Synthesis method and application of trialkynyl monomer 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-triketone
A technology for the synthesis of propargyl triargyl, which is applied in the field of synthesis of alkyne-based organic monomers, can solve the problems of difficult recovery of organic solvents, complex post-treatment processes, and large environmental impact, and achieves low cost, low production cost, and economical production. resource effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
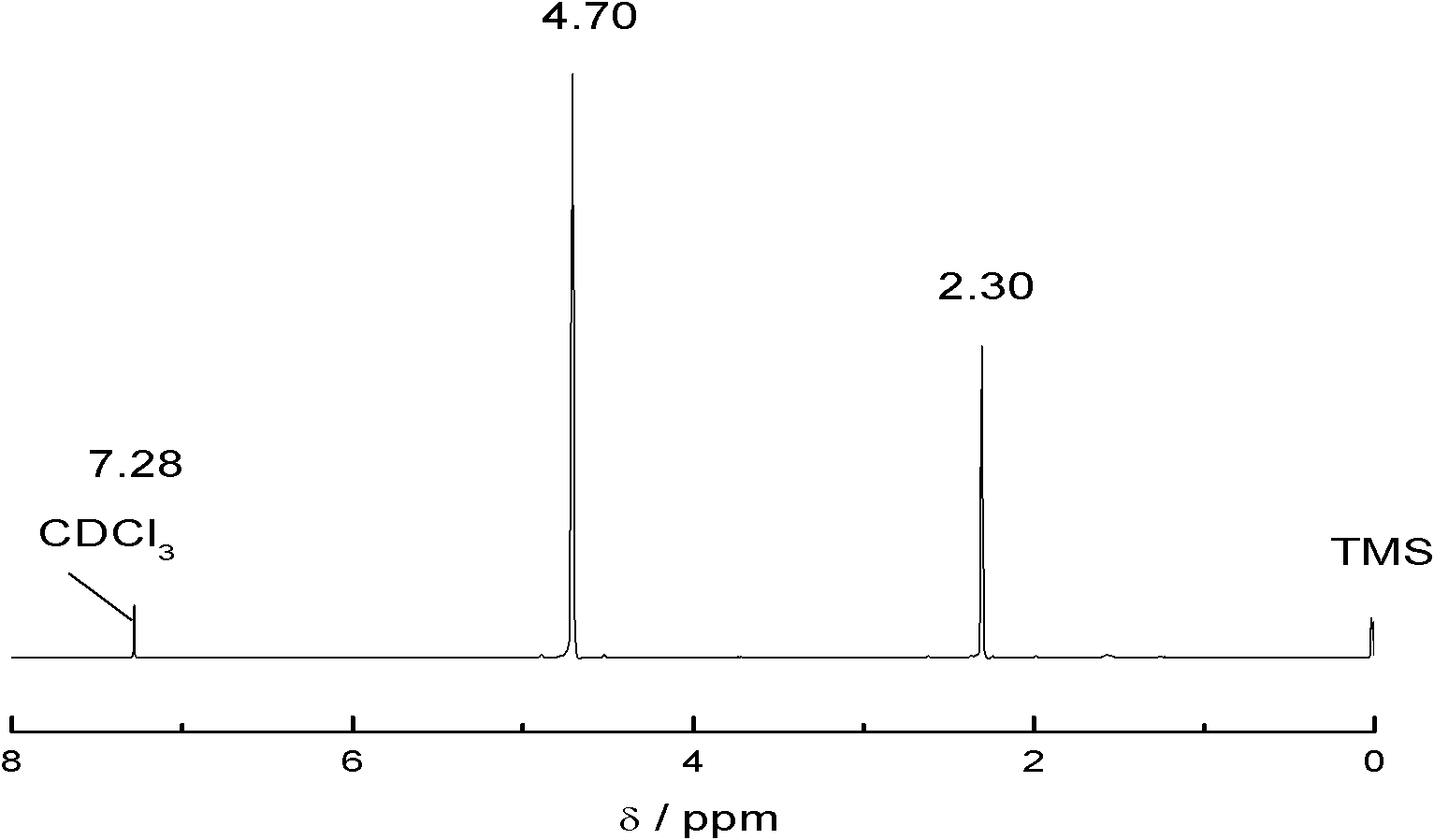
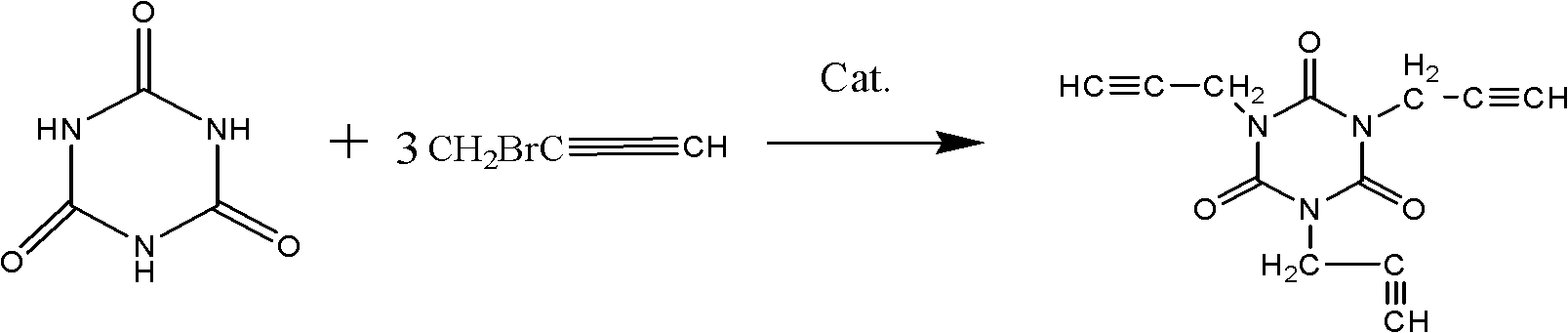
[0026] In the present embodiment, the synthetic method of 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-trione is carried out according to the following process:
[0027] Add 6.45g (0.05mol) of isocyanuric acid, 8g (0.2mol) of sodium hydroxide, 2.78g (0.01mol) of tetrabutylammonium chloride and 100mL of water into a 250mL round-bottomed three-neck flask, heat up and stir until the isocyanuric acid Dissolve, then add 47.6g (0.4mol) propyne bromide dropwise with a constant pressure dropping funnel, react at 75°C for 20 hours, cool to room temperature after the reaction is complete, separate the product and the water phase, wash the product with distilled water, and wash away NaBr, NaOH, etc., and then washed with absolute ethanol, and dried under vacuum at 55° C. for 6 hours to obtain 7.2 g of a white solid product with a yield of 59.26%.
[0028] In this embodiment, the preparation method of poly 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-trione is to take 1,3,5-tripropargyl-1 , 3,5-tr...
Embodiment 2
[0031] In this example, the preparation process of 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-trione is the same as Example 1, except that the reaction temperature is 80°C , the reaction time was 15 hours, and 7.6 g of 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-trione was prepared with a yield of 62.55%.
[0032] The preparation method of poly-1,3,5-tripropargyl-1,3,5-triazine-2,4,6-trione in this example is the same as that in Example 1.
Embodiment 3
[0034] In this embodiment, the preparation process of 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-trione is the same as in Example 1, except that the amount of propyne bromide is added It was 35.67g (0.3mol), and 5.8g of 1,3,5-tripropargyl-1,3,5-triazine-2,4,6-trione was prepared with a yield of 47.74%.
[0035] The preparation method of poly-1,3,5-tripropargyl-1,3,5-triazine-2,4,6-trione in this example is the same as that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
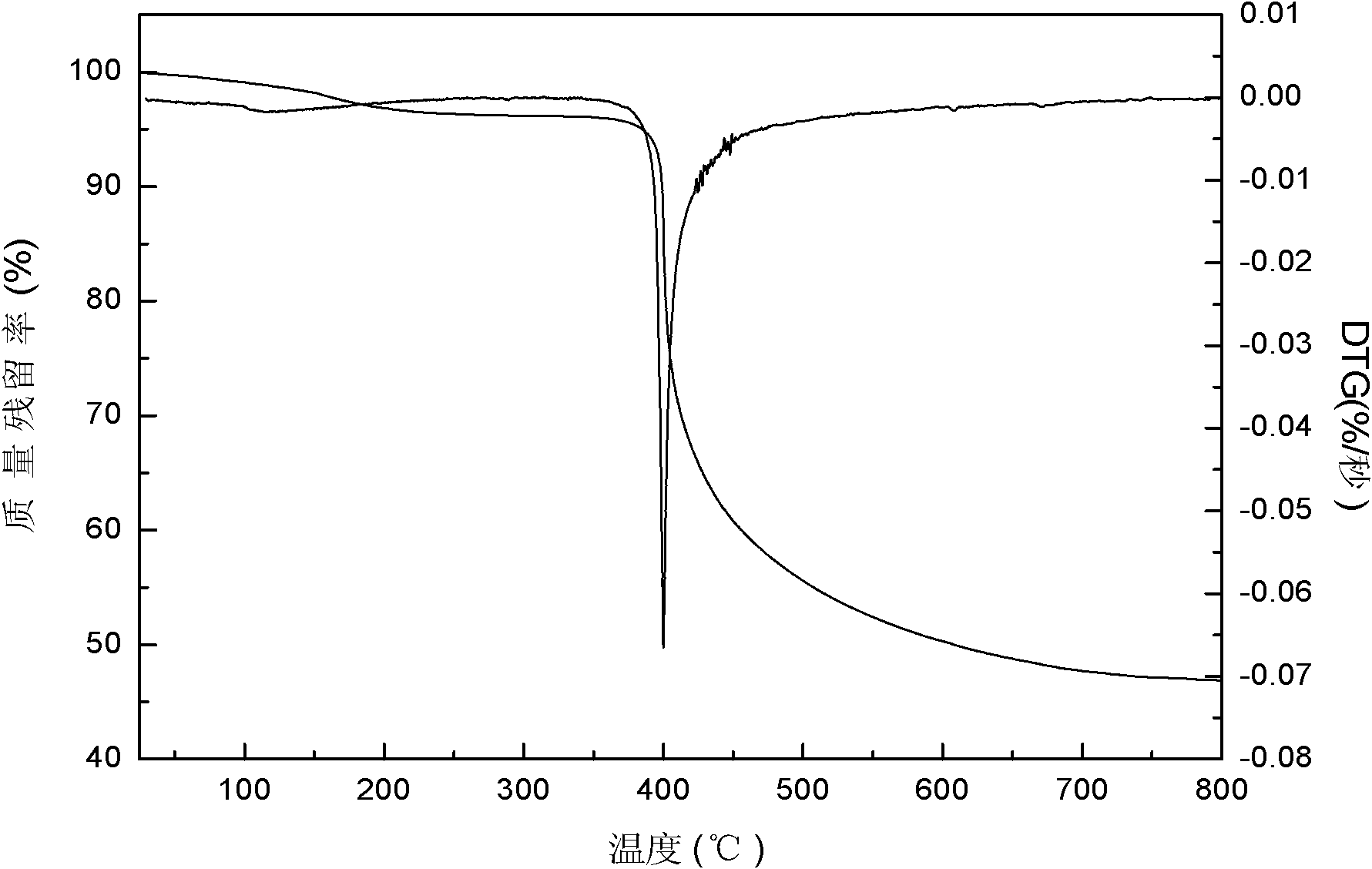
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com