Novel preparation method for butyrolactone derivative
A technology for butyrolactone and derivatives, which is applied in the field of new preparation of butyrolactone derivatives, can solve problems such as difficulty in maintaining extremely high chiral purity, and achieve the effects of good stereoselectivity, simple operation, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] The embodiments of the present invention are described in detail below. This embodiment is implemented on the premise of the technical solution of the present invention, and detailed implementation methods and specific operating procedures are provided, but the protection scope of the present invention is not limited to the following implementation example.
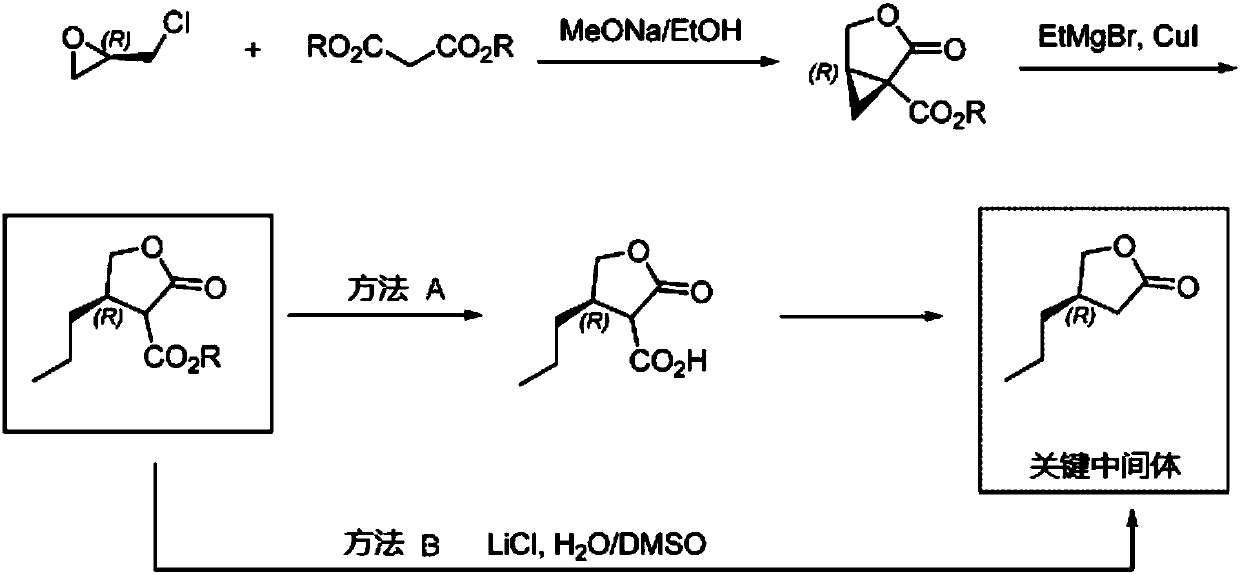
[0037] The synthesis of the butyrolactone derivative shown in embodiment 1 formula (I)
[0038] The synthetic route is as follows:
[0039]
[0040] (1) Diisopropylamine (6.1 g, 3 equivalents) was dissolved in 30 mL of tetrahydrofuran, cooled to -78 ° C, and 40 mL of n-butyl lithium in n-hexane (1.6M n-hexane solution, 3.2 equivalents) was added dropwise, After stirring at this temperature for 30 minutes, add 20 mL of tetrahydrofuran solution of valeric acid represented by formula (II) (2.0 g valeric acid, 1 equivalent), then stir at -40°C for 1 hour, then cool to -78°C, add 20mL ethyl bromoacetate ether solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com