compound shown as formula (III); (2) splitting the compound shown as formula (III) with chiral phenylethylamine to obtain a compound shown as formula (IV); (3) subjecting the compound shown as formula (IV) to reduction of carboxyl by borane so as to obtain a compound shown as formula (V); and (4) carrying out cyclization reaction on the compound shown as formula (V) to obtain a butyrolactone derivative shown as formula (I). The brand new butyrolactone derivative synthesis method provided by the invention has the advantages of low cost of synthetic raw material pentanoic acid, need of just 4-step reaction, and good stereoselectivity, and can obviously reduce the production cost. And the synthesis route is shown as the specification.
Owner:安徽华胜医药科技有限公司
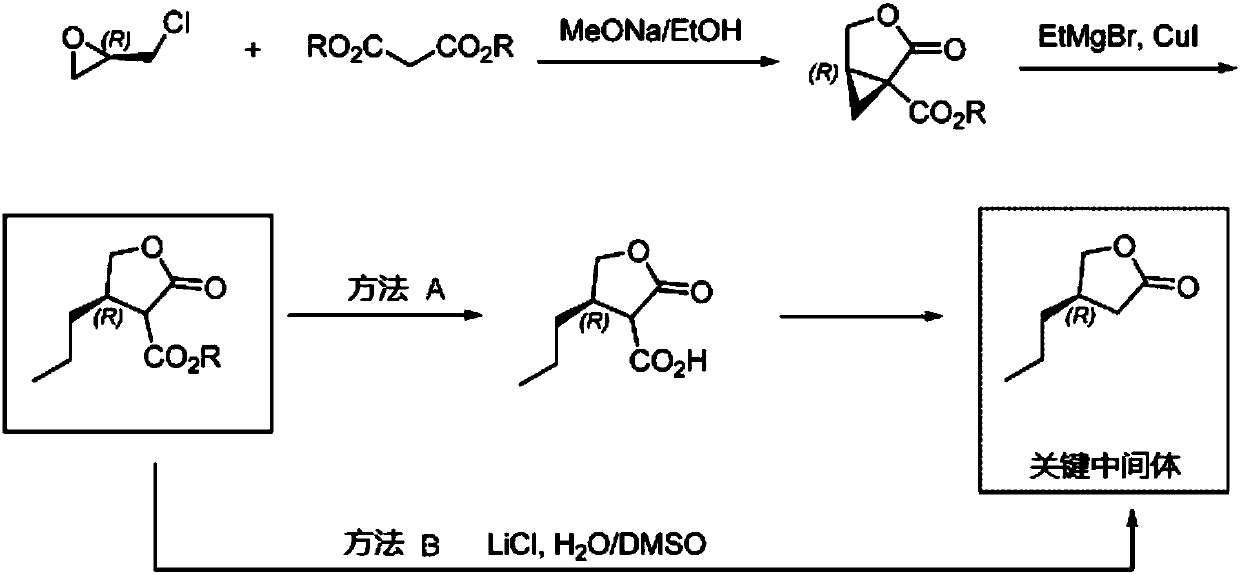
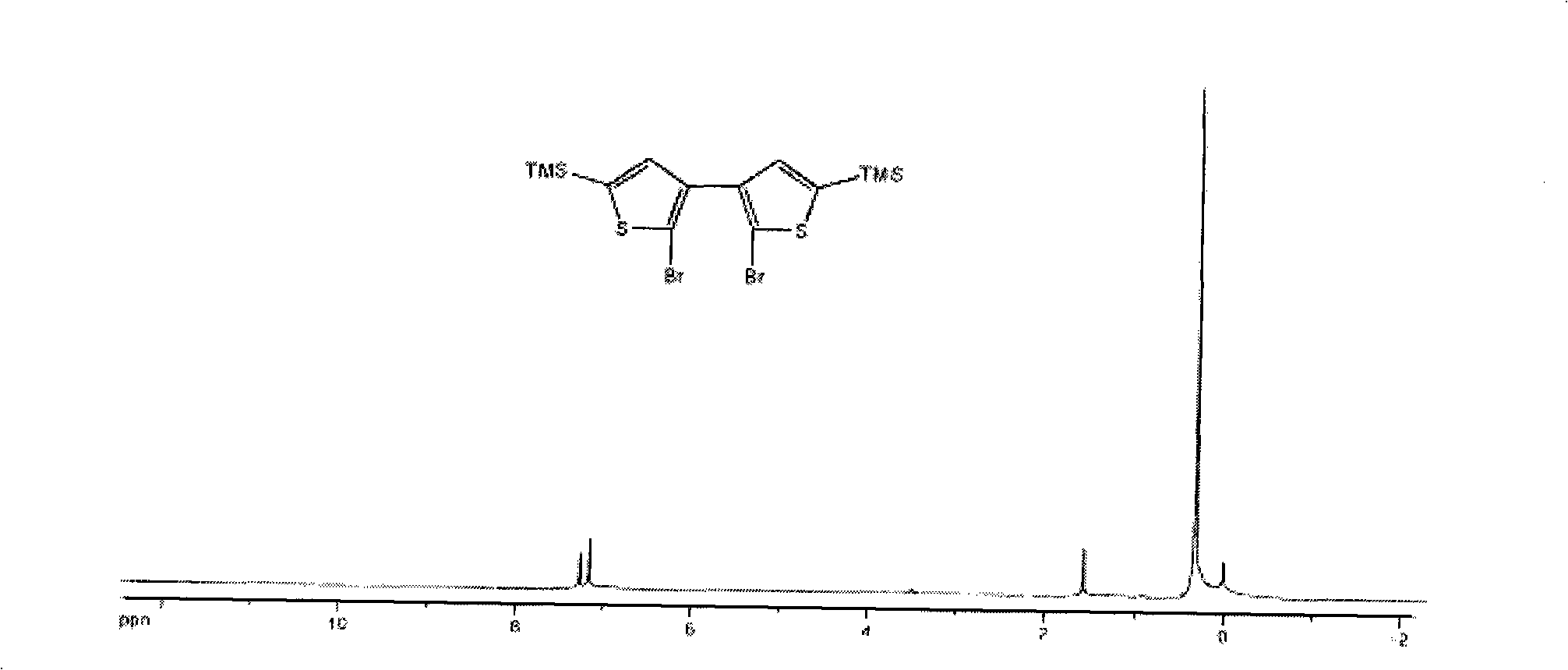
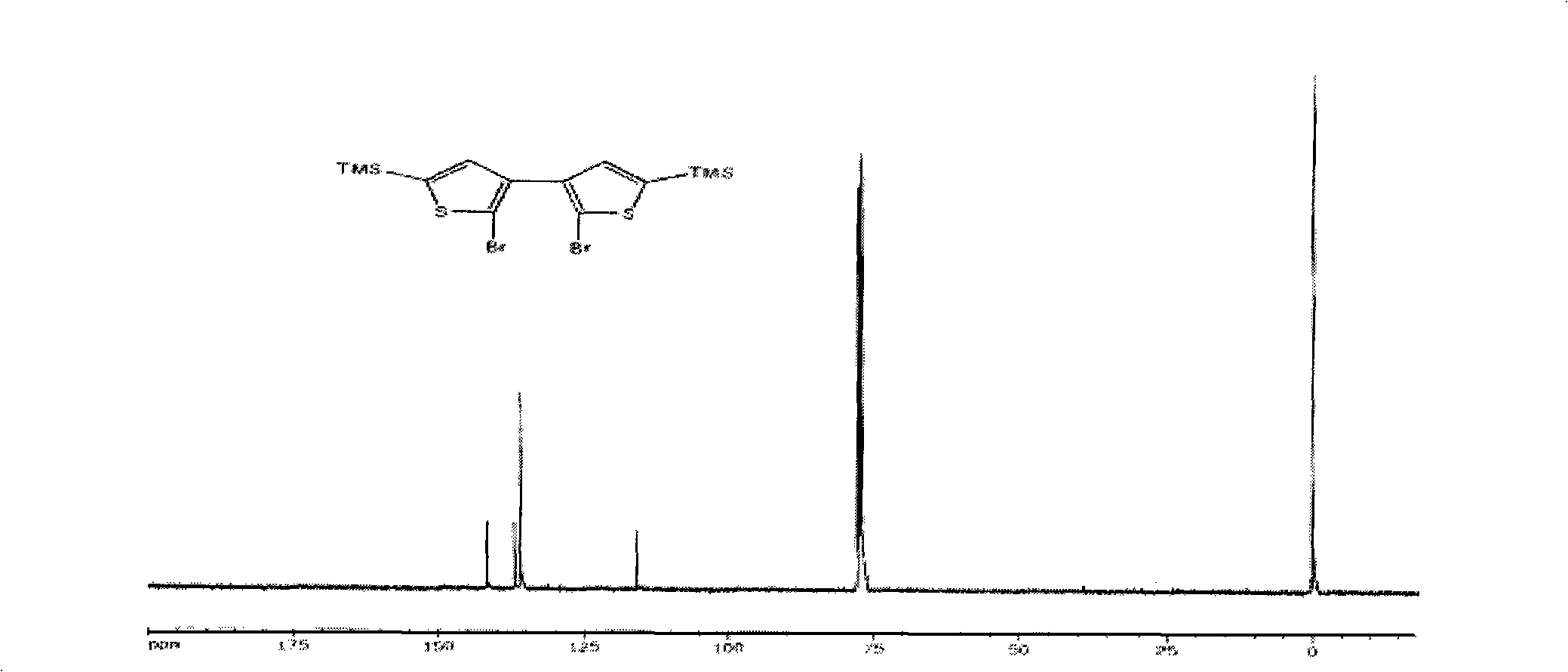
Thiophene macrocyclic compound and preparation method for its derivant
Owner:HENAN UNIVERSITY
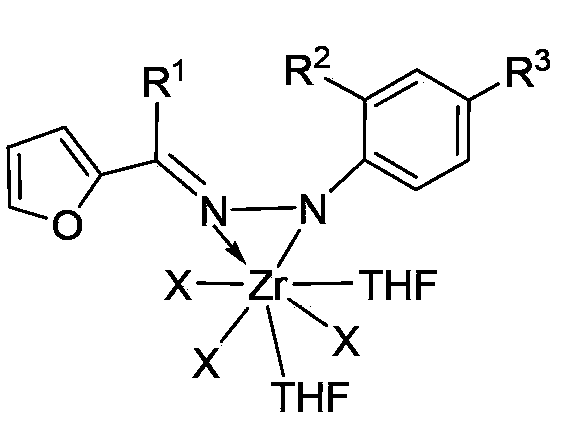
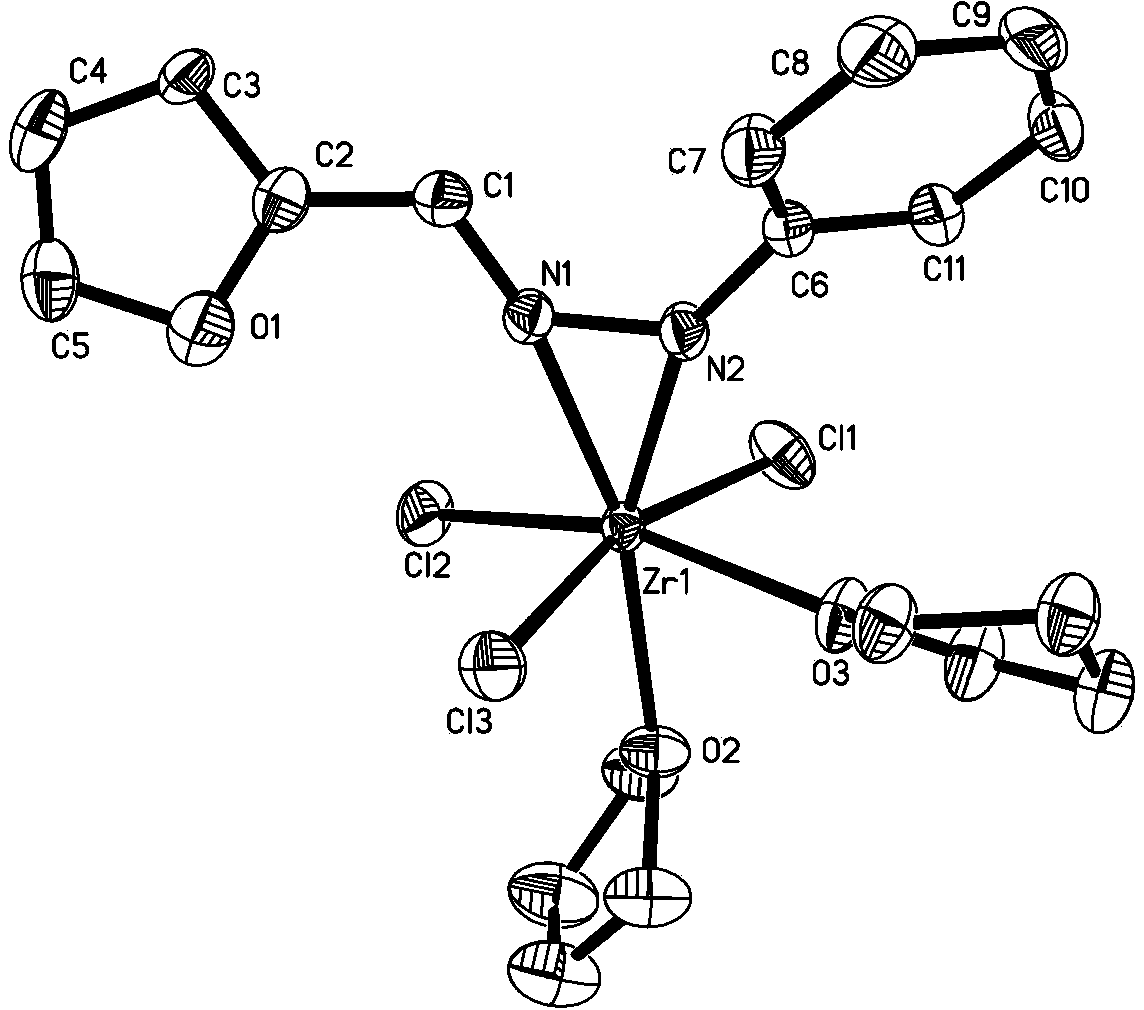
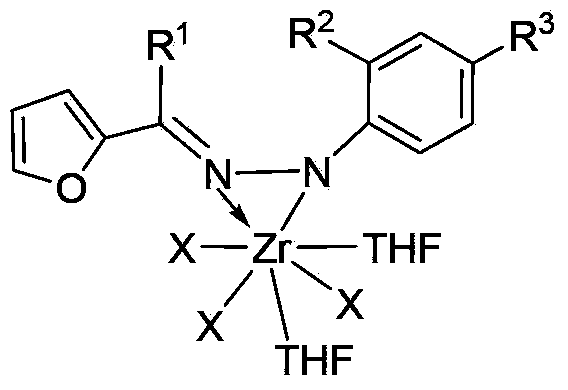
Zirconium metal complex of furan aldehyde (ketone) hydrazone as well as preparation method and application of zirconium metal complex
InactiveCN103396428ASimple manufacturing methodHigh yieldGroup 4/14 element organic compoundsBulk chemical productionFuranAluminoxane
The invention provides a zirconium metal complex of furan aldehyde (ketone) hydrazone, a preparation method of the zirconium metal complex and an application of the complex as a catalyst to the preparation of ultra-high molecular weight polyethylene. The preparation method of the complex comprises the following steps of: subjecting the furan aldehyde (ketone) hydrazone and lithium diisopropylamide (LDA) to dehydrogenation reaction, then, subjecting the furan aldehyde (ketone) hydrazone and a tetrahydrofuran solution of ZrX4 to reaction to obtain the complex, wherein the tetrahydrofuran solution of ZrX4 is same as the furan aldehyde (ketone) hydrazone in molar weight. The complex and alkylaluminoxane are formed into a catalytic system for catalyzing vinyl polymerization. Experimental results show that the catalytic system adopted by the invention can be used for preparing ultra-high molecular weight polyethylene with the weight-average molecular weight ranging from 2.3*106g / mol to 7.3*106g / mol, and has moderate catalytic activity. The complex provided by the invention is simple and easy to prepare, easy for obtaining raw materials, high in yield, easy for purification and the like.
Owner:SHANXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com