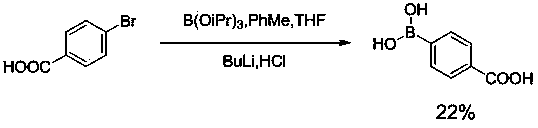Preparation method of p-carboxyphenylboronic acid
A technology of carboxyphenylboronic acid and p-bromobenzoic acid, applied in the field of chemistry, can solve problems such as low yield, low success rate, difficult post-processing, etc., and achieve the effects of high yield, low reaction cost, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
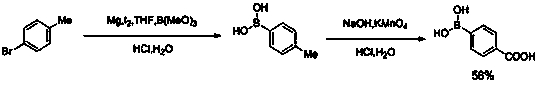
Image
Examples
Embodiment 1
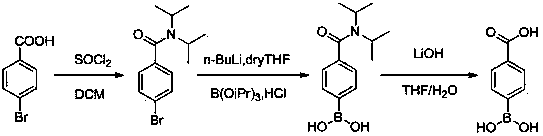
[0031] A preparation method for p-carboxyphenylboronic acid, comprising the steps of:
[0032] (1) Synthesis of 4-bromo-N,N-diisopropylbenzamide (Ⅲ)
[0033] Add 90 g of p-bromobenzoic acid into the reactor, add 450 g of thionyl chloride, heat and reflux for 2.5 hours, and detect that the reaction is complete by thin-layer chromatography, spin dry thionyl chloride with a dried rotary evaporator, add dichloromethane 320g to form A liquid; add 49.8g diisopropylamine and 350g dichloromethane to another reactor, under ice bath, drop into A liquid, after dropping, react at room temperature for 2 hours, the reaction is complete by thin layer chromatography, add 500g The aqueous phase was extracted once with 200 g of dichloromethane, the organic phases were combined, washed once with 410 g of saturated sodium bicarbonate, washed once with 400 g of saturated saline, dried with anhydrous sodium sulfate for 2 hours, filtered, and concentrated to obtain a yellow liquid 4 -Bromo-N,N-diis...
Embodiment 2
[0041] (1) Synthesis of 4-bromo-N,N-diisopropylbenzamide (Ⅲ)
[0042] Add 90 g of p-bromobenzoic acid into the reactor, add 620 g of thionyl chloride, heat and reflux for 2.3 hours, and detect that the reaction is complete by thin-layer chromatography, spin dry thionyl chloride with a dried rotary evaporator, add dichloromethane 400g to form A liquid; add 62.5g diisopropylamine and 460g dichloromethane to another reactor, drop into A liquid under ice bath, after dropping, react at room temperature for 1.8 hours, the reaction is complete by thin layer chromatography, add 570g The aqueous phase was extracted once with 270 g of dichloromethane, the organic phases were combined, washed once with 490 g of saturated sodium bicarbonate, washed once with 450 g of saturated brine, dried with anhydrous sodium sulfate for 2 hours, filtered, and concentrated to obtain a yellow liquid 4 -Bromo-N,N-diisopropylbenzamide 120.24g, yield 94.5%.
[0043] (2) Synthesis of (4-(diisopropylcarbamoy...
Embodiment 3
[0050] (1) Synthesis of 4-bromo-N,N-diisopropylbenzamide (Ⅲ)
[0051] Add 90 g of p-bromobenzoic acid into the reactor, add 710 g of thionyl chloride, heat and reflux for 2.2 hours, and detect that the reaction is complete by thin-layer chromatography, spin dry thionyl chloride with a dried rotary evaporator, add dichloromethane 450g to form A liquid; add 79.2g diisopropylamine and 520g dichloromethane to another reactor, under ice bath, drop into A liquid, after dropping, react at room temperature for 1.5 hours, the reaction is complete by thin layer chromatography, add 610g The aqueous phase was extracted once with 310 g of dichloromethane, the organic phases were combined, washed once with 530 g of saturated sodium bicarbonate, washed once with 500 g of saturated saline, dried with anhydrous sodium sulfate for 2 hours, filtered, and concentrated to obtain a yellow liquid 4 -Bromo-N,N-diisopropylbenzamide 122.66g, yield 96.4%.
[0052] (2) Synthesis of (4-(diisopropylcarbam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com