A method of preparing 2,4,6-trifluorobenzaldehyde
A technology of trifluorobenzaldehyde and trifluorobenzene, which is applied in the field of compound preparation, achieves the effects of high yield, easy availability of raw materials, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
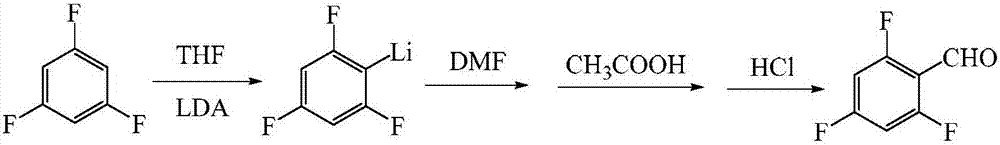
[0030] (1) Add 2000mL tetrahydrofuran and 320g diisopropylamine into the reaction flask, cool the system to -50°C with liquid nitrogen, stir under nitrogen protection, add dropwise 1240mL n-butyllithium (concentration is 2.5mol / L, the same below ), control the temperature not to exceed -10°C, and after the dropwise addition is completed, a tetrahydrofuran solution of lithium diisopropylamide is obtained, which is kept at a temperature below -10°C for use.
[0031] (2) Take another reaction bottle, add 3000mL tetrahydrofuran and 396g 1,3,5-trifluorobenzene, cool the system to -80°C with liquid nitrogen, and drop the standby lithium diisopropylamide under the protection of nitrogen The tetrahydrofuran solution, the temperature is controlled not to exceed -75 ° C, after the dropwise addition is completed, the reaction is kept for 3 hours.
[0032] (3) Add 225 g of dimethylformamide dropwise to the reaction solution, control the temperature not to exceed -75° C., and keep the reac...
Embodiment 2
[0036] (1) Add 1800mL tetrahydrofuran and 310g diisopropylamine into the reaction flask, cool the system to -50°C with liquid nitrogen, stir under nitrogen protection, add 1240mL n-butyllithium dropwise, control the temperature not to exceed -10°C, drop After the addition is complete, a tetrahydrofuran solution of lithium diisopropylamide is obtained, which is kept at -10°C until use.
[0037] (2) Take another reaction bottle, add 3000mL tetrahydrofuran and 400g 1,3,5-trifluorobenzene, cool the system to -80°C with liquid nitrogen, and drop the ready-to-use lithium diisopropylamide under the protection of nitrogen The tetrahydrofuran solution, the temperature is controlled not to exceed -75°C, and the reaction is kept for 4 hours after the dropwise addition is completed.
[0038] (3) Add 225 g of dimethylformamide dropwise to the reaction solution, control the temperature not to exceed -75° C., and keep the reaction for 1 hour after the dropwise addition is completed.
[0039...
Embodiment 3
[0042] (1) Add 1800mL tetrahydrofuran and 310g diisopropylamine into the reaction flask, cool the system to -55°C with liquid nitrogen, stir under nitrogen protection, add 1240mL n-butyllithium dropwise, control the temperature not to exceed -10°C, drop After the addition is complete, a tetrahydrofuran solution of lithium diisopropylamide is obtained, which is kept at -10°C until use.
[0043] (2) Take another reaction bottle, add 2500mL tetrahydrofuran and 400g 1,3,5-trifluorobenzene, cool the system to -80°C with liquid nitrogen, and drop the standby lithium diisopropylamide under the protection of nitrogen The tetrahydrofuran solution, the temperature is controlled not to exceed -75 ° C, and the temperature is kept for 5 hours after the dropwise addition is completed.
[0044] (3) Add 228 g of dimethylformamide dropwise to the reaction solution, control the temperature not to exceed -75° C., and keep the reaction for 1 hour after the dropwise addition is completed.
[0045...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com