Amino acid compound containing diaziridine group and synthesis method thereof
A technology of diaziridine and amino acids, applied in the field of organic synthetic chemistry, can solve the problems of expensive reagents, use, unfriendly environment, etc., and achieve the effects of novel structure and cheap and easy-to-obtain synthetic raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
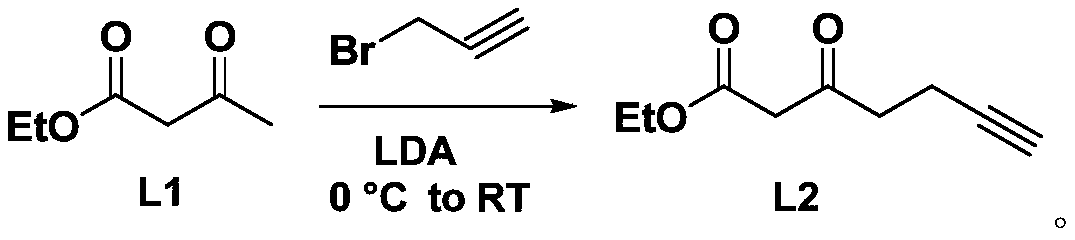
[0027] The synthesis of intermediate product L2, its synthetic route is as follows:
[0028]
[0029] Put a magnetic stirring bar in a flask with a branched mouth, seal it with a rubber stopper, heat it with a heat gun or a high-temperature flame gun under vacuum, and slowly cool it under vacuum. Charge and discharge argon three times. Under an ice-water bath, add ultra-dry tetrahydrofuran solvent (22ml), diisopropylamine (3ml, 22mmol), and slowly add n-butyllithium (22mmol) dropwise, and stir for 30min under an ice-water bath. Then ethyl acetoacetate (1.27ml, 10mmol) was added dropwise through a syringe, stirred at 0°C for more than 15min, then propyne bromide (0.75ml, 10mmol) was added, and the mixture was slowly raised to room temperature for overnight reaction. Then the reaction solution was poured into saturated ammonium chloride solution to quench, extracted with ethyl acetate, the solvent was evaporated in vacuo, purified by silica gel column chromatography, and cha...
Embodiment 2
[0040] The synthesis of target product L6, its synthetic route is as follows:
[0041]
[0042] Dissolve L-histidine (0.33mmol, 51mg) in water and 1,4-dioxane mixed solvent (1.5ml:1.5ml), add sodium bicarbonate (0.66mmol, 55mg), and then add the intermediate product L5 (0.36mmol, 90mg), stirred overnight at room temperature. The solvent was spinned off in vacuo, filtered, and rinsed several times with methanol. The filtrate was collected, spin-dried in vacuo, and purified by HPLC preparation. 1 H NMR (500MHz, CD 3 OD_SPE)δ8.20(s,1H),7.10(s,1H),4.70(dd,J=9.1,5.0Hz,1H),3.18(dd,J=15.1,5.1Hz,1H),3.00(dd, J=15.1,9.2Hz,1H),2.31(d,J=15.3Hz,1H),2.24(t,J=2.5Hz,1H),2.19(d,J=15.3Hz,1H),1.98(dt, J=7.6,3.7Hz,2H),1.60(dtt,J=22.6,15.1,7.5Hz,2H).ESI-MS Calculated for C 13 h 15 N 5 o 3 :290.13([M+H] + ), Found: 290.04.
Embodiment 3
[0044] The synthesis of target product L7, its synthetic route is as follows:
[0045]
[0046] Dissolve L-isoleucine (0.33mmol, 43mg) in water and 1,4-dioxane mixed solvent (1.5ml:1.5ml), add sodium bicarbonate (0.66mmol, 55mg), and then add the intermediate product L5 (0.36mmol, 90mg), stirred overnight at room temperature. The solvent was spun off in vacuo, extracted with water and ethyl acetate, the organic phase was kept, and the solvent was spun dry. Preparatively purified by HPLC. NMR characterization. 1 HNMR (500MHz, CD 3 OD_SPE) δ4.32(d, J=5.6Hz, 1H), 2.38(d, J=15.2Hz, 1H), 2.28(d, J=15.2Hz, 1H), 2.23(t, J=2.7Hz, 1H ),2.05–1.99(m,2H),1.84(dd,J=12.8,6.4Hz,1H),1.74–1.60(m,2H),1.52–1.42(m,1H),1.27–1.16(m,1H ), 0.91 (dd, J=14.4, 7.1Hz, 6H). ESI-MS Calculated for C 13 h 19 N 3 o 3 :288.13([M+Na] + ), Found: 288.07.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com