Preparation method of glycopolymer with lateral chains containing heterogeneous sugar units
A sugar unit and polymer technology, which is applied in the synthesis field of sugar-containing polymers to achieve the effects of regular structure, narrow molecular weight distribution and stable synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] The synthesis of poly-A-Man-OH and poly-A-Man-Man-OH poly-A-Man-Man-OH polymers containing heterogeneous sugar units in the side chain, the specific steps are as follows:
[0090] 1. Synthesis of Compound 1
[0091] Weigh 2-amino-2-methyl-1,3-propanediol (20.0g, 0.19mol) and dissolve it in 200mL (MeOH:t-BuOH=1:1) solution, add dropwise BOC anhydride (57mL, 0.247mol ), reacted at room temperature for 18 h, and after the reaction was completed, the solvent was spin-dried, and recrystallized from ethyl acetate and petroleum ether to obtain 31.9 g of a white solid (Compound 1), with a yield of 82%. 1 H NMR (500MHz, CDCl 3 )δ=4.97(s,1H),3.77(dd,J=11.3,5.4Hz,2H),3.61(dd,J=11.3,7.1Hz,2H),1.69(s,2H),1.44(s,9H ),1.16(s,3H).
[0092] 2. Synthesis of compound 2
[0093] Weigh compound 1 (24.0g, 0.117mol) and dissolve it in 200mL of N,N-dimethylformamide, slowly add propargyl bromide (35.1mL, 0.468mol) dropwise at 0°C, and continue the reaction for 10min. Then add potassium hy...
Embodiment 2
[0114] The synthesis of the sugar-containing polymer poly-A-Man-Glu-OH containing heterogeneous sugar units in the side chain, the specific steps are as follows:
[0115] 1. Synthesis of Compound 1
[0116] As in step 1 in Example 1.
[0117] 2. Synthesis of compound 2
[0118] As in step 1 in Example 1.
[0119] 3. Synthesis of α-D-mannose and β-D-glucose heterogeneous sugar addition compound BOC-A-Man-Glu-OAc
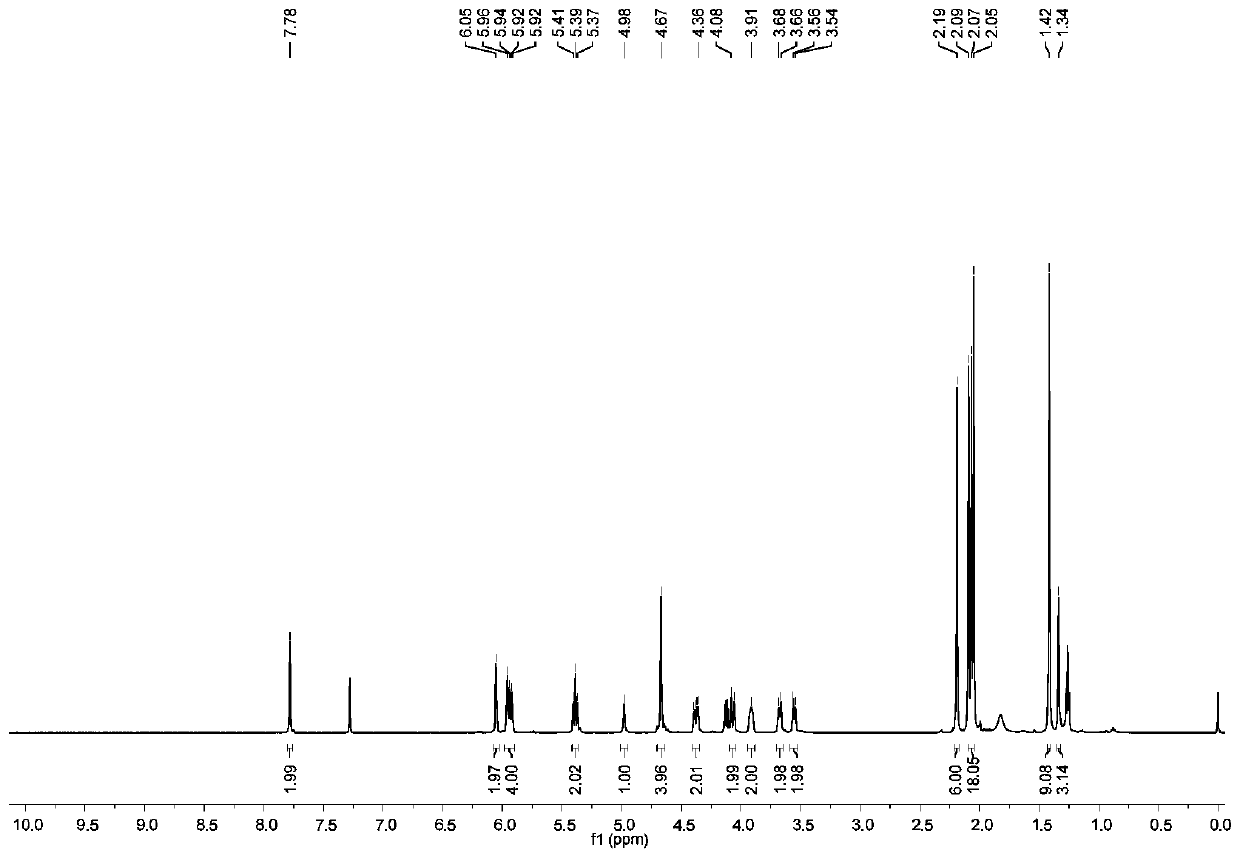
[0120] Take BOC-A-Man-OAc (0.95g, 1.45mmol) and β-D-glucose azide compound (0.6g, 1.6mmol) and dissolve in 10mL of (t-BuOH:H 2 O=1:1) in the mixed solvent, then add the catalyst copper sulfate pentahydrate (0.18g, 0.73mmol) and sodium ascorbate (0.29g, 1.45mmol), after stirring at room temperature for 2h, dichloromethane and saturated salt Extracted with water, the collected organic phase was dried with anhydrous sodium sulfate, and the product was subjected to column chromatography to obtain 1.21 g of a white solid (BOC-A-Man-Glu-OAc), with a yield of 81%. The h...
Embodiment 3
[0128] The synthesis of the poly-A-Man-Gal-OH poly-A-Man-Gal-OH polymer containing heterogeneous sugar units in the side chain, the specific steps are as follows:
[0129] 1. Synthesis of Compound 1
[0130] As in step 1 in Example 1.
[0131] 2. Synthesis of compound 2
[0132] As in step 1 in Example 1.
[0133] 3. Synthesis of α-D-mannose and β-D-galactose heterogeneous sugar addition compound BOC-A-Man-Gal-OAc
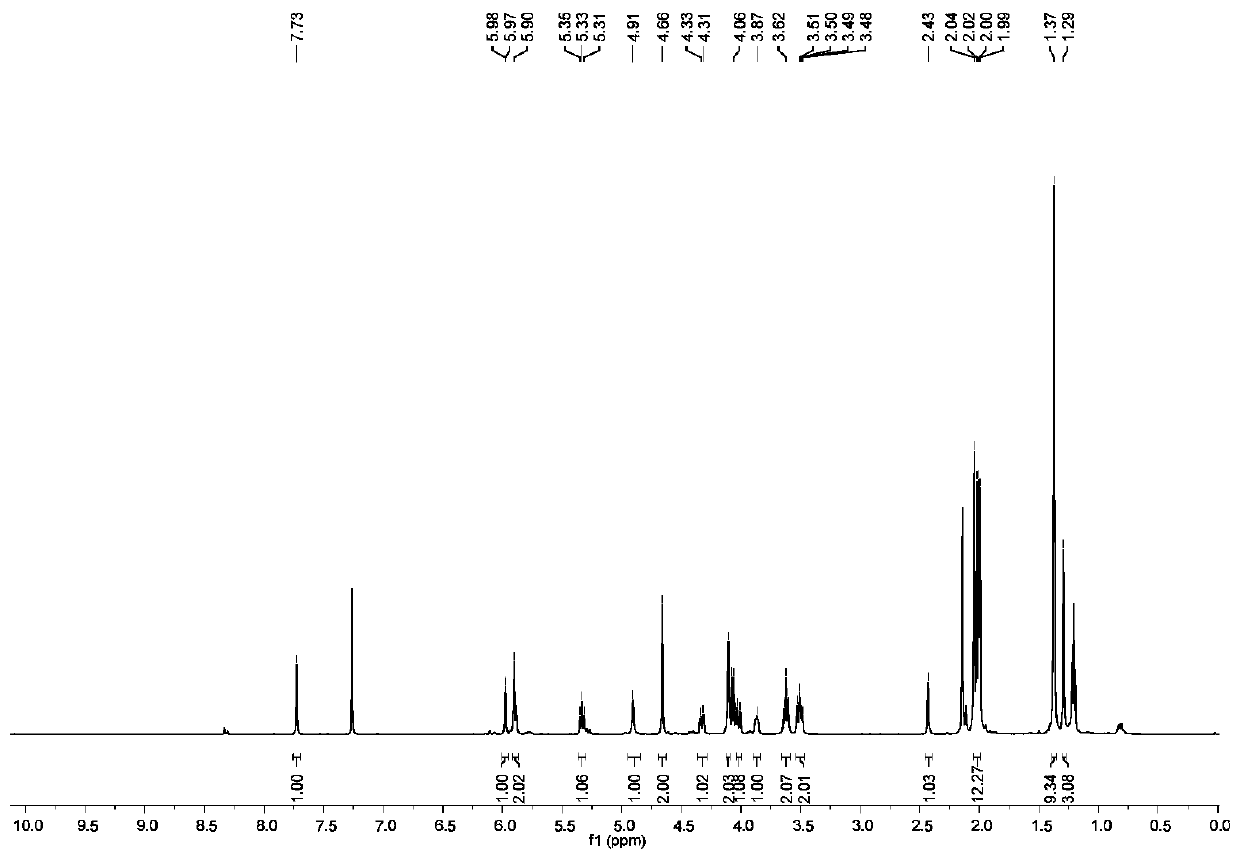
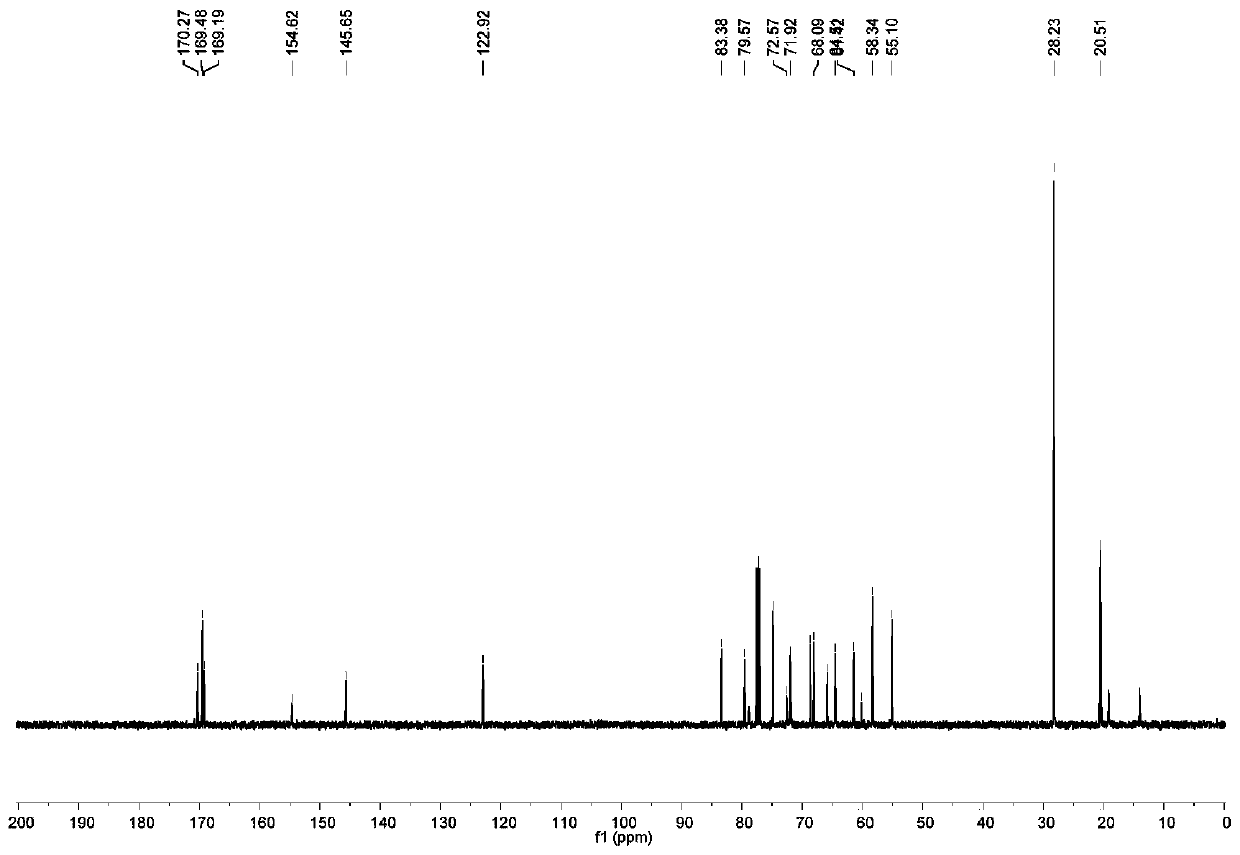
[0134] In the same synthesis method as BOC-A-Man-Glu-OAc, BOC-A-Man-OAc reacts with β-D-galactose azide to prepare BOC-A-Man-Gal-OAc, and obtains a white solid with a yield of was 66%. The hydrogen spectrum and carbon spectrum of BOC-A-Man-Gal-OAc are as follows Figure 24 with Figure 25 shown. 1 H NMR (500MHz, CDCl 3)δ=7.79-7.72(m,2H),6.02(d,J=8.2Hz,1H),5.88-5.80(m,3H),5.49-5.42(m,2H),5.29(t,J=9.1Hz ,2H),5.21(d,J=7.2Hz,1H),4.91(s,1H),4.52(d,J=20.1Hz,5H),4.24(s,2H),4.14-3.90(m,4H) ,3.82(s,1H),3.54(d,J=8.6Hz,1H),3.45(dd,J=17.1,10.6Hz,3H),3.31(d,J=9.3Hz,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com