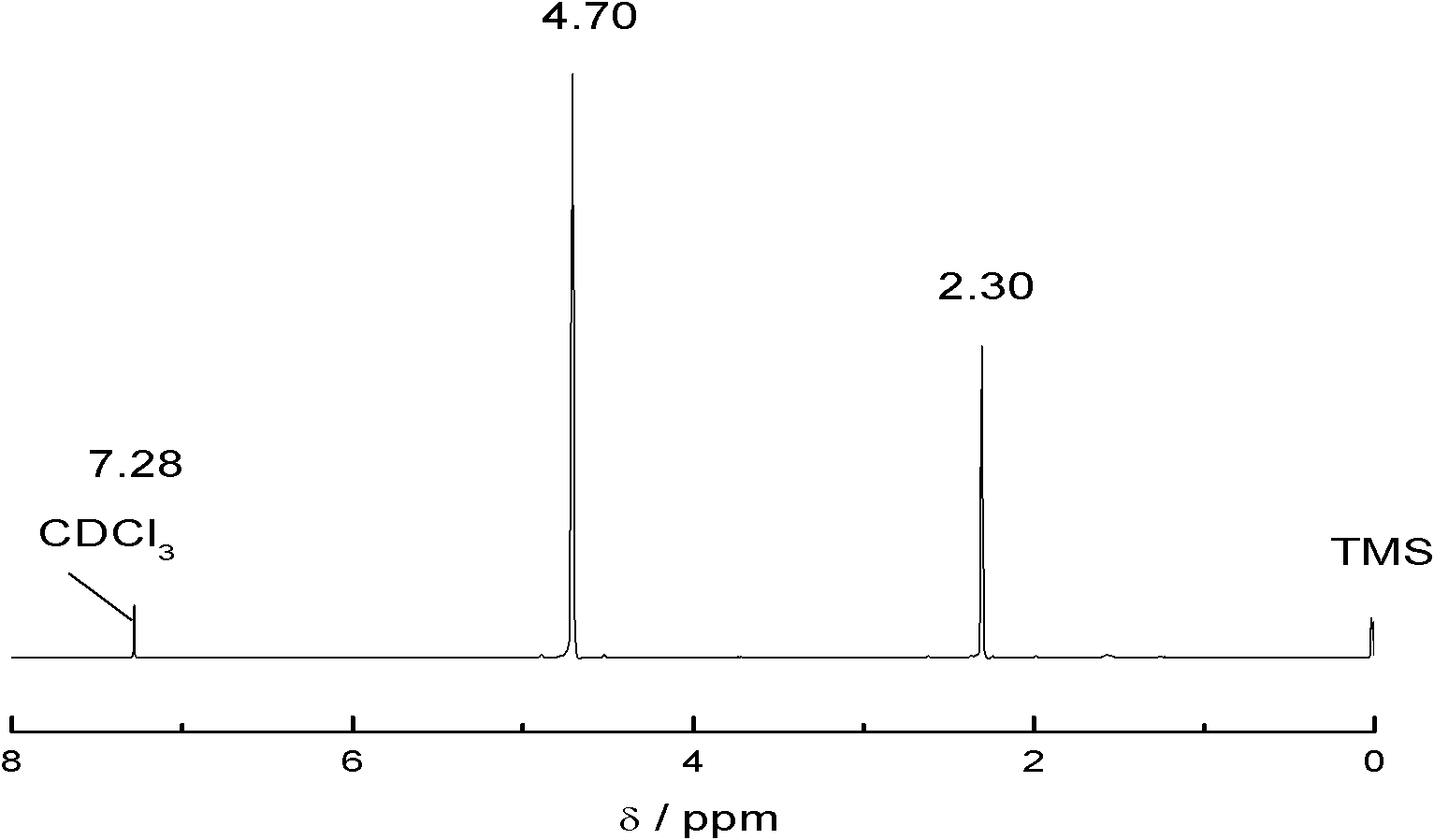
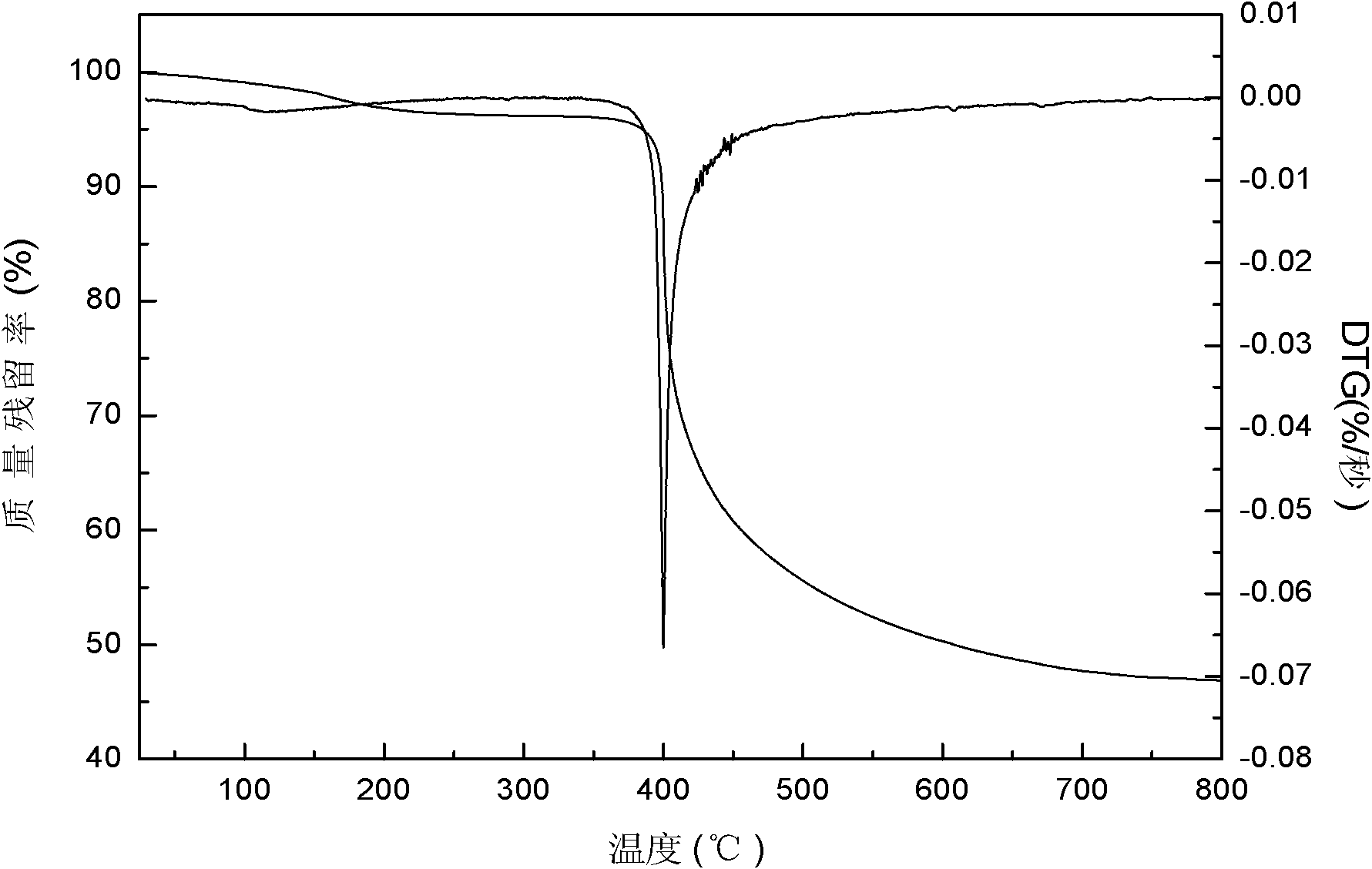
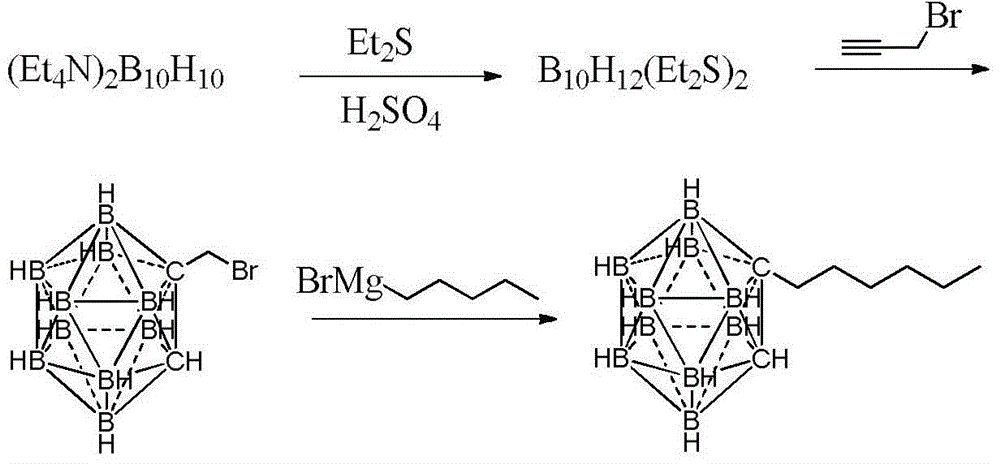Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
109 results about "Propargyl bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Propargyl bromide, also known as 3-bromo-1-propyne, is an organic compound with the chemical formula CHCCH₂Br. It is a halogenated organic compound consisting of propyne with a bromine substituent on the methyl group. It has a lachrymatory effect, like related compounds. The compound is a useful reagent in organic synthesis.
Cyclodextrin chiral chromatogram fixed phase and preparation method thereof
InactiveCN101306354AHigh column efficiencyHigh selectivityOther chemical processesAzirineChemical reaction
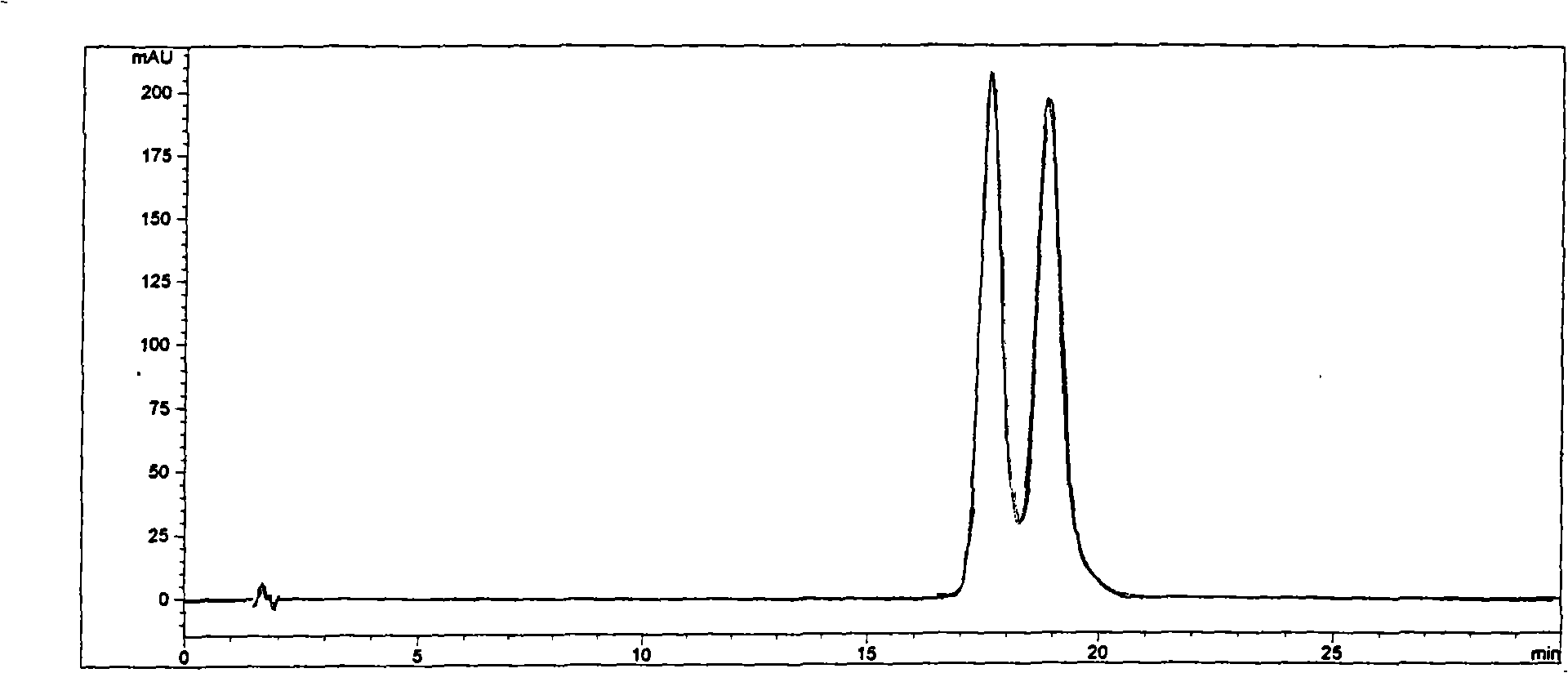
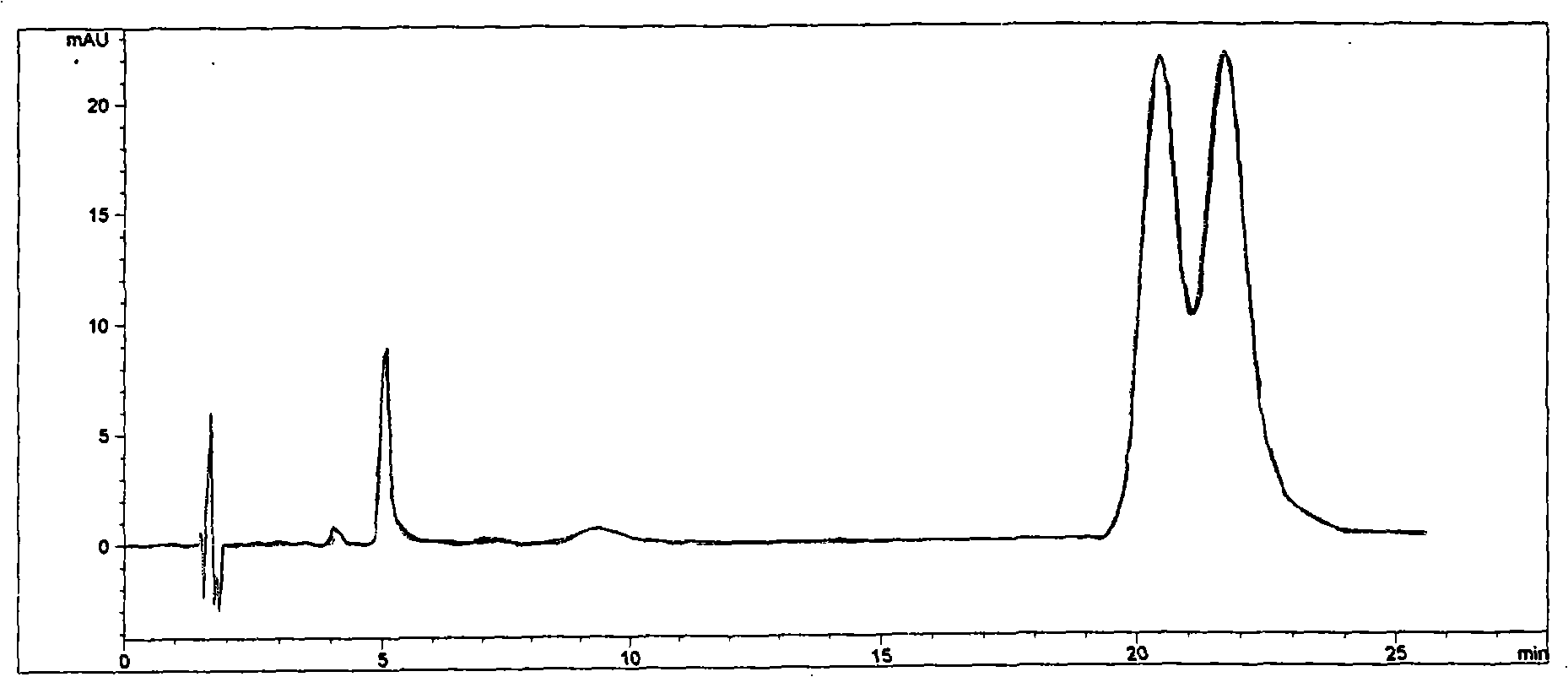
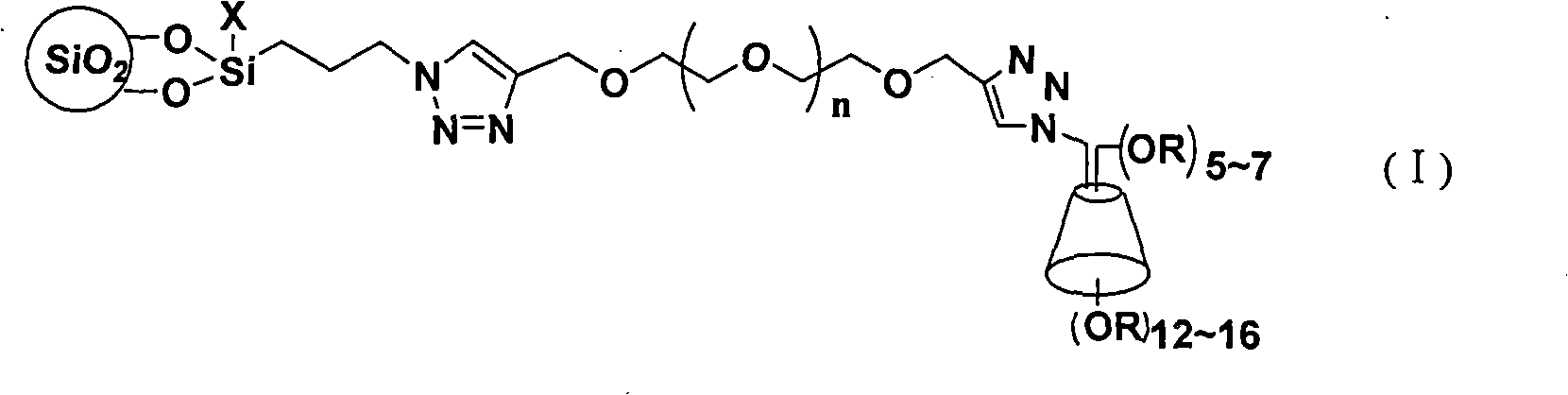
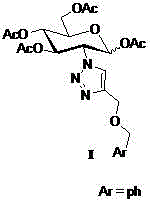
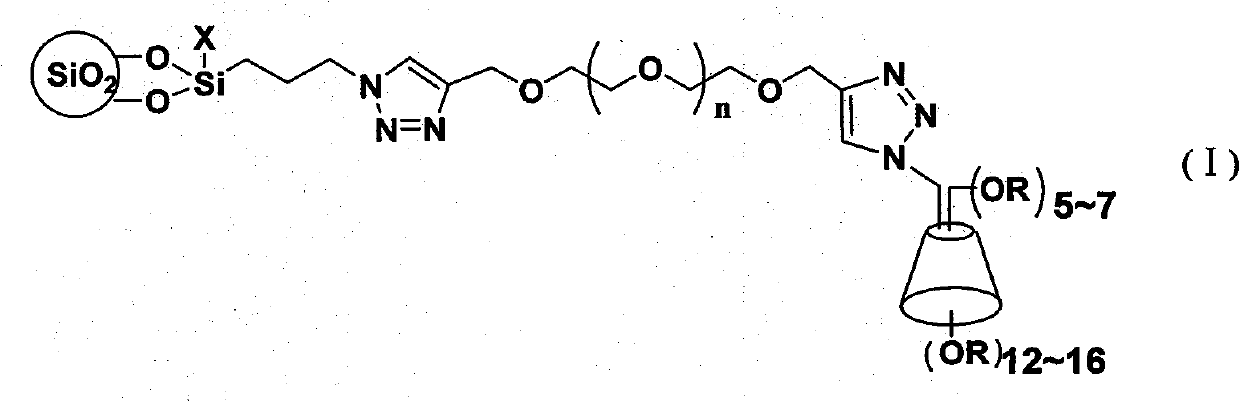
The invention discloses a cyclodextrin chiral stationary phase, the structure of which is shown in the general formula (I), wherein X is -OCH3 or -OCH2CH3, n is equal to 1-7, and R is -H, -CH3, -COCH3, -COC6H5 and -CONHC6H5. The preparation method of the stationary phase comprises the following steps: a silane coupling agent, sodium azide and a catalyst are added into an organic solvent, then spheroidal silicon is added for preparing azide silica gel derivant; oligomeric ethylene glycol, sodium hydride and propargyl bromide are added into tetrahydrofuran for preparing bialkynyl oligomeric ethylene glycol; monosubstituted nascent and derivative cyclodextrin containing azid groups is prepared; finally, the click chemistry reaction method is used for bonding the cyclodextrin. The cyclodextrin chiral stationary phase has the advantages that the selectivity of the bonding reaction is high, and the surface bonded amount is large; the chiral separation ability is strong, thereby being especially suitable for the chiral separation of a high efficiency liquid chromatography in the reversed-phase mode; the preparation method is simple and has less steps, the bonding reaction is the click chemistry reaction, the reaction condition is mild, and the reaction is carried out in the water solution.
Owner:EAST CHINA UNIV OF SCI & TECH
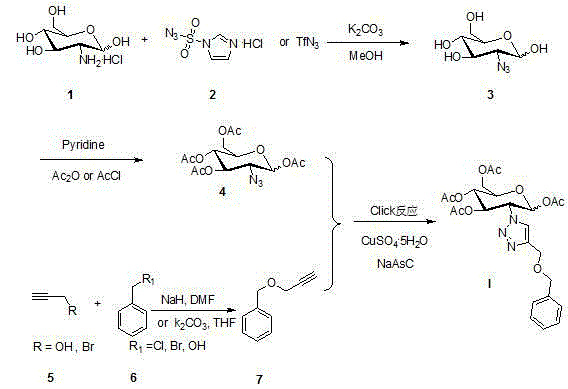
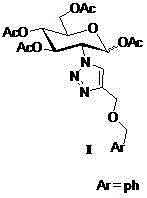
2-(1',2',3'-triazole-4'-benzyloxy)-1,3,4,6-O-acetyl-D-glucose and preparation method and application thereof
ActiveCN104817605AGood anti-rectal cancer activitySugar derivativesSugar derivatives preparationTriazole derivativesPharmaceutical Substances
The core structure of 2-(1',2',3'-triazole-4'-benzyloxy)-1,3,4,6-O-acetyl-D-glucose with an anti-colorectal carcinoma activity is that a 1,2,4-triazole derivative substitutes 2-site of 1,3,4,6-O- acetyl-D-glucose. The above compound has a good activity of inhibiting colorectal carcinoma cells, and can be used as an anti-colorectal carcinoma medicine. A synthesis method of the above compound comprises the following steps: 2-amino-D-glucose hydrochloride and an azidation reagent are used as raw materials to generate a 2-azido-1,3,4,6-O-acetyl-D-glucose intermediate under an alkaline condition; 3-propargyl bromide and phenylcarbinol react under the action of sodium hydride to generate phenyl propargyl ether; and the 2-azido-1,3,4,6-O-acetyl-D-glucose intermediate and phenyl propargyl ether undergo a click reaction in a solvent under the catalysis of monovalent copper to generate 2-(1',2',3'-triazole-4'-benzyloxy)-1,3,4,6-O-acetyl-D-glucose.
Owner:HUBEI ENG UNIV
Benzoin oxime derivative and preparation method thereof
ActiveCN104447396AComplex structureBroad use prospectsImino compound preparationChemical industryMalonate
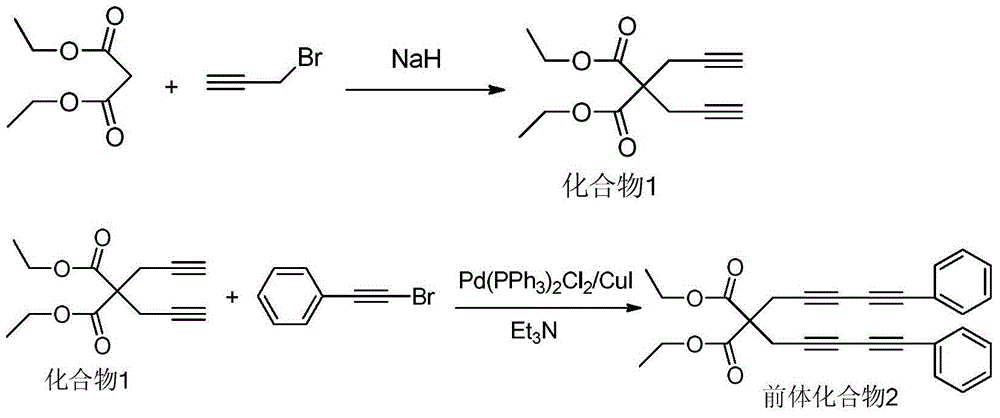
The invention relates to a benzoin oxime derivative and a preparation method thereof. A structural formula of the benzoin oxime derivative is shown in the specification. The preparation method comprises the following steps: reacting malonate with propargyl bromide in anhydrous acetonitrile under the catalysis of sodium hydride to obtain a white solid product; by taking triethylamine as alkali, reacting the white solid product with phenylethynyl bromine or substituted phenylethynyl bromine in anhydrous acetonitrile under the catalysis of Pd(PPh3)2Cl2 / CuI to obtain a light brown solid product; reacting the light brown solid product with benzoin oxime in toluene at 95-105 DEG C to obtain the benzoin oxime derivative. A brand new synthetic method of polysubstituted benzoin oxime is provided, and a series of new benzoin oxime derivatives are generated. Compared with normal benzoin oxime derivatives, the benzoin oxime derivative prepared by virtue of the preparation method has relatively complex and diverse structures by virtue of multiple cycles, and presents relatively wide application prospects in chemical industry production and clinic medicines.
Owner:ANHUI NORMAL UNIV
Fluorescent ion probe reagent used in cadmium ion detection and preparation method thereof
InactiveCN101555296ANon-destructiveThe synthetic route is simpleFluorescence/phosphorescenceSodium ascorbate8-Hydroxyquinoline
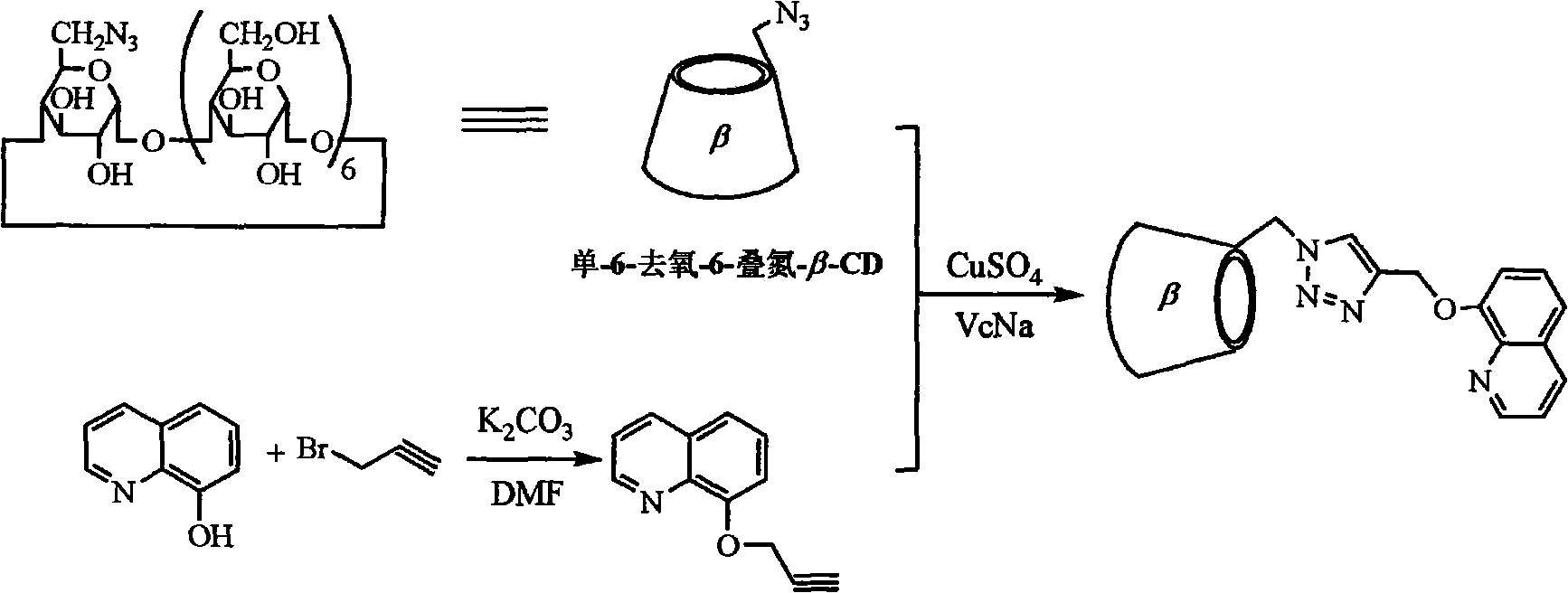
The invention relates to a fluorescent ion probe reagent used in cadmium ion detection, which is the derivate of 8-hydroxyquinoline and triazolyl modified Beta-cyclodextrin; wherein 8-hydroxyquinoline is adopted as probe molecule, and nitrogen oxide atom in quinoline ring and nitrogen atom in triazole ring are taken as the bonding lotus of cadmium (II) ion. The method comprises the steps of: synthesizing 8-hydroxyquinoline alkynyl intermediate by 8-hydroxyquinoline, 3-propargyl bromide and the like firstly; and synthesizing a target product by the intermediate, mono-6-deoxy-6-azido-Beta-cyclodextrin, sodium ascorbate and the like. The invention has the advantages that as water-soluble cadmium ion fluorescent probe, the prepared cyclodextrin derivate has simple synthesizing process and high yield, and is suitable for further enlargement synthesis and practical production application; the sensing operation to cadmium ion is simple and practical; and the sensitivity is high and displayed by fluorescence spectrum. As cadmium ion fluorescent probe, the invention exactly explains the coordinate bonding mechanism to cadmium (II) ion and has wide application prospect.
Owner:NANKAI UNIV
Dibenzo selenophene derivative and preparation method thereof
The invention provides a dibenzo selenophene derivative and a preparation method thereof. The preparation method comprises the following steps that sodium hydride is used as a catalyst, malonic ester and propargyl bromide are added into an acetonitrile solvent, and a reaction is performed in an ice-water bath for 8-14 h to obtain a compound a; on the oxygen-free and water-free conditions, the compound a and a substituted complex of phenyl acetylene bromide are subjected to a reaction in an acetonitrile solvent under the effect of a catalyst and alkali at the temperature of 20-30 DEG C for 8-14 h to obtain a compound b; the compound b and diphenyl diselenide are subjected to a reaction at the temperature of 95-105 DEG C, and after separation and purification, the dibenzo selenophene derivative can be obtained. Compared with the prior art, the novel polysubstitution dibenzo selenophene synthesis method is provided, a series of novel selenophene derivatives are generated, multi-loops exist in the obtained dibenzo selenophene derivative compared with a common selenophene derivative, the structure is more complex and diversified, and wider application prospect is represented in chemical production and clinic medicine.
Owner:ANHUI NORMAL UNIV
Preparation method of (R)-(+)-N-propargyl-1-indan amines
InactiveCN101381314AStrong response specificityHigh yieldAmino preparation by functional substitutionBulk chemical productionN-propargylSide effect
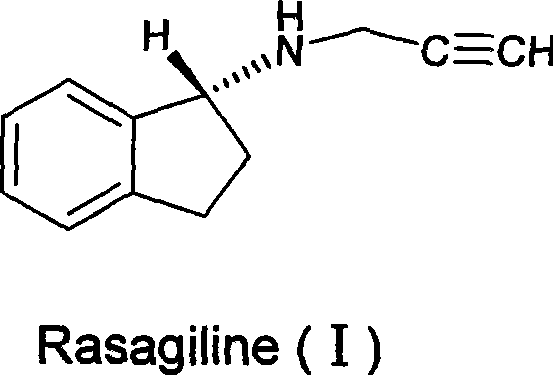
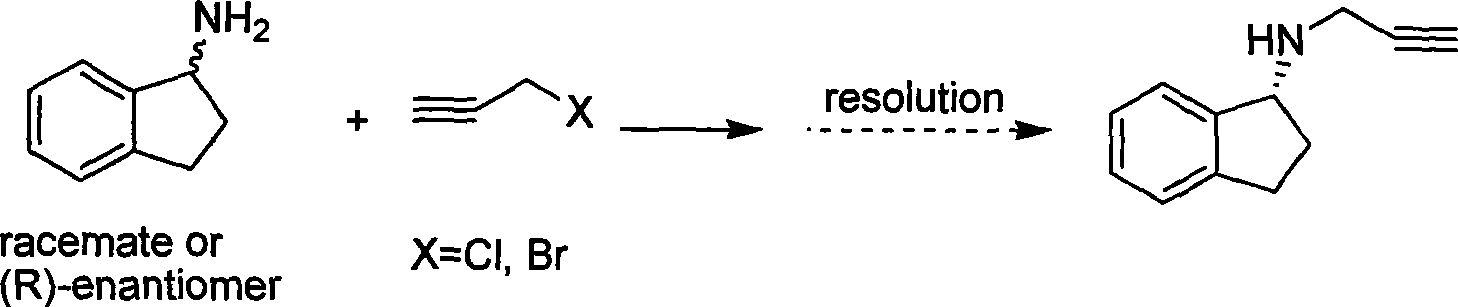
The invention discloses a method for preparing rasagiline which has simple and convenient operation and is suitable for industrialized production. In the method, primary amine group on 1-indan amine is protected by o-Nos, and the 1-indan amine removes the protecting group after the 1-indan amine is substituted by propargyl chloride (or propargyl bromide); compared with the prior method, two steps of reactions are added in the method, but after the 1-indan amine is protected by the o-Nos, the reaction specificity of the 1-indan amine and the propargyl chloride (or the propargyl bromide) is greatly improved; and the protecting group can be easily removed after reaction without other side effects, so a single product is generated. Besides, raw materials and reaction reagent used in the method is cheap and easily obtained. The method has the advantages of simple and easy operation, mild reaction condition, easy control, good reaction selectivity, total yield improvement and cost reduction, and has excellent industrialized prospect.
Owner:成都和康药业有限责任公司
2-(1',2',3'-triazolyl-4'-oxymethylenepyridyl)-1,3,4,6-O-acetyl-D-glucose and its preparation method and use
ActiveCN104945456AGood anti-rectal cancer activitySugar derivativesSugar derivatives preparationTriazole derivativesOxomethylene
The invention discloses 2-(1',2',3'-triazolyl-4'-oxymethylenepyridyl)-1,3,4,6-O-acetyl-D-glucose with anti-rectal cancer activity. The 2-(1',2',3'-triazolyl-4'-oxymethylenepyridyl)-1,3,4,6-O-acetyl-D-glucose has a core structure of 1,3,4,6-O-acetyl-D-glucose subjected to 1,2,4-triazole derivative 2-site substitution. The above compound has good rectal cancer cell inhibition activity and can be used as a drug for resisting rectal cancer. A compound synthesis method comprises that 2-amino-D-glucose hydrochloride and an azidation reagent as raw materials undergo a reaction under alkaline conditions to produce a 2-azido-1,3,4,6-O-acetyl-D-glucose intermediate, 3-propargyl bromide and aromatic methanol undergo a reaction under the action of sodium hydride to produce aromatic propargyl ether, and the 2-azido-1,3,4,6-O-acetyl-D-glucose intermediate and the aromatic propargyl ether undergo a click reaction in a solvent in the presence of monovalent copper as a catalyst to produce 2-(1', 2', 3'-triazolyl-4'-oxymethylenepyridyl)-1,3,4,6-O-acetyl-D-glucose.
Owner:HUBEI ENG UNIV
Florescent ion probe reagent for zinc ion detection and preparation method thereof
InactiveCN103242827AEfficient detectionNon-destructiveFluorescence/phosphorescenceLuminescent compositionsFluorescence spectraSodium ascorbate
The invention discloses a florescent ion probe reagent for zinc ion detection, which contains triazole phenanthroline bridge type bis(beta-cyclodextrin), adopts phenanthroline as a probe molecule and takes the nitrogen atom on the phenanthroline ring and the nitrogen atom on the triazole ring as bonding sites of bivalent zinc ions. A preparation method comprises the following steps of: firstly synthesizing a 2,9-dipropargyl-1,10-phenanthroline intermediate from 2,9-dihydroxymethyl-1,10-phenanthroline, 3-propargyl bromide and the like; and then synthesizing a target product from the intermediate, uni-6-deoxy-6-azido-beta-cyclodextrin, sodium ascorbate and the like. As a water-soluble zinc ion florescent probe, the synthesis path of the cyclodextrin derivative is simple, the production cost is low, and the florescent ion probe reagent is suitable for amplification synthesis and practical production application; the zinc ion identifying operation is simple and practical and has high sensitivity, and the zinc ion is displayed by a fluorescence spectrum; and as a zinc ion florescent probe, the florescent ion probe reagent disclosed by the invention can effectively detect zinc ions in cells and has broad application prospects.
Owner:NANKAI UNIV
Method for preparing polyurethane material grafted by methoxy polyethylene glycol
The invention discloses a method for preparing a polyurethane material grafted by methoxy polyethylene glycol, comprising the following steps: first, reacting dimethyl 5-hydroxyisophthalate with 3-propargyl bromide to obtain 5- propargyloxy dimethyl isophthalate; slowly adding a tetrahydrofuran solution in a tetrahydrofuran solution of lithium aluminum hydride for reacting to obtain 3,5-dihydroxytoluene-1-propargyloxy benzene; reacting methoxy polyethylene glycol with bromo-2-methylpropionyl bromide to obtain methoxy polyethylene glycol having an end group of bromine atoms; converting methoxy polyethylene glycol having the end group of bromine atoms into methoxy polyethylene glycol having an end group of azido by using sodium azide; reacting methoxy polyethylene glycol having the end group of azido by using sodium azide with dihydric alcohol 3,5-dihydroxytoluene-1-propargyloxy benzene by click chemistry; and reacting dihydric alcohol containing methoxy polyethylene glycol long tail with diisocyanate to obtain polyurethane having a main chain grafted by methoxy polyethylene glycol.
Owner:SOUTHEAST UNIV
Alkynyl quaternary ammonium salt multifunctional surfactants and preparation method thereof
InactiveCN106187790AHas viscoelastic surfactant functionGood corrosion inhibitionOrganic compound preparationDrilling compositionViscous liquidQuaternary ammonium cation
The invention discloses a series of alkynyl quaternary ammonium salt multifunctional surfactants and a preparation method thereof. A structural formula of quaternary ammonium salt is shown in the description, wherein R represents a C4-C22 linear, branched or cycloalkyl, aryl or alkenyl group. The preparation method comprises the following steps: (1) carrying out alkylation on ethanol amine under alkaline and acetonitrile reflux conditions by virtue of bromo-hydrocarbon; and (2) carrying out further alkylation on tertiary amine under an ethanol reflux condition by virtue of propargyl bromide, so as to obtain alkynyl quaternary ammonium salt, namely reddish brown semitransparent viscous liquid or solid. The alkynyl quaternary ammonium salt multifunctional surfactants have very high surface activity, good corrosion resistance and excellent viscoelasticity, the preparation method is simple and feasible, the raw material cost is low, the operation is easy, the yield is high, and the surfactants are environmentally friendly.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
Method for preparing mannose-containing derivatives capable of being used for post-polymerization modification through double-click chemistry combination
ActiveCN112592376AThe synthesis method is stableEfficient synthesis methodSugar derivativesSugar derivatives preparationPolymer scienceClick chemistry
The invention relates to a method for preparing mannose-containing derivatives capable of being used for post-polymerization modification by double-click chemistry combination. The method comprises the following steps: under the actions of propargyl bromide and acryloyl chloride respectively, preparing a compound with terminal alkyne and terminal olefin by using a Williessen ether-forming reaction; and then performing sulfydryl-alkene addition reaction on terminal olefin and sulfydryl compounds, and performing CuAAC reaction on terminal alkyne and acetyl-protected alpha-D-pyranomannose azide.Compared with the prior art, mercaptan substances with different structures are successfully combined with the alpha-D-pyranomannose azide through a mercapto-alkene addition reaction and GuAAC combined method for the first time, the mannose-containing derivative capable of being applied to post-polymerization modification is prepared, and the synthesis method is stable, efficient and high in yield. A considerable way is provided for obtaining a sugar-containing homopolymer with controllable molecular weight and narrower molecular weight distribution in the later period.
Owner:SHANGHAI INST OF TECH
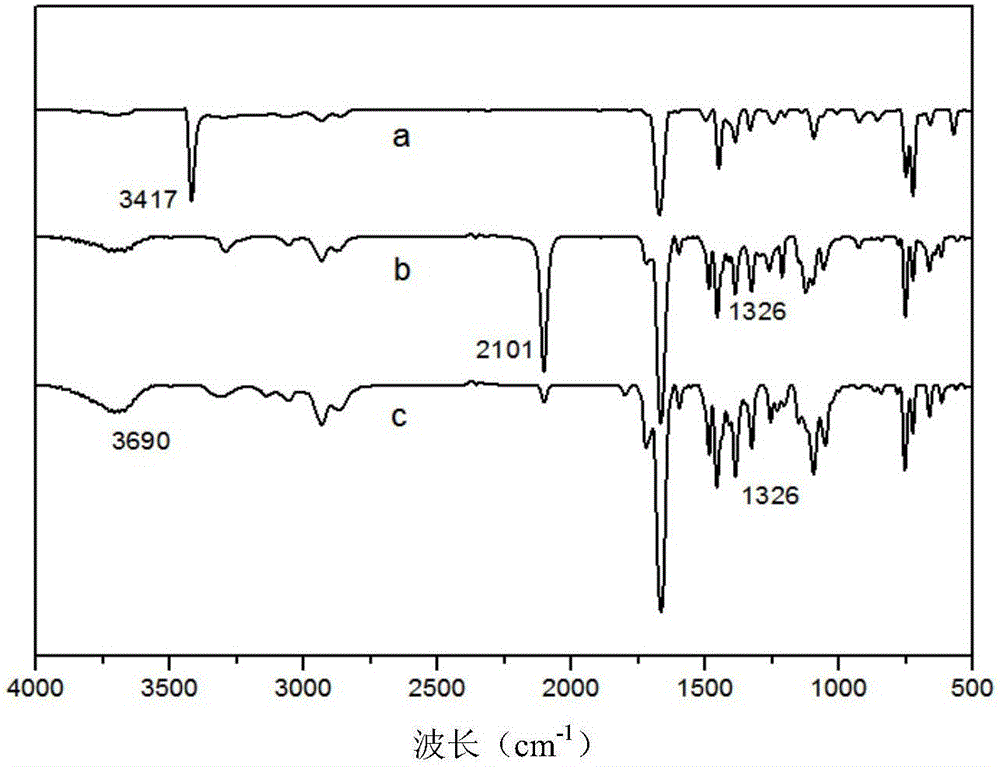
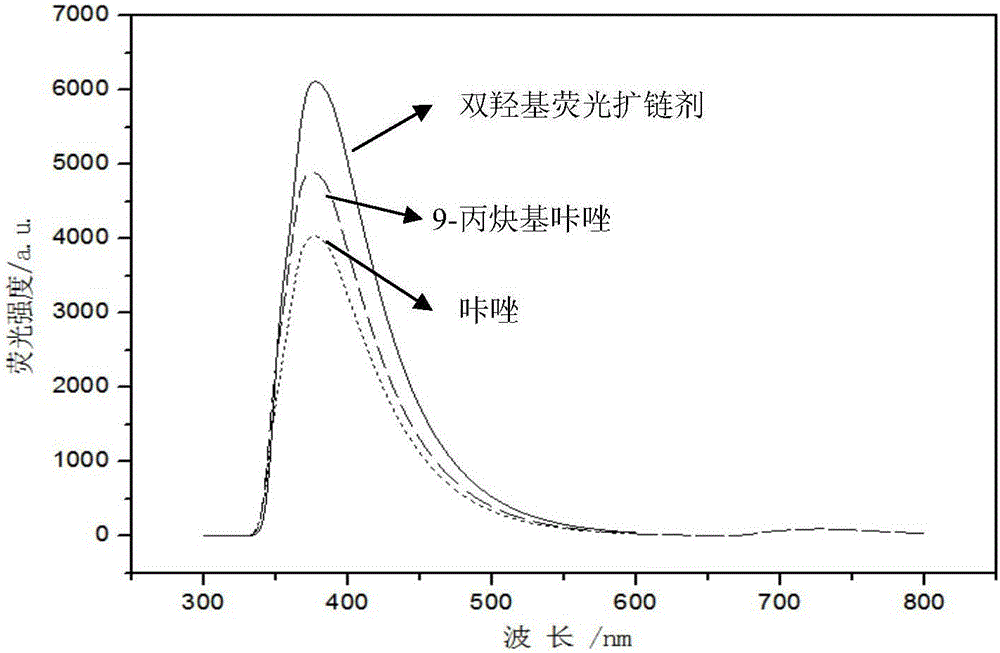
Dihydroxy fluorescence chain extender based on carbazole derivative and preparation and application thereof
ActiveCN106832175AHigh yieldGood UV stabilityOrganic chemistryLuminescent compositionsOrganic solventEmulsion
The invention belongs to the technical field of organic materials and discloses a dihydroxy fluorescence chain extender based on a carbazole derivative and preparation and application thereof. The method comprises the following steps: (1) under alkaline condition, an organic solvent is used as a reaction medium, carbazole and propargyl bromide react and subsequent processing is carried out to obtain an intermediate 9-propynyl carbazole; the molar ratio of carbazole to propargyl bromide is 1: 1.1-2; and (2) in the atmosphere of inert gas and in the presence of a catalyst and a reducing agent, the intermediate 9-propynyl carbazole and dihydroxy azide react in a solvent, and subsequent processing is carried out to obtain the dihydroxy fluorescence chain extender based on the carbazole derivative. The chain extender has high yield, has high fluorescence intensity and good anti-ultraviolet stability, and is used in manufacturing of a fluorescence WPU emulsion.
Owner:SOUTH CHINA UNIV OF TECH
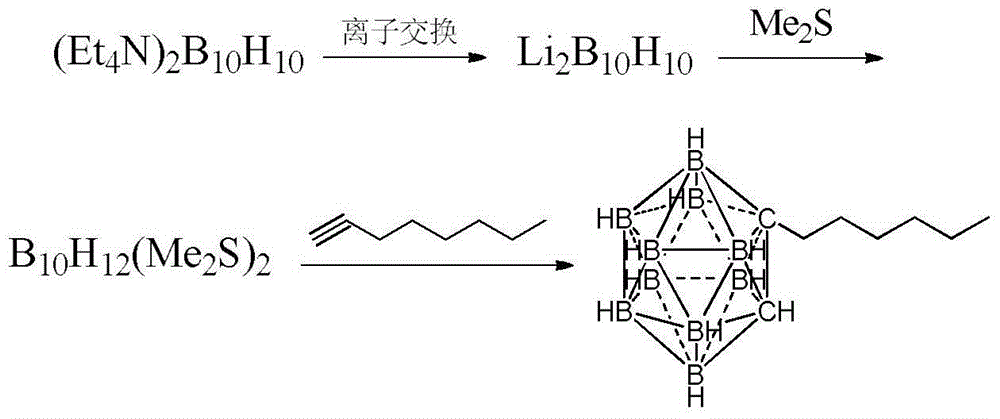
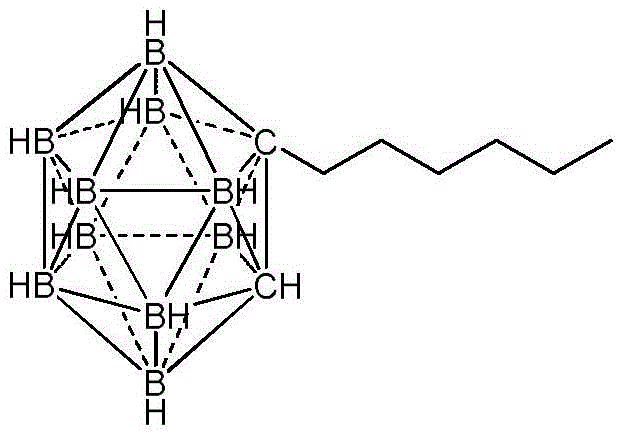
Preparation method of n-hexyl carborane
ActiveCN104017010AReduce manufacturing costGroup 3/13 element organic compoundsGrignard reagentBromine
The invention provides a preparation method of n-hexyl carborane, and the preparation method comprises the following steps: by taking decahydro-decaborate ditetraethylammonium as a raw material and ethyl thioether as a reaction raw material and a solvent, reacting the materials in the presence of a concentrated sulfuric acid used as a catalyst, so that a dodecahydrogen-decabo-diethyl thioether complex is obtained; by taking methylbenzene as a solvent, reacting the dodecahydrogen-decabo-diethyl thioether complex with propargyl bromide, so that brooethyl carborane is obtained; by taking tetrahydrofuran as a solvent, reacting the brooethyl carborane with a grignard reagent of bromopentane, so that n-hexyl carborane is obtained. The method is cheap and easily available in raw materials, relatively high in yield and low in preparation cost, and the method disclosed by the invention is applied to the preparation of n-hexyl carborane.
Owner:XIAN MODERN CHEM RES INST
Chrysin non-natural amino acid derivative as well as preparation method and application thereof
ActiveCN108101892AHas anticancer effectOrganic chemistryAntineoplastic agentsSodium ascorbateBromine
The invention discloses a chrysin non-natural amino acid derivative or pharmacologically-acceptable hydrate and salt thereof. The chrysin non-natural amino acid derivative comprises a stereoisomer ora tautomer. The chrysin non-natural amino acid derivative comprises the following preparation steps: preparing 5-hydroxy-7-propargylflavone by chrysin and propargyl bromide under the action of alkali;preparing (L / D)-N3-Boc amino acid methyl ester by (L / D)-halogenated-Boc amino acid methyl ester and NaN3 under the action of CuI / L-proline sodium; reacting the 5-hydroxy-7-propargylflavone and the (L / D)-N3-Boc amino acid methyl ester under the action of CuSO4.5H2O / sodium ascorbate, removing Boc groups and hydrolyzing to prepare the chrysin amino acid derivative. The chrysin non-natural amino acidderivative disclosed by the invention has an anti-cancer effect and can be used in preparation of an anti-cancer drug.
Owner:SHIJIAZHUANG UNIVERSITY
Preparation method of glycopolymer with lateral chains containing heterogeneous sugar units
ActiveCN110305238AMolecular weight controllableThe synthesis method is stableSugar derivativesSugar derivatives preparationSolubilityChemical reaction
The invention relates to a preparation method of glycopolymer with lateral chains containing heterogeneous sugar units. The preparation method includes: using 2-amino-2-methyl-1,3-propanediol as the raw material, using BOC to perform amino protection, then allowing the 2-amino-2-methyl-1,3-propanediol to have Williamson etherification reaction with propargyl bromide to prepare a compound with terminal alkyne, using an acetyl protected carbohydrate compound containing azido as the raw material to prepare a compound containing heterogeneous sugar through click chemical reaction, removing BOC protection, polymerizing with RAFT, using modification after polymerization to prepare glycopolymer with lateral chains containing heterogeneous sugar units, and then removing OAc protection to prepare glycopolymer containing heterogeneous sugar units. Compared with the prior art, the preparation method is simple in polymer monomer synthesizing method and controllable in polymer lateral chain structure, and the prepared glycopolymer is controllable in molecular weight, narrow in molecular weight distribution, good in water solubility and applicable to fields such as high polymer materials and biological medicine.
Owner:SHANGHAI INST OF TECH
Ligand, preparation method thereof, fluorescent probe, and preparation method and application of fluorescent probe
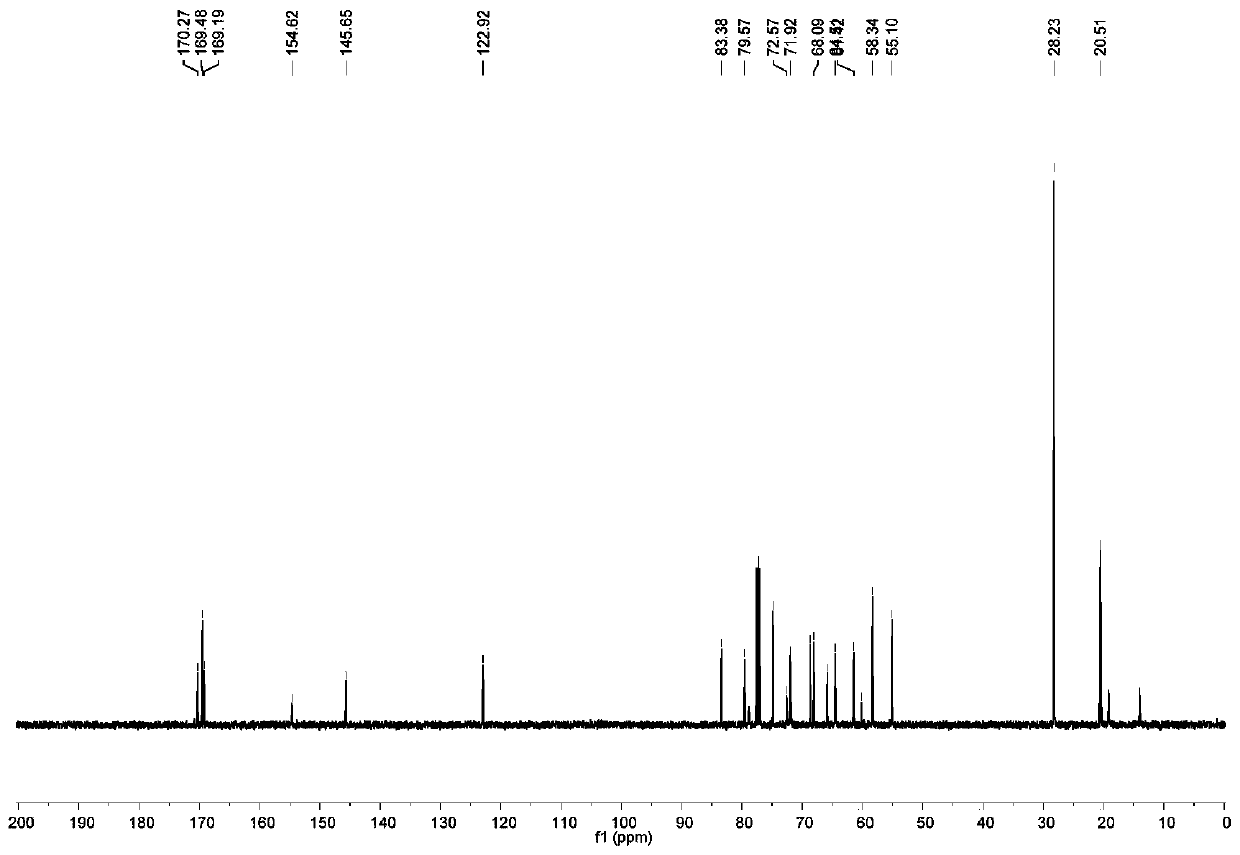
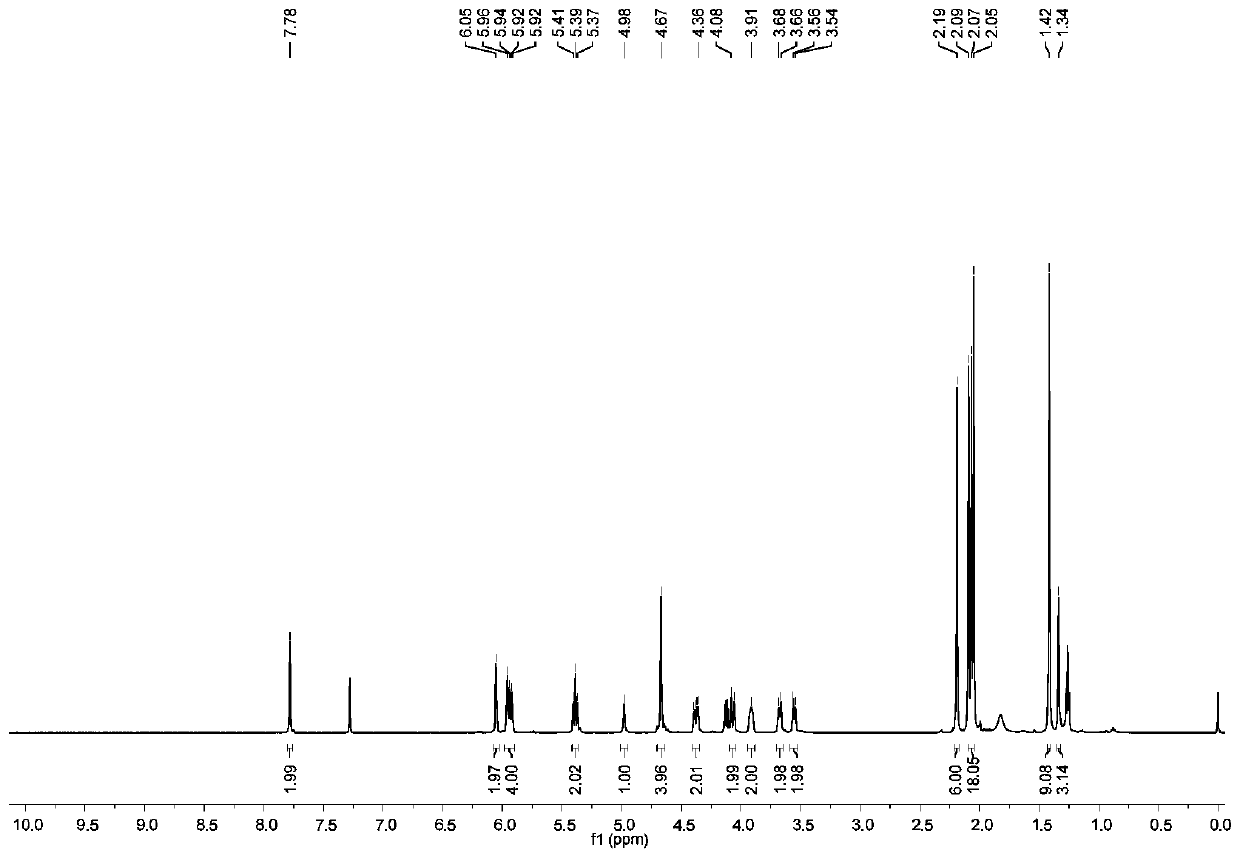
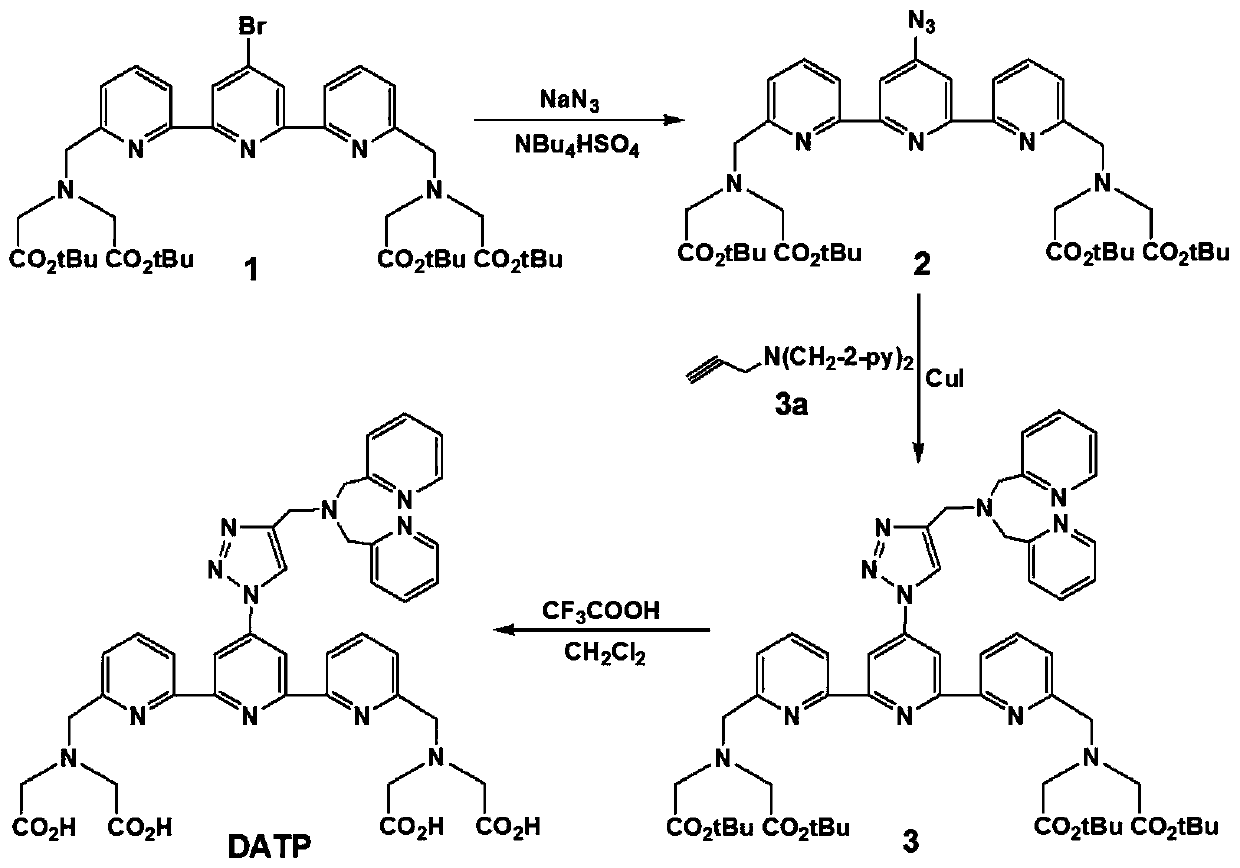
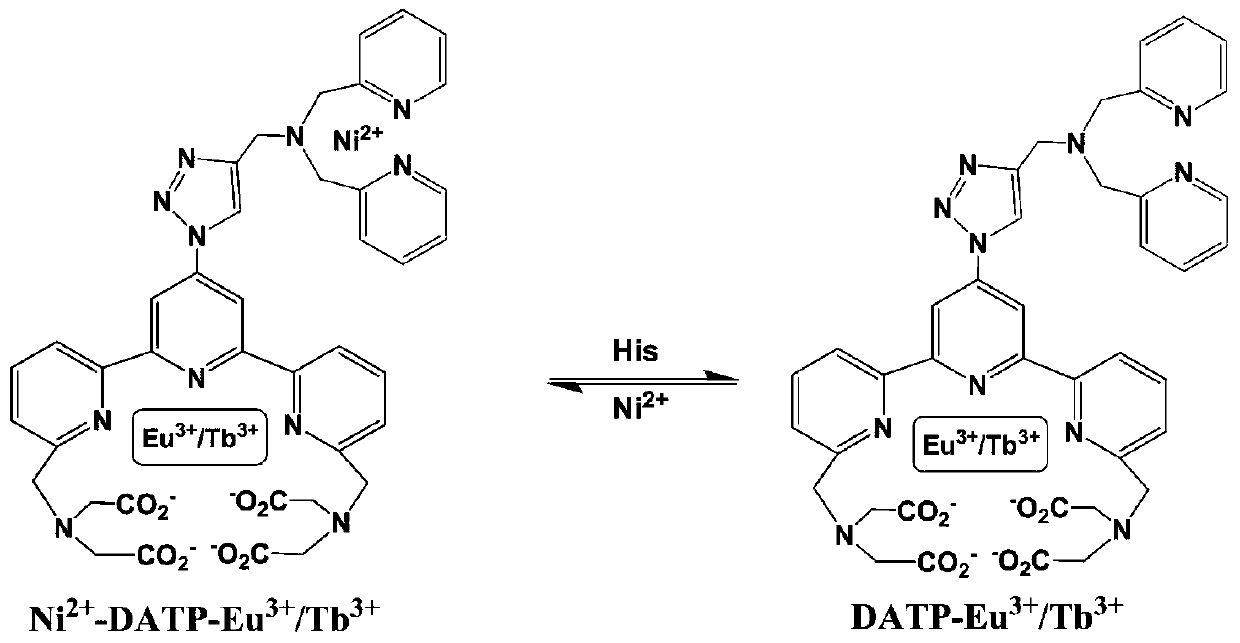
InactiveCN110330482AImprove signal-to-noise ratioHigh sensitivityGroup 8/9/10/18 element organic compoundsFluorescence/phosphorescenceSolubilityFluorescence
The invention discloses a preparation method of a ligand, a fluorescent probe, and a preparation method and an application of the fluorescent probe. The ligand is DATP, and is obtained by reacting a compound 1, NaN3, tetrabutylammonium hydrogen sulfate, bis(2-pyridylmethyl)amine, propargyl bromide, CuI, and a CH2Cl2 and CF3COOH mixed solvent. The fluorescent probe adopts DATP and Ni<2+> as cores,Eu<3+> and Ni<2+> are linked with the ligand through a Ni-N coordination bond, and Tb<3+> is linked with the ligand trough Tb-O and Tb-N coordination bonds. The fluorescent probe of the invention is prepared with DATP as the core, and a Eu<3+> / Tb<3+> complex can be used in time-resolved fluorescence detection to effectively eliminate interference from samples and short-lived fluorescence such as scattered light, so the prepared fluorescent probe has the advantages of good water solubility, extremely high sensitivity, excellent selectivity to histidine, simple preparation method and mild conditions, and is suitable for detecting histidine in biological systems.
Owner:湖南艾科瑞生物工程有限公司
Compound immobilized enzyme carrier material and preparation method and application thereof
ActiveCN107254014AEasy to prepareImprove stabilityOn/in organic carrierCarbon-oxygen lyasesPectinaseChemical reaction
The invention discloses a compound immobilized enzyme carrier material and a preparation method and application thereof. The carrier material is prepared as follows: after alkynyl groups are introduced into a reaction of UiO-66-NH2 and 3-propargyl bromide, a click chemistry reaction occurs with poly(tert-butylmethacrylate) of which the end group is nitrine, and then hydrolyzing to obtain nano-particles with the grain diameter of 280 to 320 nm. Through electrostatic incorporation between carboxyl and amino, pectinase is fixed onto the carrier material, so that the active sites capable of being combined with pectinase are also greatly increased while the stability of the pectinase is improved, the adsorbing capacity of the pectinase is high and reaches 89.7% at most; the immobilized pectinase has higher pH, temperature and storage stability and preferable reusability, the relative enzyme activity after eight times of reusability is still 81%, and the carrier material has huge application prospect in the field of immobilized enzymes.
Owner:SHAANXI NORMAL UNIV
2-metridazloe derivative as well as application and preparation method thereof
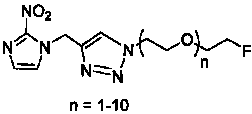
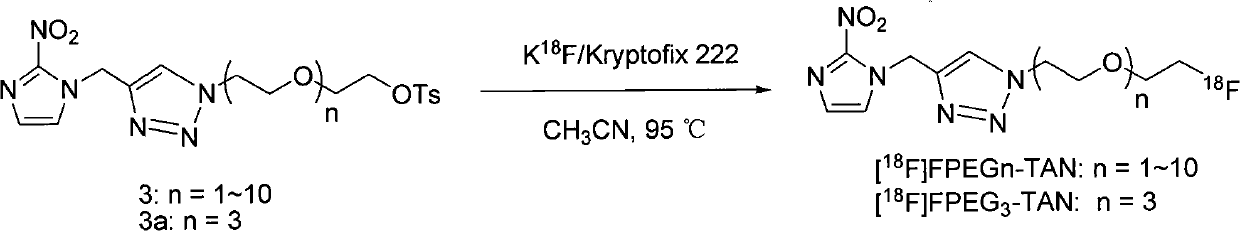
InactiveCN101921263AIncrease water solubility of moleculesGood compatibilityOrganic chemistryRadioactive preparation carriersNitroimidazoleRadioactive drug
The invention discloses a radioactive drug and a preparation method thereof. The compound is fluo-polyethylene glycol-triazole ring-methylene-2metridazloe, and the structural general formula of the compound is disclosed in the specification, wherein n is 1-10, and F is 18F. The preparation method comprises the following steps of: adding 2-metridazloe, propargyl bromide and K2CO3 to CH3CN; heating for refluxing; adding a product obtained after sufficient reaction, a product obtained by adding di-p-tolyl-sulphonate of polyethylene glycol and NaN3 to DMF (Dimethyl Formamide) and heating with boiled water, CuI and Et3N to THF (Tetrahydrofuran); and finally carrying out a clink reaction on the two products to finally obtain a [18F]FPEGn-TAN solution.
Owner:LANZHOU UNIVERSITY
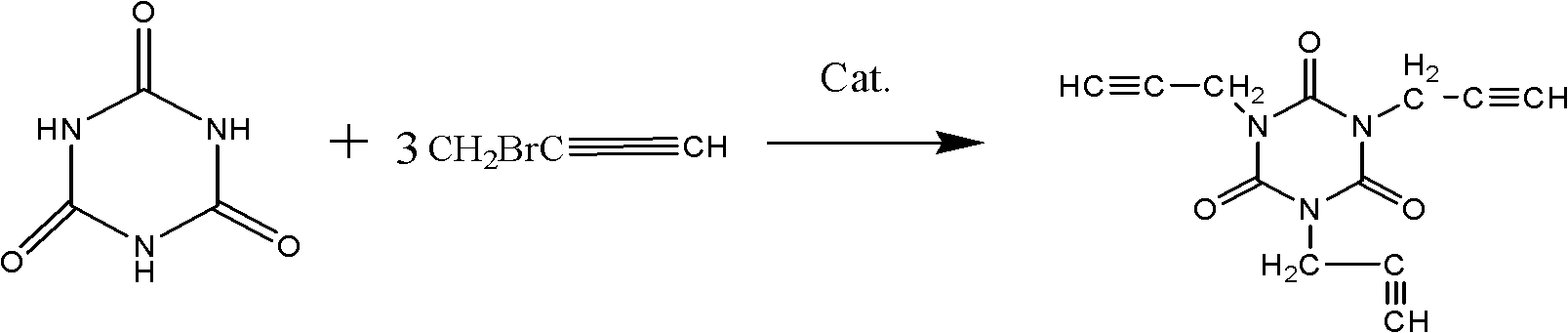
Synthesis method and application of trialkynyl monomer 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-triketone
InactiveCN102030717AReduce manufacturing costSimple processOrganic chemistry1,3,5-TriazineSynthesis methods
The invention discloses a synthesis method and application of a trialkynyl monomer 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-triketone. The synthesis method comprises: isocyanuric acid and propargyl bromide as raw materials are utilized to synthesize the trialkynyl monomer in the presence of a phase transfer catalyst; and poly 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-triketone is obtained after 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-triketone is cured at high temperature. The synthesis method has the beneficial effects of high safety, low raw material cost and mild reaction conditions, is simple in operation and easy for scale production.
Owner:HEFEI UNIV OF TECH
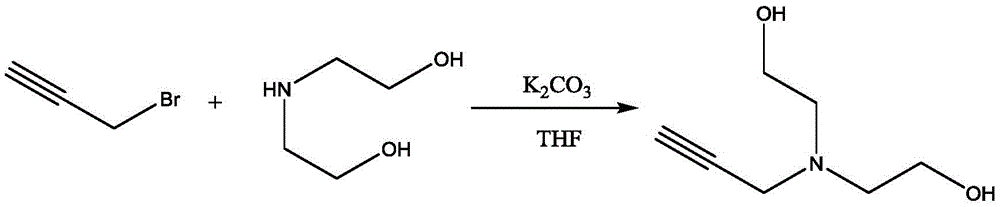
Method for synthesis and purification of bonding agent N-propargyl diethanol amine
InactiveCN105481704AShort reaction timeHigh yieldOrganic compound preparationAmino-hyroxy compound preparationN-propargylDistillation
The invention discloses a method for synthesis and purification of the bonding agent N-propargyl diethanol amine. The structure of the compound is shown in the description. The bonding agent N-propargyl diethanol amine is expected to be applied to energetic binder type propellants. The method comprises the steps of adding 3-propargyl bromide to a diethanol amine tetrahydrofuran solution dropwise with triethylamine as the acid-binding agent under the ice-bath condition, conducting filtration after three hours of reaction, conducting filtrate concentration and reduced pressure distillation in sequence, and collecting the product. The method has the advantages of being high in yield and environmentally friendly.
Owner:XIAN MODERN CHEM RES INST
Benzodihydropyran ring derivative and preparation method thereof
The invention discloses a benzodihydropyran ring derivative and a preparation method thereof. The malonate and propargyl bromide are used as initial raw materials to synthesize a benzodihydropyran ring derivative through a three-step reaction; and the raw material is simple and easy to obtain, the synthesis method is convenient and fast, and an efficient and fast synthesis path is provided for the preparation of the benzodihydropyran ring derivative.
Owner:ANHUI NORMAL UNIV
Amino acid compound containing diaziridine group and synthesis method thereof
The invention discloses an amino acid compound containing a diaziridine group and a synthesis method thereof. The method comprises the following steps: with ethyl acetoacetate as a raw material, performing a reaction under the action of lithium diisopropylamide; performing a reaction with propargyl bromide to generate 3-oxoheptyl-6-acetylenic acid ethyl ester; then hydrolyzing the reaction productto generate 3-oxoheptyl-6-acetylenic acid, so as to obtain 3-oxoheptyl-6-acetylenic acid; then performing a reaction with hydroxylamine-O-sulfonic acid in an ammonia gas methanol solution; performingiodine elementary substance oxidation to generate 2-(3-(butyl-3-acetylene-1-yl)-3H-diaziridine-3-yl)acetic acid; then carrying out a reaction on 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and N-hydroxysuccinimide to generate 2,5-dioxopyrrolidine-1-yl-2-(3-(butyl-3-yl-1-yl)-3H-diaziridine-3-yl)acetate, and then carrying out a reaction with amino acid molecules to generate the targetproducts. The compound synthesized by the invention contains diaziridine groups and amino acid molecules with biological activity, and can be used in biological activity test and analysis.
Owner:NANJING UNIV OF SCI & TECH
Acyl chloride and alkyne addition product and preparing method thereof
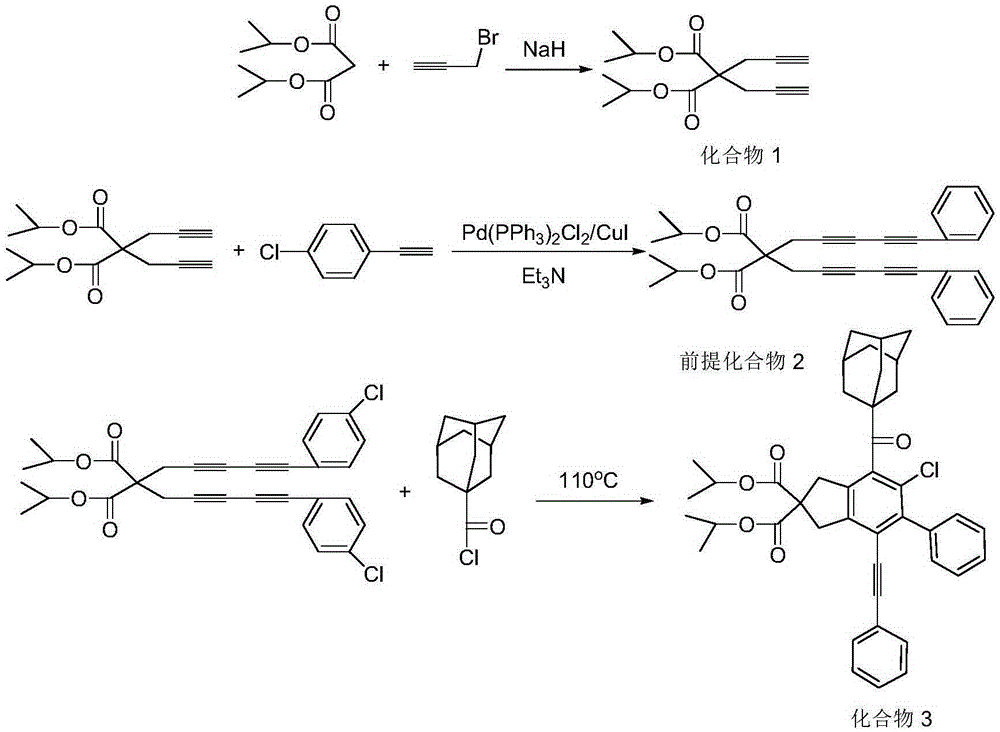
ActiveCN105348094AHigh yieldComplex structureOrganic compound preparationCarboxylic acid esters preparationChemical industryAlkyne
The invention discloses an acyl chloride and alkyne addition product and a preparing method thereof. The preparing method includes the steps that sodium hydride is used as a catalyst, diisopropyl malonate and propargyl bromide are added into anhydrous acetonitrile, the mixture is put in an ice-water bath and stirred for reacting, and a white solid product is obtained after purification and separation; the product phenyl ethynyl bromide is mixed in an anhydrous oxygen-free catalytic system of Pd(PPh3)2Cl2 / CuI, triethylamine is used as alkali, anhydrous acetonitrile is used as solvent, a light brown solid product is obtained through stirring reacting at the room temperature as well as purification and separation, the light brown solid product reacts with adamantanecarbonyl-chloride in methylbenzene, and the acyl chloride and alkyne addition product is obtained through purification and separation. Compared with the prior art, the prepared beta-chloro-alpha, beta unsaturated ketone does not need a metal catalyst, is high in yield and more complex and verified in structure and will show broader application prospects in chemical industry production and clinic medicine.
Owner:ANHUI NORMAL UNIV
Cyclodextrin chiral chromatogram fixed phase and preparation method thereof
InactiveCN101306354BHigh column efficiencyHigh selectivityOther chemical processesChemical reactionClick chemistry
Owner:EAST CHINA UNIV OF SCI & TECH
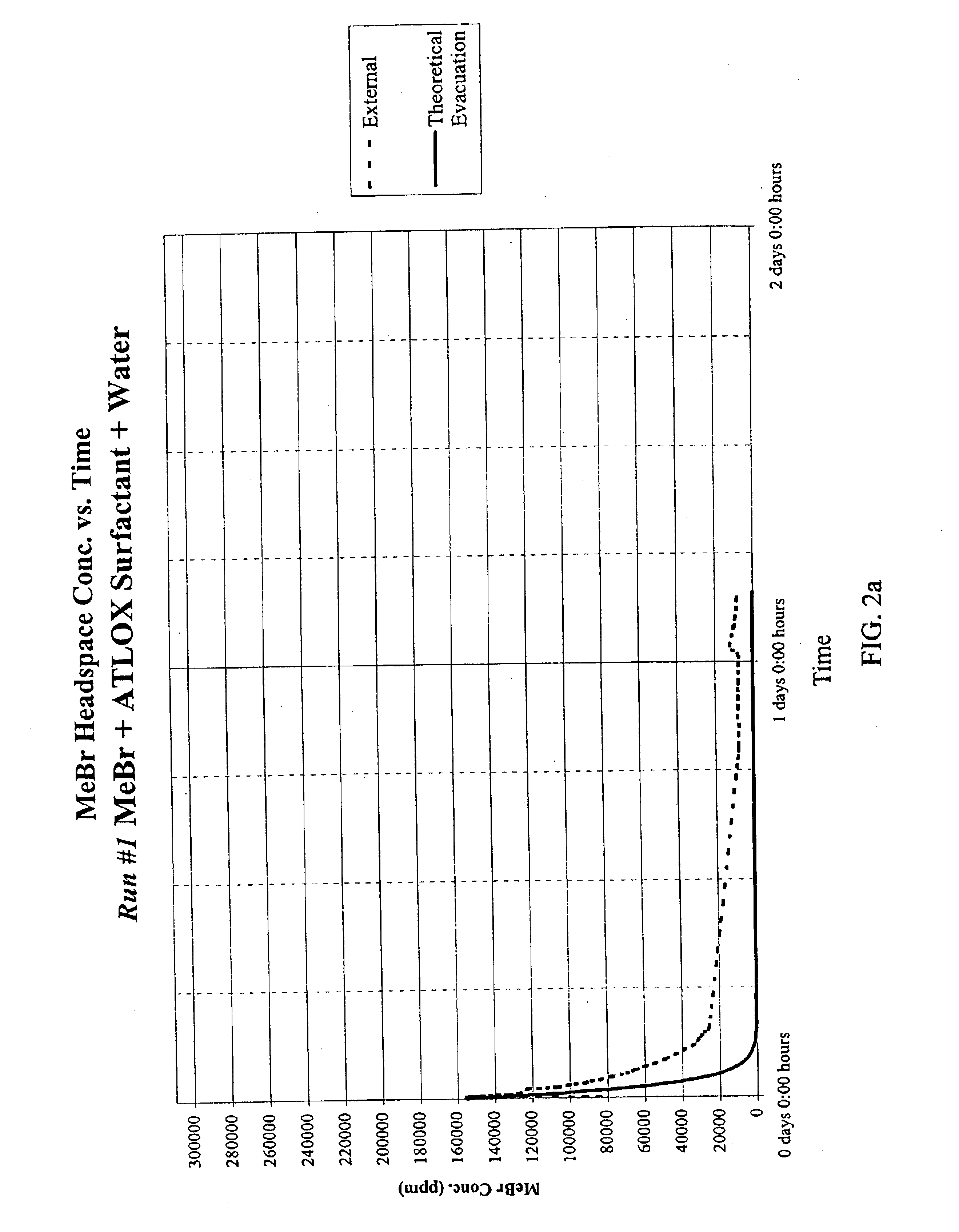
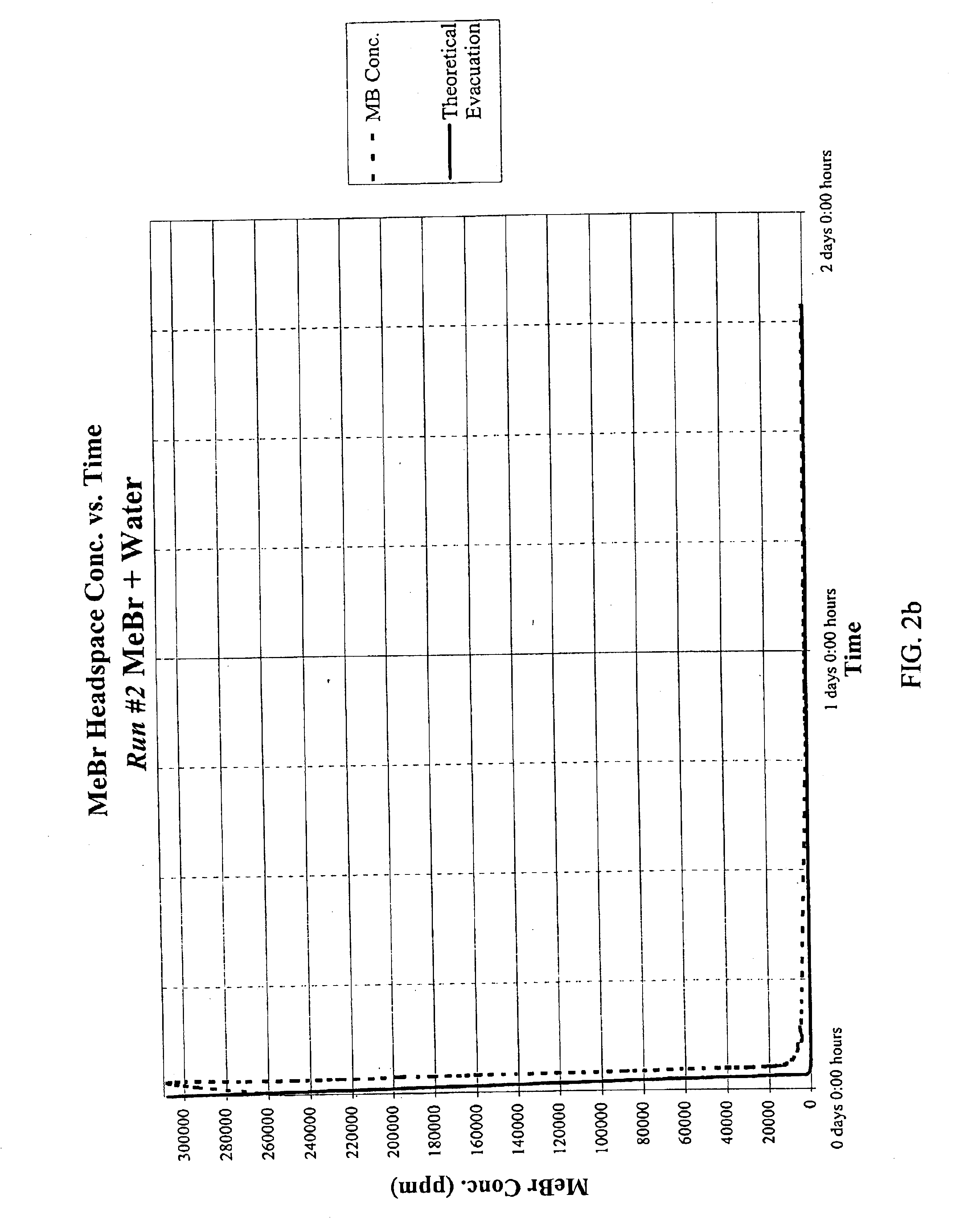
Emulsified soil biocides used in drip irrigation systems
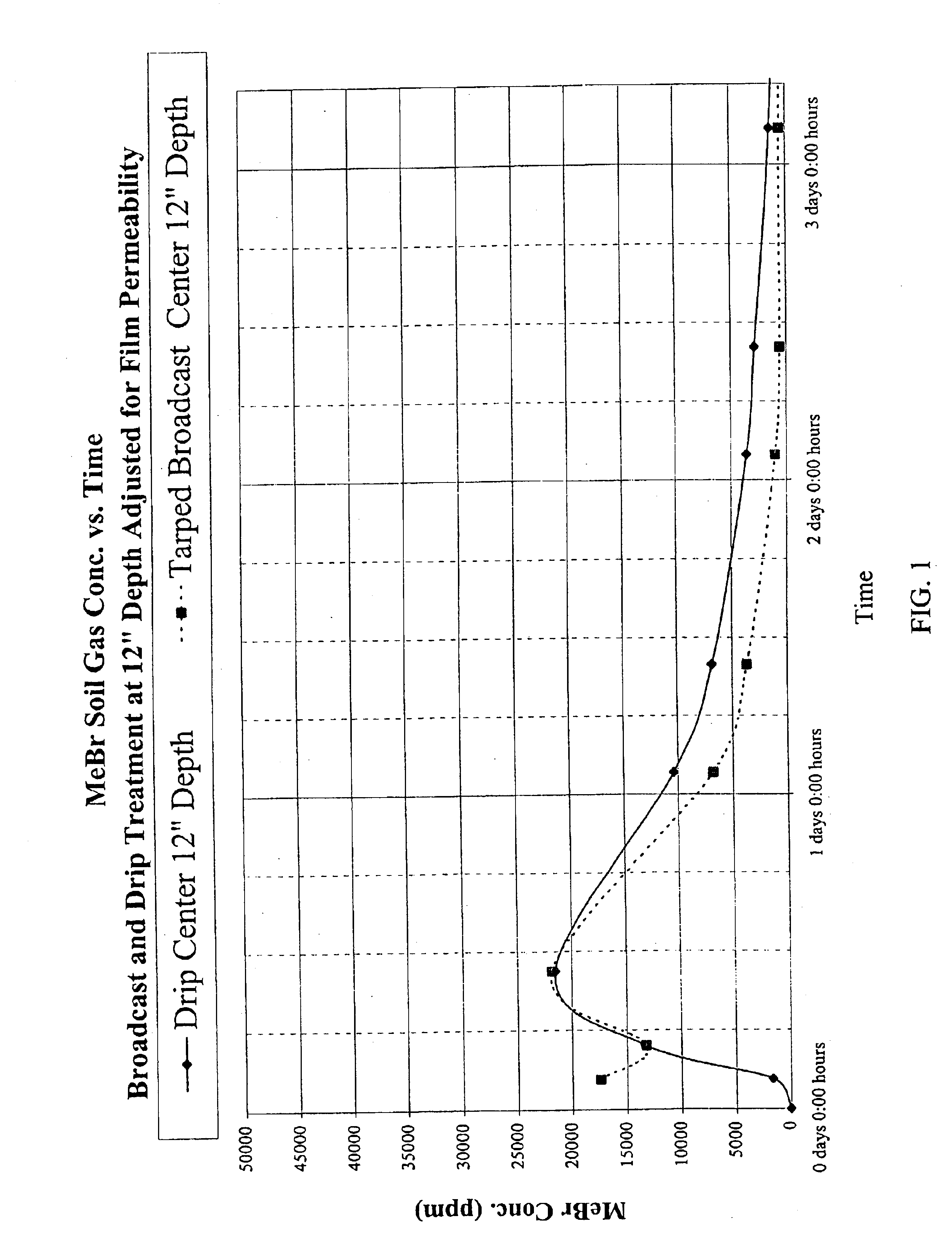
InactiveUS6923937B2Good curative effectMinimizing corrosionBiocideDead animal preservationDrip irrigationNon ionic
A soil biocide formulation for aqueous delivery comprising from about 50 to 99% by weight of the formulation of a fumigant preferably selected from the group consisting of methyl bromide, chloropicrin, 1-3 dichloropropene (Telone), propargyl bromide, dimethyl disulphide methylisothiocyanate and mixtures of them; and from about 50 to 1% emulsifier with the emulsifier being comprised of non-ionic and anionic surfactants.
Owner:TRICAL
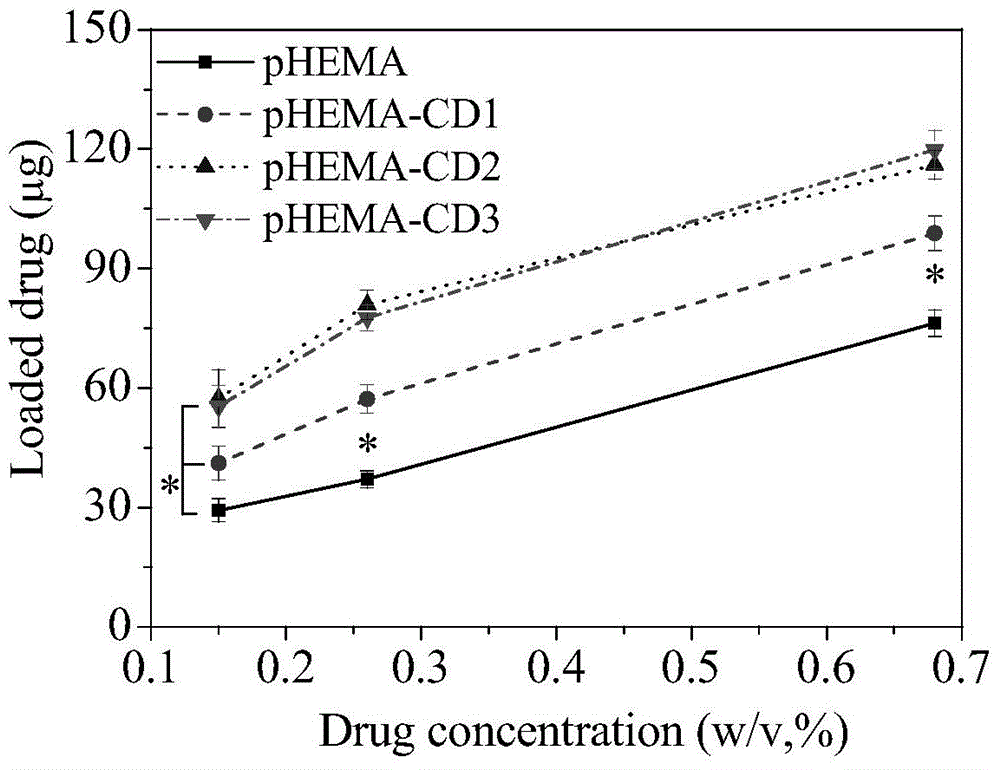
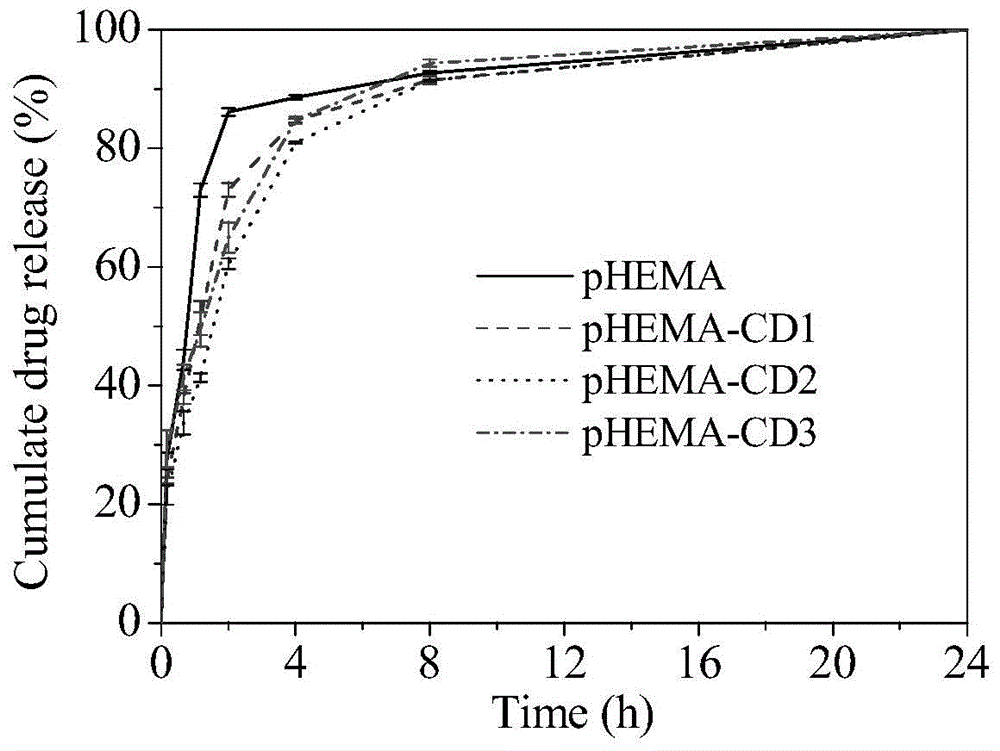
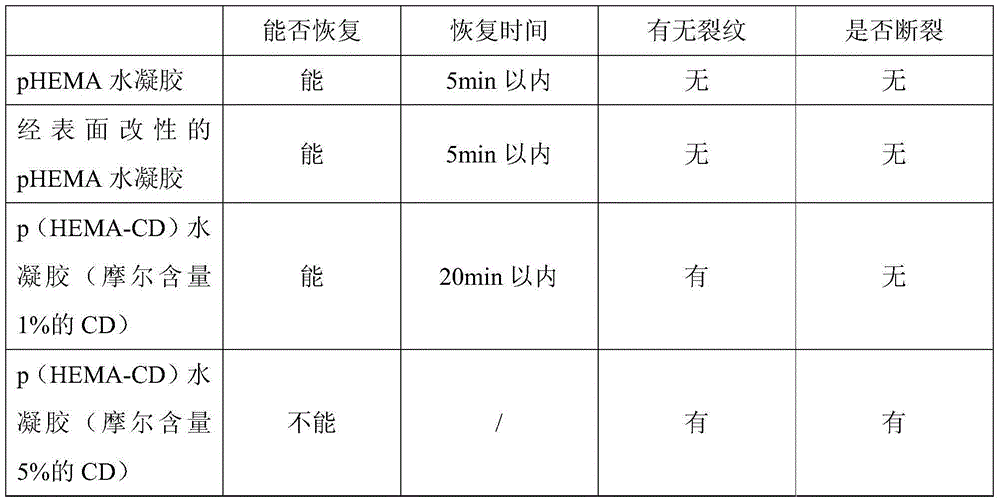
Environment-friendly surface modification method for preparing hydrogel drug carrier
ActiveCN104888229ASurface Equilibrium Water Content DecreasedStay resilientMacromolecular non-active ingredientsPolymer scienceDrug carrier
The invention discloses an environment-friendly surface modification method for preparing a hydrogel drug carrier. The method comprises the following steps that after a hydrogel sheet is mixed with propargyl bromide, catalysts are added, and a reaction is achieved by stirring under the sealed environment; after the reaction, the hydrogel sheet is taken out, monomers and the catalysts which have no reaction on the hydrogel sheet are washed out, and the hydrogel sheet is obtained after alkynylation; the hydrogel sheet obtained after alkynylation, mono-(6-sulfydryl-6-deoxidation)-beta-cyclodextrin and photoinitiators are evenly mixed and sealed, and are placed in the sun to react; after the reaction, the hydrogel sheet is taken out, the monomers and the catalysts which have no reaction on the hydrogel sheet are washed out, and the hydrogel sheet with a cyclodextrin function is obtained. According to the surface method for the hydrogel with the good drug control releasing capacity, the basic performance of the hydrogel is kept in the performance of the obtained product, and drug releasing can be controlled. Great social and economic benefits are achieved.
Owner:JINLING INST OF TECH
1-substitution phenyl-4-polysubstitution phenyl-5-methylmercapto-1H pyrazole compound with anti-hepatoma activity
The invention relates to the synthesis and anti-hepatoma activity study of a 1-substitution phenyl-3-methyl-4-(2-fluorine-4-chlorine-5-propargyloxyphenyl)-5-methylmercapto-1H pyrazole compound (A). The compound (A) is prepared through the following steps of reducing 2-chlorine-4-fluorine-5-nitro phenyl ethyl carbonate with iron powder, carrying out diazotization and Meerwein arylation reaction and reacting with carbon disulfide and dimethyl sulfate to obtain 2-chlorine-4-fluorine-5-(1,1-dimethylthio-3-oxo-1-alkene-2-group) phenyl ethyl carbonate (5), hydrolyzing the (5), then reacting with propargyl bromide to obtain 4,4-dimethylthio-3-(2-fluorine-4-chlorine-5-propargyloxyphenyl) butane-3-alkene-2-ketone (6), and finally with ethyl alcohol as a solvent, reacting (6) with different substituted phenylhydrazine hydrochlorides under reflux condition to generate the compound (A). Test results indicate that both the degrees of the compound A for inhibiting HepG2 cancer cells and promoting cell apoptosis are greater than or equal to control drug cyclophosphamide.
Owner:NANKAI UNIV
Method for turning biomass cardanol into chloroprene rubber internal plasticizer by click chemistry
Relating to a plasticizer preparation method, the invention discloses a method for turning biomass cardanol into a chloroprene rubber internal plasticizer by click chemistry. The method includes: firstly reacting cardanol with propargyl bromide to prepare propargyl ether cardanol and chloroprene rubber with an azido structure, then carrying out click chemistry reaction to realize reaction of the propargyl ether cardanol and chloroprene rubber with an azido structure, thus realizing internal plasticization of chloroprene rubber. The method for internal plasticization of chloroprene rubber withthe natural biomass raw material by click chemistry reaction disclosed by the invention has the advantages that: the cardanol raw material is sufficient, the chemical reaction process is simple, the product has obvious plasticization effect, excellent thermal stability and near zero migration of plasticizer, therefore the chloroprene rubber internal plasticizer is a new product conforming to the green and environment-friendly circular economy concept and has very broad market prospects.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
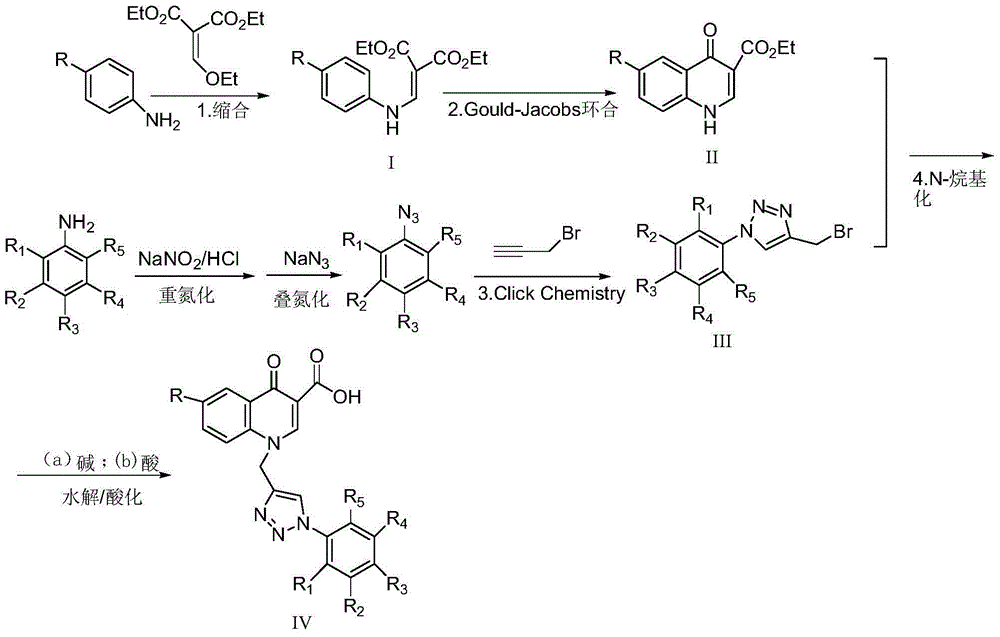
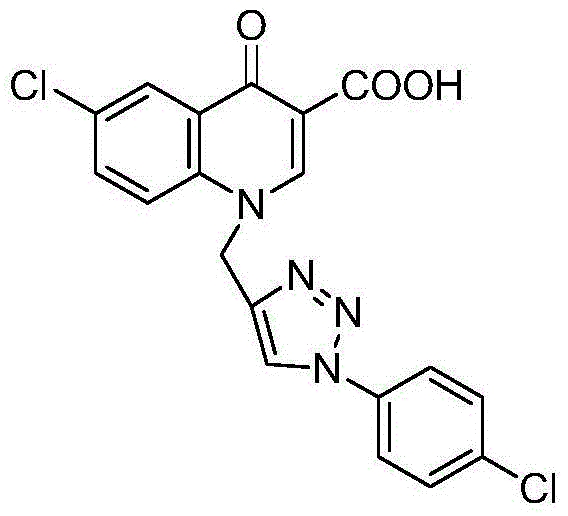
6-substituted-1-((1-substituted phenyl-1,2,3-triazole-4-yl)methyl)-4-carbonylquinoline-3-carboxylic acid or pharmaceutical salt, preparation and application
ActiveCN105237512AHigh yieldLow costOrganic chemistryAntineoplastic agentsOrganic synthesisClick chemistry
The present invention belongs to the technical field of organic synthesis, and specifically discloses 6-substituted-1-((1-substituted phenyl-1,2,3-triazole-4-yl)methyl)-4-carbonylquinoline-3-carboxylic compounds or a pharmaceutical salt thereof, and preparation and application thereof. The general formula is shown in the specification, wherein R, R1, R2, R3, R4 and R5 are the same or different, each being selected from one of H, halogen, nitro, amino, hydroxyl, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxyl, C1-C4 carboxyl and formyl. The preparation comprises: performing condensation between substituted phenyl as a raw material and diethyl ethoxymethylene malonate to produce an intermediate I, and performing Gould-Jacobs cyclization to produce an intermediate II; performing ''click chemistry'' between substituted phenyl azide and propargyl bromide to produce an intermediate III; and performing an N-alkylation reaction, alkaline hydrolysis and acidification between the intermediate II and the intermediate III to obtain a target product IV. The inventive compound or the pharmaceutically salt thereof has an excellent antitumor effect.
Owner:河南省医药科学研究院
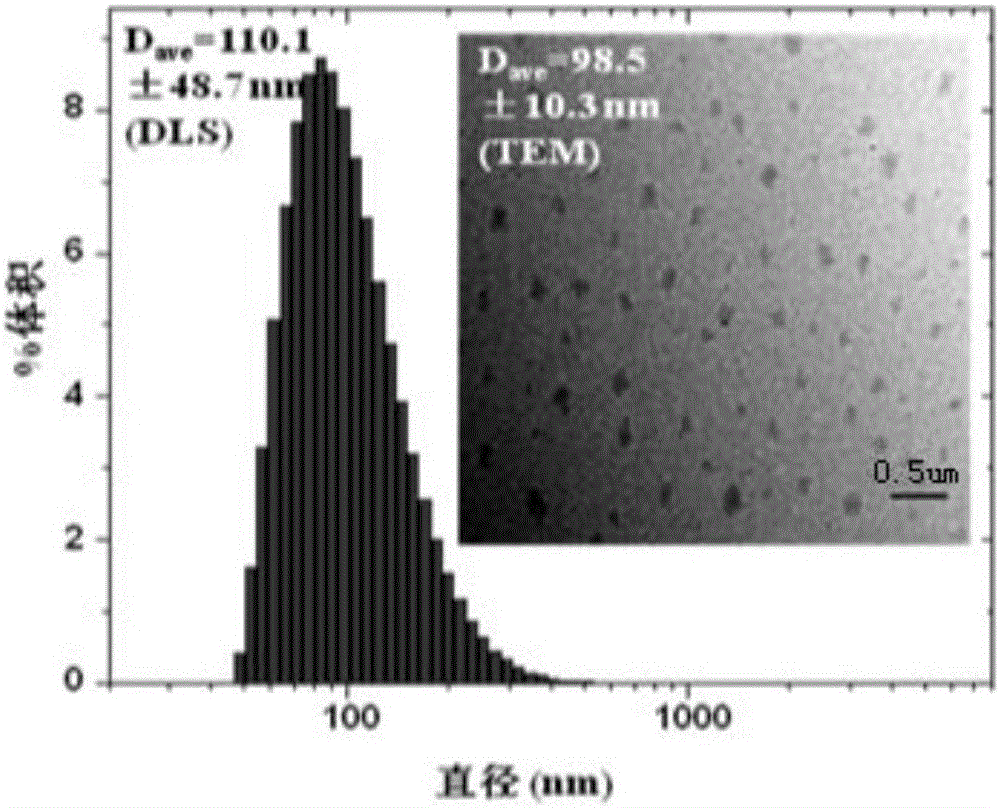
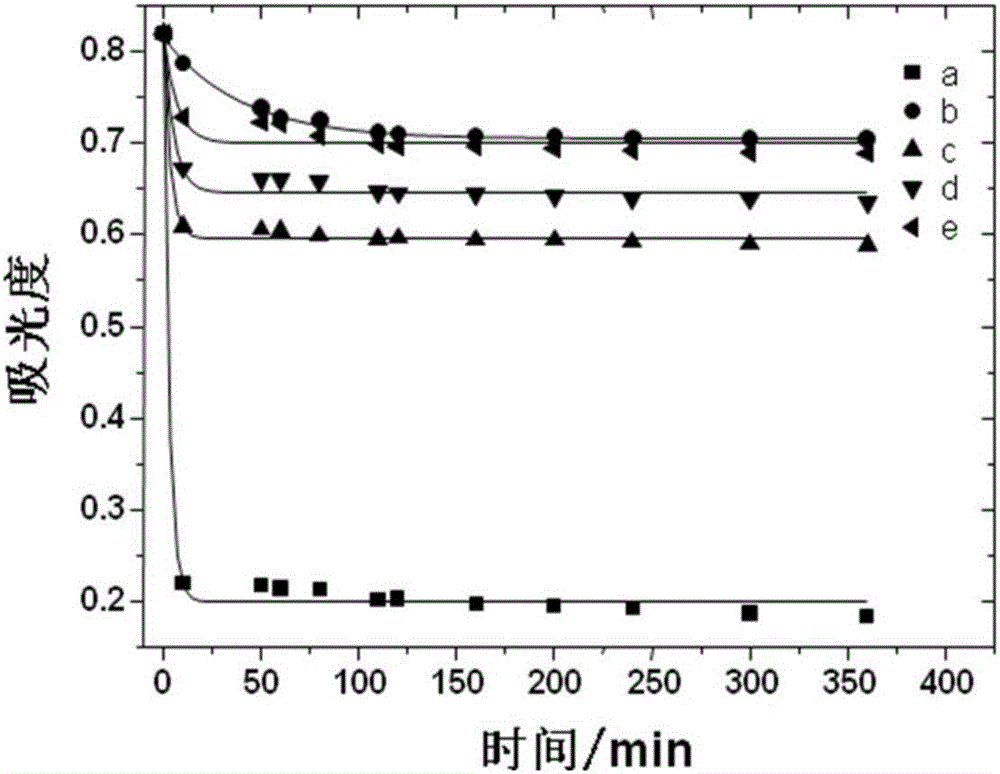
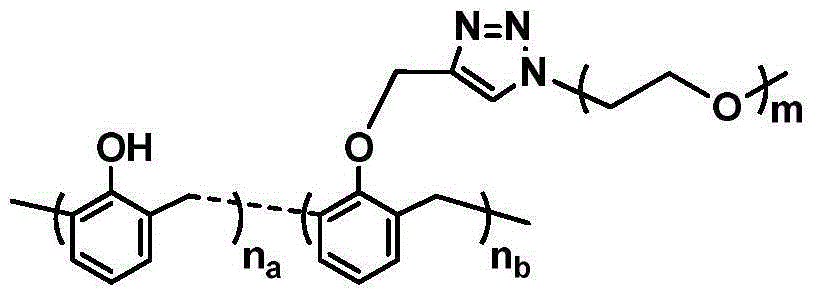
Free radical nano-capture material and preparation method thereof
The invention discloses a free radical nano-capture material. The free radical nano-capture material is amphiphilic phenol. The chemical composition general formula of the free radical nano-capture material is (C6H5OH)na-(C6H5O-PEG)nb and the structural formula of the free radical nano-capture material is shown in the description. According to the invention, linear phenol is prepared through acid catalysis, alkynyl functional groups are introduced to a phenolic chain through propargyl bromide, alkynyl and azide groupa chemically reacts through click by using methoxypolyethylene glycol with azide groups at the terminal, and finally, the nanoscale free radical nano-capture material-amphiphilic phenol is obtained; a preparation method provided by the invention is simple, the prepared free radical nano-capture material can form micelle in water and reserved phenolic hydroxyl of the free radical nano-capture material has an obvious capture effect on free radicals.
Owner:XIAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com