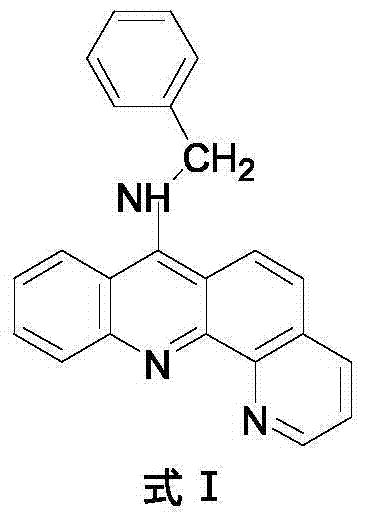
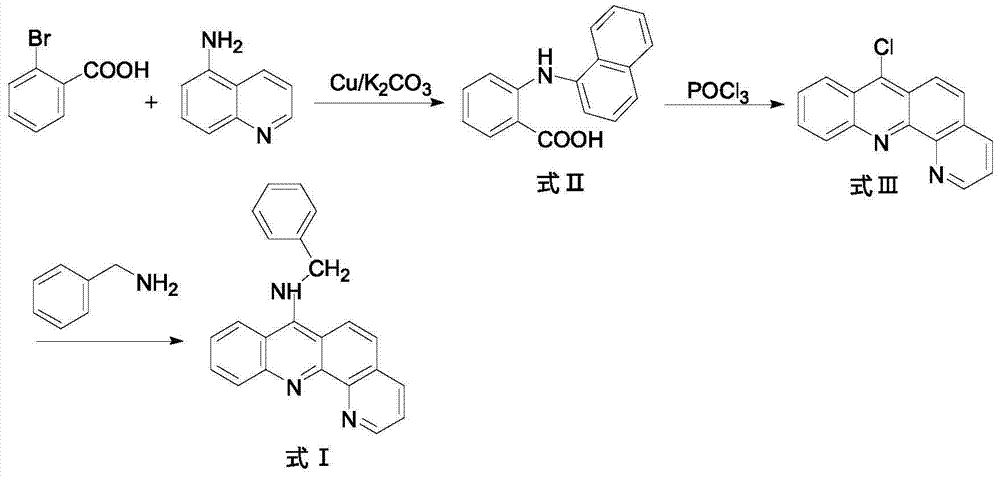
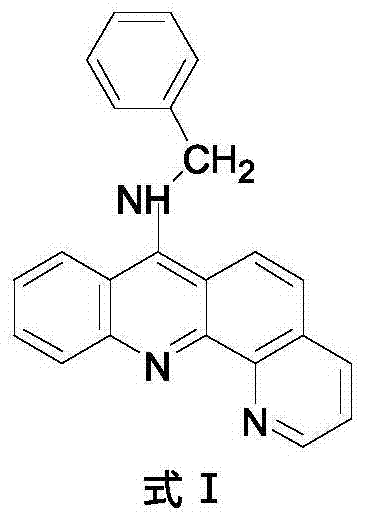
9-amino substituted pyrido acridine derivative, preparation method and uses thereof
A technology of acridine and derivatives, applied in the field of medicine, can solve the problems of short half-life, side effects of peripheral cholinergic system, unfavorable long-term use of patients, etc., and achieve the effect of good effect, simple preparation method, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
[0018] The preparation of embodiment 19-benzylaminopyridoacridine
[0019] 1) In a 250ml three-necked flask, add 5.20g (26mmol) of o-bromobenzoic acid (26mmol), 8-aminoquinoline (34mmol), 7.5g (36.2mmol) of potassium carbonate and 0.3g (4.7mmol) of copper powder, and then add 30ml Isoamyl alcohol was used as a solvent, and stirred under reflux at 140°C for 2h. After the reaction, evaporate the solvent under reduced pressure, add 600ml of water to the obtained residue, react at 80°C for 20 minutes, filter while hot, wash the filter cake, combine the water layer, acidify the water layer with concentrated hydrochloric acid to pH=2, and precipitate a large amount of light green Precipitate, filter with suction, and recrystallize the obtained solid with chloroform to obtain compound N-(quinolyl)anthranilic acid (formula II), with a yield of 51%;
[0020] 2) In a 100ml round bottom flask, add the compound represented by formula II (18mol) and 14.37ml of phosphorus oxychloride, and ...
Embodiment 2
[0022] Embodiment 2 Identification and analysis of compounds of the present invention
[0023] The 9-amino-substituted pyridoacridine derivatives obtained by the method described above 1 After H NMR nuclear magnetic resonance spectrum, fast atom bombardment mass spectrometry, melting point and other tests, its chemical structure was confirmed by analysis.
[0024] The physical and chemical properties are as follows:
[0025] 1) Appearance: light yellow powder
[0026] 2) Melting point: 201~204℃
[0027] 3) Molecular weight: 335.4
[0028] 4) Molecular formula: C 23 h 17 N 3 , the structural formula is as follows:
[0029]
[0030] 5) Fast atom bombardment mass spectrometry (FAB-MS): m / z: 336[M+H] + .
[0031] 6) 1H NMR nuclear magnetic resonance spectrum: the sample is dissolved in deuterated dimethyl sulfoxide (DMSO-d6), and measured at 400MHz, the obtained spectral data is: δ: 8.81 (d, 1H, J=8.4, - ArH), 8.34(d, 1H, J=8.4, -ArH), 8.24(t, J=9.3Hz, 2H, ArH), 8.02(...
Embodiment 3
[0032] The determination of embodiment 3 in vitro acetylcholinesterase inhibitory activity
[0033] Application of Ellman (Ellman, G.L.; Courtney, K.D.; Andres, V.; et al.Biochem.Pharmacol.1961, 7, 88.) method test compound for acetylcholinesterase inhibition IC 50 value. All tests were carried out with a Microplate reader ELX808TM microplate reader (BioTek, USA) at 37°C. The data analysis software used Origin software for data processing, and galantamine hydrobromide was used as the reference substance.
[0034] experimental method:
[0035] 1) Preparation of inhibitor stock solutions: the tested inhibitors were formulated into 10 mM DMSO solutions.
[0036] 2) Preparation of enzyme stock solution: Acetylcholinesterase (extracted from electric eel) was purchased from Sigma Company; 0.1 mg / mL and 2 mg / mL were prepared respectively with phosphate buffer solution of pH=8.0.
[0037] 3) Preparation of substrate stock solution: Acetylmercaptocholine (acetylcholinesterase subst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com