Free radical nano-capture material and preparation method thereof
A free radical and nanotechnology, applied in the field of functional polymer materials, can solve the problems of poor water solubility and limited application of ordinary polyphenolic polymer compounds, and achieve the effect of simple preparation method and significant capture effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
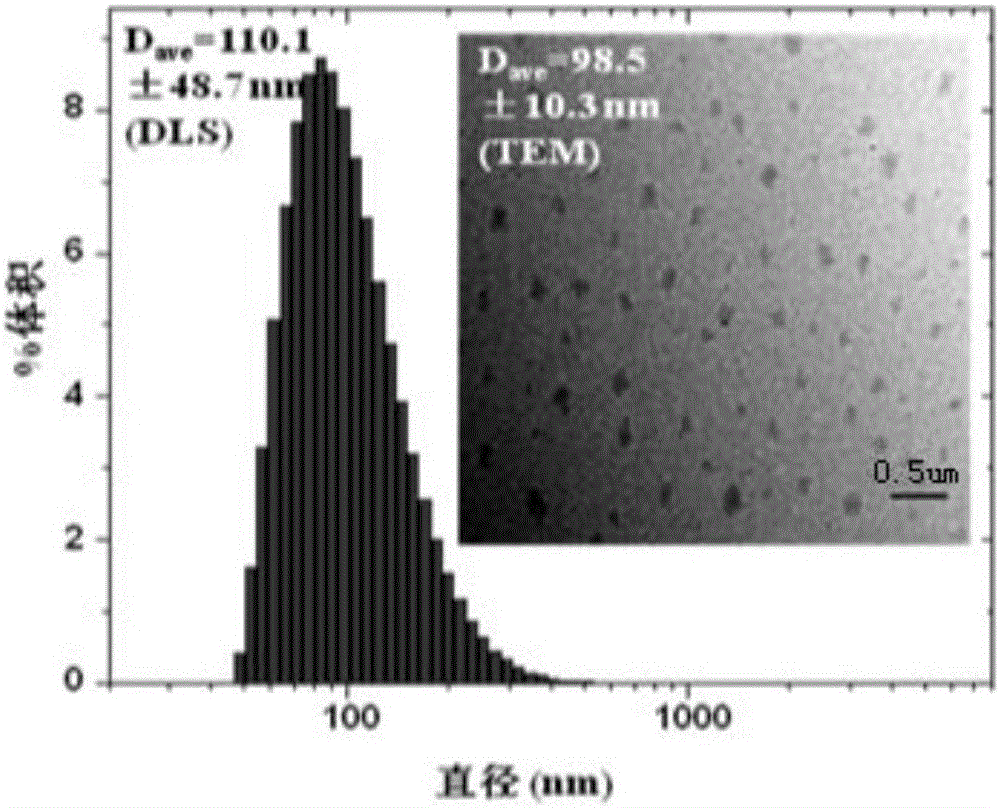
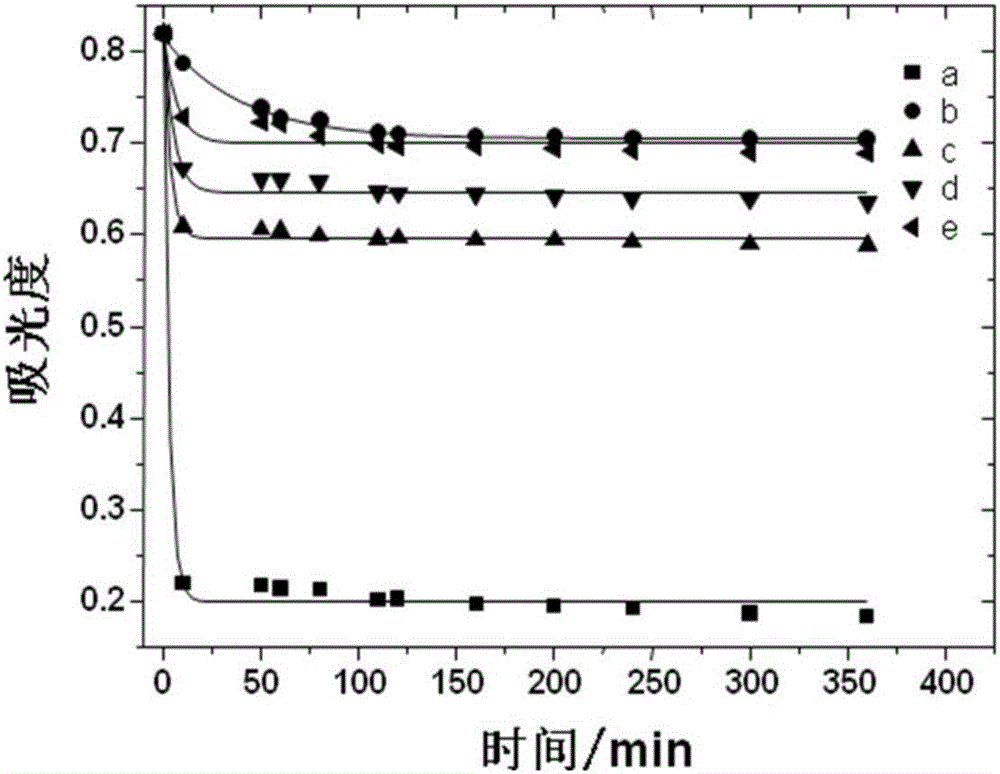
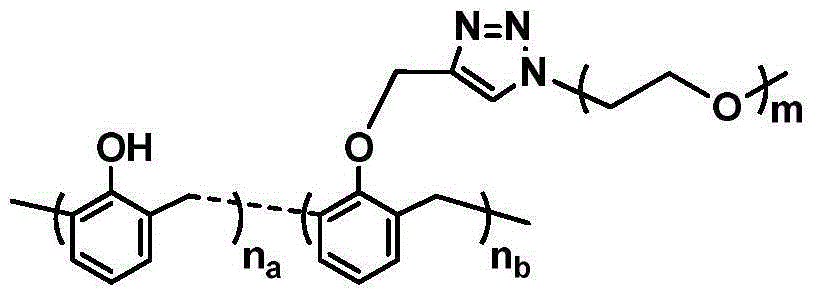
Image
Examples
Embodiment 1
[0030] Step 1: Take 14.12g of phenol and 10.96g of formaldehyde (37% aqueous solution) respectively, mix them evenly, add 1.10g of dihydrate oxalic acid, at 80-85°C, mechanically stir for 1.5h to obtain novolac resin; The obtained novolac resin was precipitated and purified twice in a mixed solvent of 25.20g petroleum ether and 7.89g ethanol, and dried in vacuum at 70°C for more than one week to obtain a dry phenolic resin; 0.50g of the obtained phenolic resin was dissolved in 2.37g of acetone and poured into it Add 0.70g propyne bromide, 0.65g anhydrous potassium carbonate, 0.005g benzyltriethylammonium chloride and 0.016g potassium iodide, remove oxygen through freeze-thaw-cycle operation, react at 60°C for 20h, and the substitution rate is 90% (NMR test) of alkyne functionalized novolac;
[0031] Step 2: Dissolve 1.00g of polyethylene glycol monomethyl ether (MW=500) and 1.14g of p-toluenesulfonyl chloride in 6.22g of tetrahydrofuran, add 0.607g of anhydrous triethylamine, ...
Embodiment 2
[0034] Step 1: take 0.50g example 1 gained phenolic resin and dissolve in 2.37g acetone, and add 0.35g propyne bromide and 0.33g anhydrous potassium carbonate, 0.005g benzyltriethylammonium chloride, 0.016g potassium iodide, Oxygen was removed by freeze-thaw-cycle operation, reacted at 60°C for 20 hours, and alkynyl-functionalized novolac with a substitution rate of 47% (NMR test) was obtained;
[0035] Step 2: Dissolve 8.00g of polyethylene glycol monomethyl ether (MW=500) and 9.15g of p-toluenesulfonyl chloride in 49.78g of tetrahydrofuran, add 4.86g of anhydrous triethylamine, and react at room temperature for 10 hours to obtain an intermediate product ; After the obtained intermediate product was purified by column chromatography, 0.50 g of the purified polyethylene glycol intermediate product was dissolved in 1.89 g of N,N-dimethylformamide, and 0.15 g of sodium azide was added to mix, and the mixture was prepared at 110° C. After reacting for 10 h, and precipitating the ...
Embodiment 3
[0038] Step 1: take 0.50g example 1 gained phenolic resin and dissolve in 2.37g acetone, and add 2.81g propyne bromide and 2.60g anhydrous potassium carbonate, 0.005g benzyltriethylammonium chloride, 0.016g potassium iodide, Oxygen removal by freeze-thaw-cycle operation, reaction at 60°C for 20h, and alkynyl-functionalized novolac with a substitution rate of 100% (NMR test);
[0039] Step 2: Weigh 8.00g polyethylene glycol monomethyl ether (MW=350) and 13.07g p-toluenesulfonyl chloride, dissolve in 49.78g tetrahydrofuran, add 7.00g anhydrous triethylamine, and react at room temperature for 10h to obtain an intermediate product; after the obtained intermediate product was purified by column chromatography, 2.00 g of the purified polyethylene glycol intermediate product was taken and dissolved in 7.55 g of N,N-dimethylformamide, 0.97 g of sodium azide was added to mix, and the mixture was heated at 110°C. The reaction was continued for 10 h, and the reaction product was precipitat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com