Method for preparing mannose-containing derivatives capable of being used for post-polymerization modification through double-click chemistry combination
A click chemistry and post-polymerization technology, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., to achieve the effects of improving the preparation method, simple experimental operation, and stable synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] (1) Preparation of compound 1
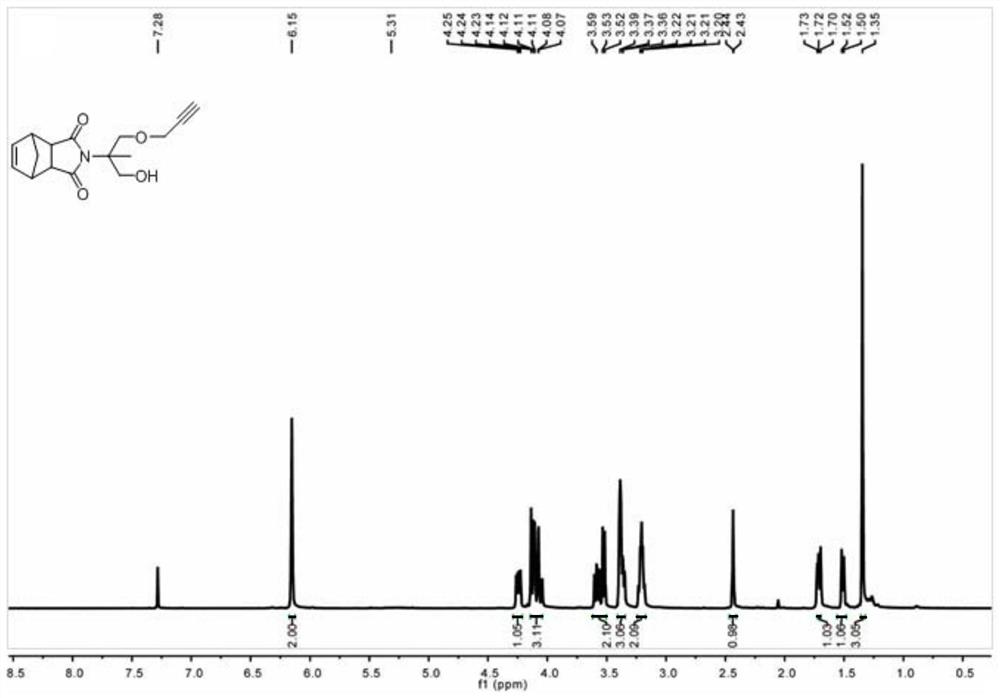
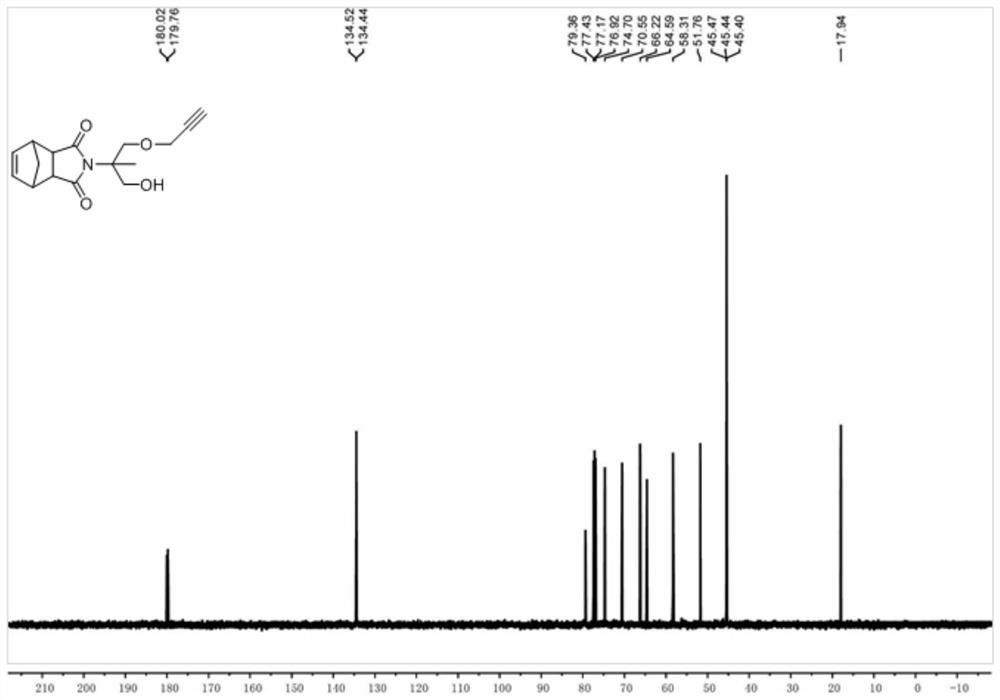
[0060] Take 5-norbornene-2,3-dicarboxylic anhydride (5.00g, 30.46mmol) and 2-amino-2-methyl-1,3-propanediol (3.80g, 36.14mmol) into a 250ml dry reaction flask In, nitrogen was introduced for 10 minutes, then 150ml of anhydrous toluene was added, refluxed at 120°C for 16 hours and a water separator was installed. After the completion of the reaction was confirmed by thin-layer chromatography, the solvent was removed by rotary evaporation, and the product was separated by silica gel column chromatography to obtain 5.21 g of a white solid with a yield of 68%.
[0061] 1 H NMR (500MHz, CDCl 3 )δ=6.15(s,2H),4.19(d,J=12.0Hz,2H),3.58-3.68(m,4H),3.40(m,2H),3.23(m,2H),1.57(d,J =9.0Hz, 1H), 1.49(d, J=8.5Hz 1H), 1.17(s, 3H). 13 C NMR (125MHz, CDCl 3 )δ=178.72,165.49,134.53,131.12,128.16,79.43,77.32,77.07,76.82,74.58,70.31,64.84,62.25,58.31,51.79,45.41,19.34. 13 h 17 NO 4 H(M+H + )calc.for:252.11576;found:252.11394.
[0062] (2) Preparation...
Embodiment 2
[0071] (4) Compound G 1 preparation of
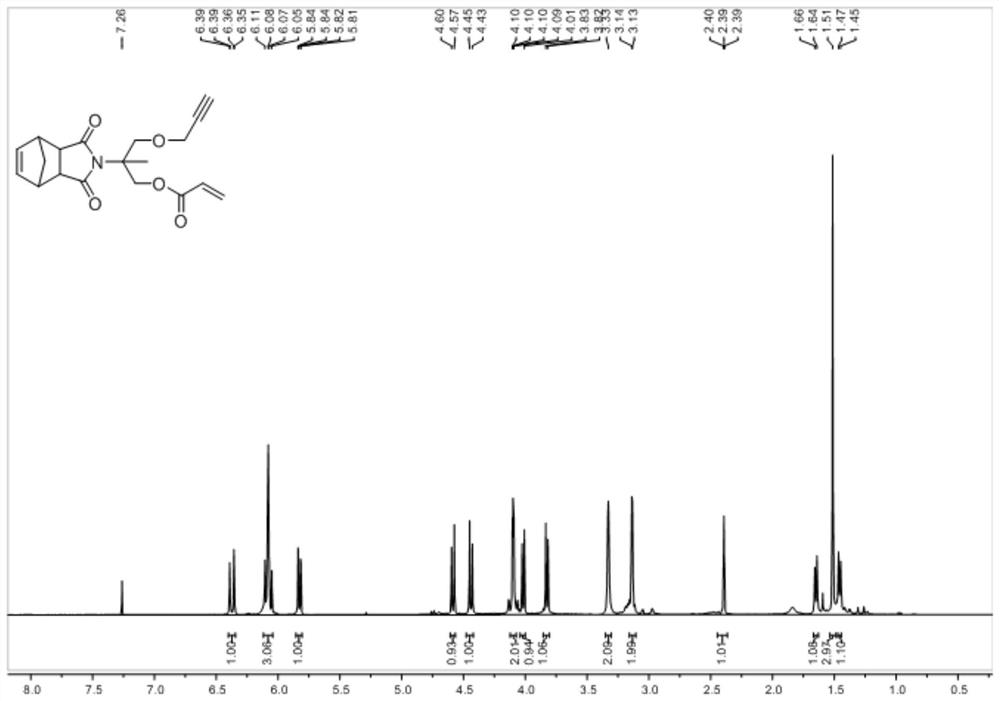
[0072]Dissolve compound 3 (1.80g, 5.24mmol) and 1-pentanethiol (0.74ml, 5.86mmol) in 20ml of anhydrous CH 2 Cl 2 , after stirring for 30 min, a dichloromethane solution of dimethylphenylphosphine (3.50ml, 7mmol%) was added (the concentration of dimethylphenylphosphine was 0.1M in CH 2 Cl 2 : Dissolve 142 μL of pure dimethylphosphorus in 10 mL of CH 2 Cl 2 prepared in). After reacting at room temperature for 3 h, thin layer chromatography confirmed whether the reaction was complete. After the reaction was completed, it was extracted with saturated brine and dichloromethane, dried with anhydrous sodium sulfate, and the product was separated by silica gel column chromatography to obtain 2.43 g of light yellow oily liquid (i.e. compound G 1 ), the yield is 98%.
[0073] The obtained compound G 1 The hydrogen NMR spectrum and the carbon NMR spectrum can be found in Figure 5 with Image 6 .
[0074] 1 H NMR (500MHz, CDCl 3 )δ6....
Embodiment 3
[0080] (6) Compound G 2 preparation of
[0081] Dissolve compound 3 (2.00g, 5.83mmol) and 3-mercapto-1-propanol (0.61ml, 7.06mmol) in 20ml of anhydrous CH 2 Cl 2 After stirring for 30 min, dimethylphenylphosphine (0.1M in CH 2 Cl 2 , 4.10ml, 7mol%) to continue the reaction, after 3h at room temperature, thin-layer chromatography confirmed whether the reaction was complete. After the reaction was completed, it was extracted with saturated brine and dichloromethane, and after drying with anhydrous sodium sulfate, the product was separated by silica gel column chromatography to obtain 2.53 g of light yellow oily liquid (i.e. compound G 2 ), the yield is 95%.
[0082] The obtained compound G 2 The H NMR spectrum and C NMR spectrum can be found in Figure 7 with Figure 8 shown.
[0083] 1 H NMR (500MHz, CDCl 3 )δ6.09(s, 2H), 4.58(d, J=11.5Hz, 1H), 4.35(d, J=11.5Hz, 1H), 4.08(t, J=2.0Hz, 2H), 3.92(d, J=9.0Hz, 1H), 3.79(d, J=9.0Hz, 1H), 3.70(t, J=6.0Hz, 2H), 3.32(s, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com