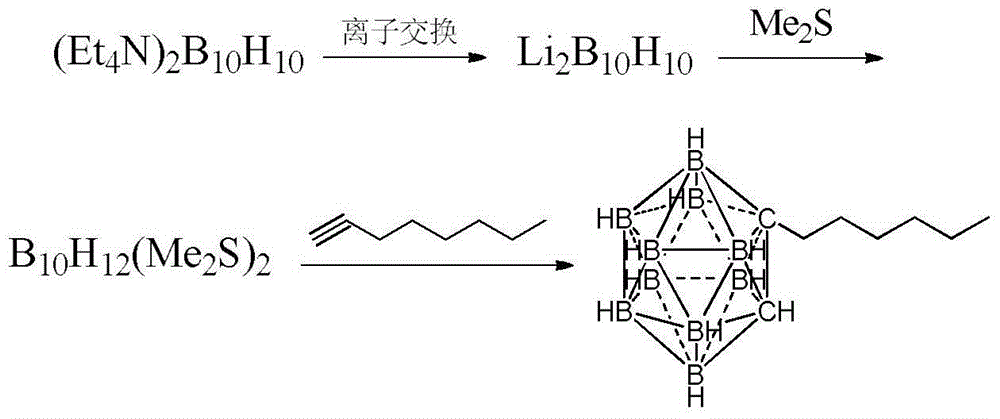
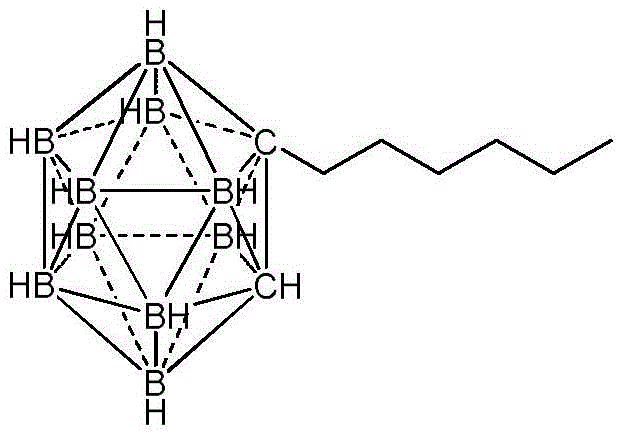
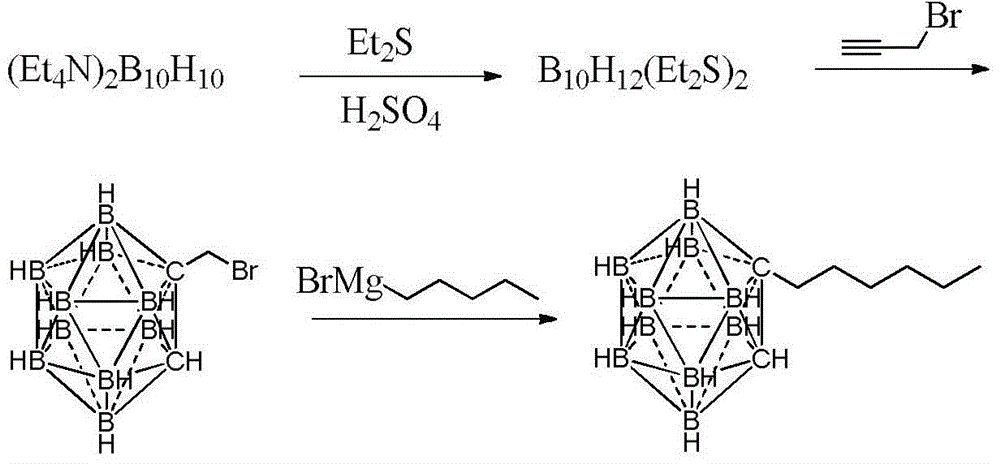Preparation method of n-hexyl carborane
A technology of n-hexyl carborane and bromomethyl carborane, which is applied in the field of preparation of n-hexyl carborane, can solve the problems such as high price of 1-octyne, and achieves reduction of production cost, small steric hindrance, and total cost reduction. high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Add 4.0g (15.1mmol) ditetraethylammonium decahydrodecaborate and 35mL diethyl sulfide into the reaction flask, add 12mL of a mixture of concentrated sulfuric acid and diethyl sulfide in an equal volume ratio at 0°C, and then After the reaction was completed for 6 hours, the supernatant was poured out, neutralized with triethylamine to pH ≈ 8, filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain 2.76 g of dodecahydrodecaboron bis-diethylsulfide complex. The yield was 87.0%.
[0024] (2) Add 3.0 g (10 mmol) of dodecahydrodecaboron bis-diethyl sulfide complex, 15 mL of toluene, and 3 g (20 mmol) of propynyl bromide toluene solution with a mass fraction of 80% into the reaction flask, and heat at 85° C. Reacted for 9 hours, after the reaction was completed, filtered, the filtrate was neutralized with triethylamine to pH ≈ 8, and the solvent was distilled off under reduced pressure to obtain 1.90 g of bromomethylcarborane with a yield of ...
Embodiment 2
[0035] (1) Add 4.0g (15.1mmol) ditetraethylammonium decahydrodecaborate and 30mL diethyl sulfide into the reaction flask, add 10mL of concentrated sulfuric acid and diethyl sulfide equal volume mixture at 10°C, and then 15°C After the reaction was completed for 5 hours, the supernatant liquid was poured out, neutralized with triethylamine to pH ≈ 8, filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain 2.38 g of dodecahydrodecaboron bis-diethylsulfide complex. The yield was 75.0%.
[0036] (2) 3.0 g (10 mmol) of dodecahydrodecaboron bis-diethylsulfide complex, 15 mL of toluene, and 1.5 g (10 mmol) of bromopropynyl toluene solution with a mass fraction of 80% were added to the reaction flask, and After reaction at ℃ for 10 h, after the reaction, filter, the filtrate was neutralized with triethylamine to pH ≈ 8, and the solvent was distilled off under reduced pressure to obtain 1.88 g of bromomethylcarborane with a yield of 79.2%.
[0037] (3) Re...
Embodiment 3
[0039] (1) Add 4.0g (15.1mmol) ditetraethylammonium decahydrodecaborate and 30mL diethyl sulfide into the reaction flask, add 15mL of concentrated sulfuric acid and ethyl sulfide equal volume mixture at 5°C, and then 10°C React for 5.5 hours. After the reaction is over, pour out the supernatant, neutralize with triethylamine to pH ≈ 8, filter, and evaporate the filtrate to dryness under reduced pressure to obtain 2.71 g of dodecahydrodecaboron bis-diethylsulfide complex , and the yield was 85.3%.
[0040] (2) 3.0g (10mmol) of dodecahydrodecaboron bis-diethylsulfide complex, 15mL toluene, and 4.5g (30mmol) of bromopropynyl toluene solution with a mass fraction of 80% were added to the reaction flask, and After reaction at ℃ for 9 hours, after the reaction, filter, the filtrate was neutralized with triethylamine to pH ≈ 8, and the solvent was distilled off under reduced pressure to obtain 1.88 g of bromomethylcarborane with a yield of 79.3%.
[0041] (3) React 1.31 mL (10.5 mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com