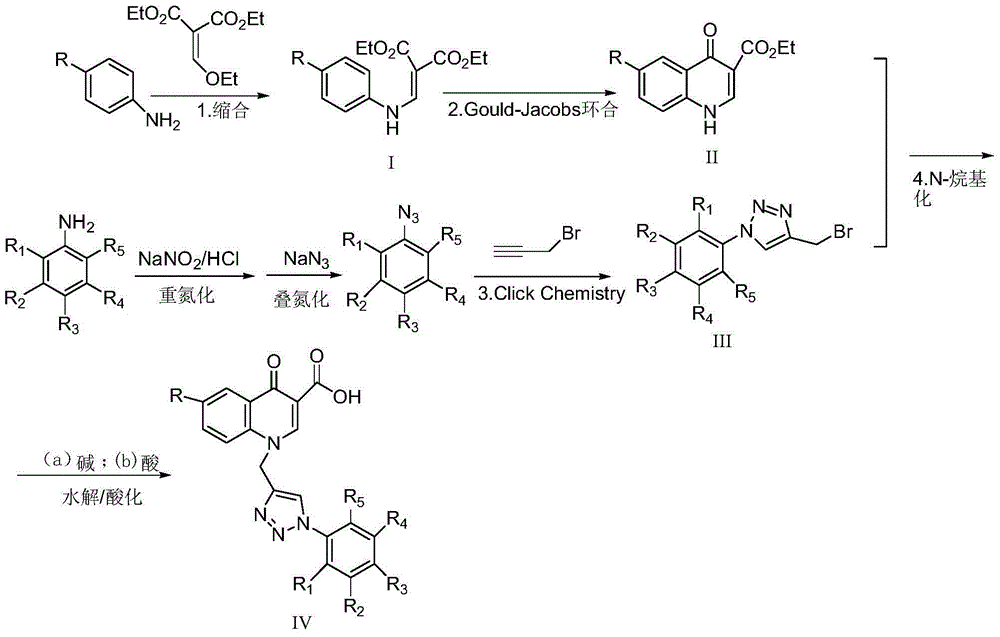
6-substituted-1-((1-substituted phenyl-1,2,3-triazole-4-yl)methyl)-4-carbonylquinoline-3-carboxylic acid or pharmaceutical salt, preparation and application
A carbonyl quinoline and medicinal salt technology, applied in the field of organic synthesis, can solve the problems of unsatisfactory prevention and treatment effects, low efficiency of anti-tumor drugs, narrow therapeutic window, etc., achieve excellent anti-tumor activity, easy post-processing, and simple steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
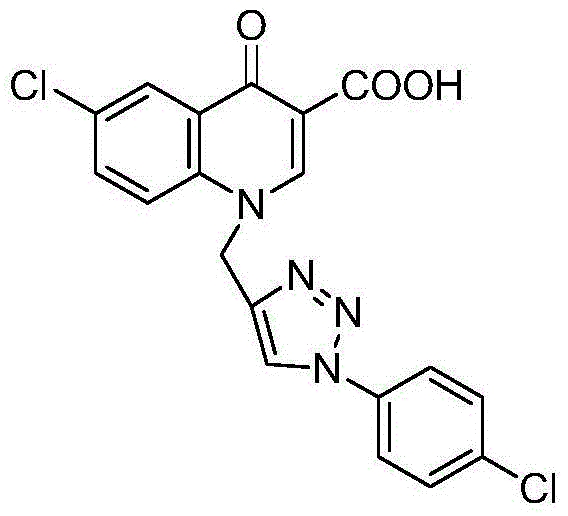
[0050] 6-Chloro-1-((1-(4-chlorophenyl)-1,2,3-triazol-4-yl)methyl)-4-carbonylquinoline-3-carboxylic acid (IV-1) synthesis
[0051]
[0052] (1) Synthesis of 2-((4-chloroaniline) methylene) diethyl malonate (I-1)
[0053] In a 100mL three-necked flask equipped with a thermometer and a reflux condenser, add 3.8g (0.030mol) of p-chloroaniline and 6.5mL (0.031mol) of diethyl ethoxymethylene malonate, add 15mL of toluene to dissolve, and heat To reflux, TLC tracking, developer V (ethyl acetate): V (petroleum ether) = 1:4, react for 6 hours, stop the reaction, stand at room temperature, filter with suction, and recrystallize from ethanol to obtain 2-((4-chloro Aniline) methylene) malonate (I-1), 7.56 g of white crystals, 87% yield, m.p.80-81°C, 1 HNMR (400MHz, CDCl 3 )δ: 8.46 (d, J = 13.2Hz, 1H, CHofVinylic,), 7.34 (d, J = 8.8Hz, 2H, Ar-H), 7.07 (d, J = 8.8Hz, 2H, Ar-H), 4.30(q, J=6.8Hz, 2H, CH 2 ,ofOCH 2 CH 3 ), 4.24 (q, J=7.2Hz, 2H, CH 2 ,ofOCH 2 CH 3 ), 1.38(t, J=7.2H...
Embodiment 2
[0061] 6-Methyl-1-((1-(4-chlorophenyl)-1,2,3-triazol-4-yl)methyl)-4-carbonylquinoline-3-carboxylic acid (IV-2) Synthesis
[0062]
[0063] According to the method for embodiment 1, p-toluene and diethyl ethoxymethylene malonate are condensed to obtain 2-((4-methylaniline) methylene) diethyl malonate: white solid, Yield 80.3%; Gould-Jacobs cyclization produced 6-methyl-4-carbonylquinoline-3-ethyl formate: white powder, yield 73.1%, and then with 4-bromomethyl-1-(4- Chlorophenyl)-1,2,3-triazole undergoes N alkylation reaction, NaOH hydrolysis and hydrochloric acid acidification to obtain 6-methyl-1-((1-(4-chlorophenyl)-1,2 ,3-triazol-4-yl)methyl)-4-carbonylquinoline-3-carboxylic acid (IV-2), white solid, yield 71.2%, m.p.223-224°C. The structure of IV-2 is characterized as follows: IR(cm -1 ) ν: 3470 (O-H), 3163 (quinoline=C-H), 3115 (triazole=C-H), 1745 (C=O, 1516, 1465 (C=C). 1 HNMR (400MHz, DMSO)δ:2.45(s,3H,-CH 3 ),5.65(s,2H,CH 2 of NCH 2 ),7.55(d,2H,ArH,J=7.2Hz),7....
Embodiment 3
[0065] 6-fluoro-1-((1-(4-chlorophenyl)-1,2,3-triazol-4-yl)methyl)-4-carbonylquinoline-3-carboxylic acid (IV-3) synthesis
[0066]
[0067]Using p-fluoroaniline as raw material, according to the method of Example 1, condensed with ethoxymethylene malonate diethyl ester to obtain 2-((4-fluoroaniline) methylene) malonate diethyl ester: white solid , yield 71.2%; Gould-Jacobs cyclization obtains 6-fluoro-4-carbonylquinoline-3-ethyl formate: white powder, yield 71.7%; and 4-bromomethyl-1-(4- Chlorophenyl)-1,2,3-triazole was subjected to N alkylation reaction, NaOH hydrolysis, and hydrochloric acid acidification to obtain 6-fluoro-1-((1-(4-chlorophenyl)-1,2, 3-triazol-4-yl)methyl)-4-carbonylquinoline-3-carboxylic acid (IV-3), white solid, yield 71.2%, m.p.218-219°C. The structural characterization of IV-3 is as follows: IR(cm -1 )ν: 3495(O-H), 3179(quinoline=C-H), 3125(triazole=C-H), 1753(C=O, 1530, 1470(C=C); 1 HNMR(400MHz,DMSO)δ:5.70(s,2H,CH 2 of NCH 2 ),7.60(d,2H,ArH,J=8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com