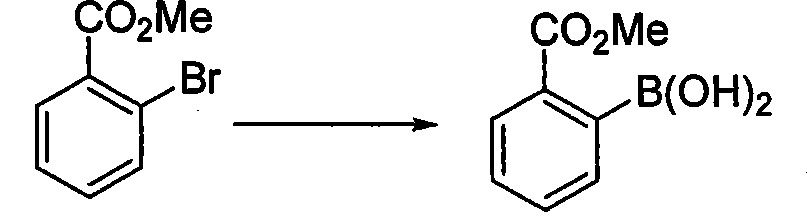
Method for preparing phenylboronic acid-2-methyl formate
A technology of methyl benzoate and methyl formate, which is applied in the field of preparing methyl phenylboronic acid-2-formate, and achieves the effects of stable process conditions, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
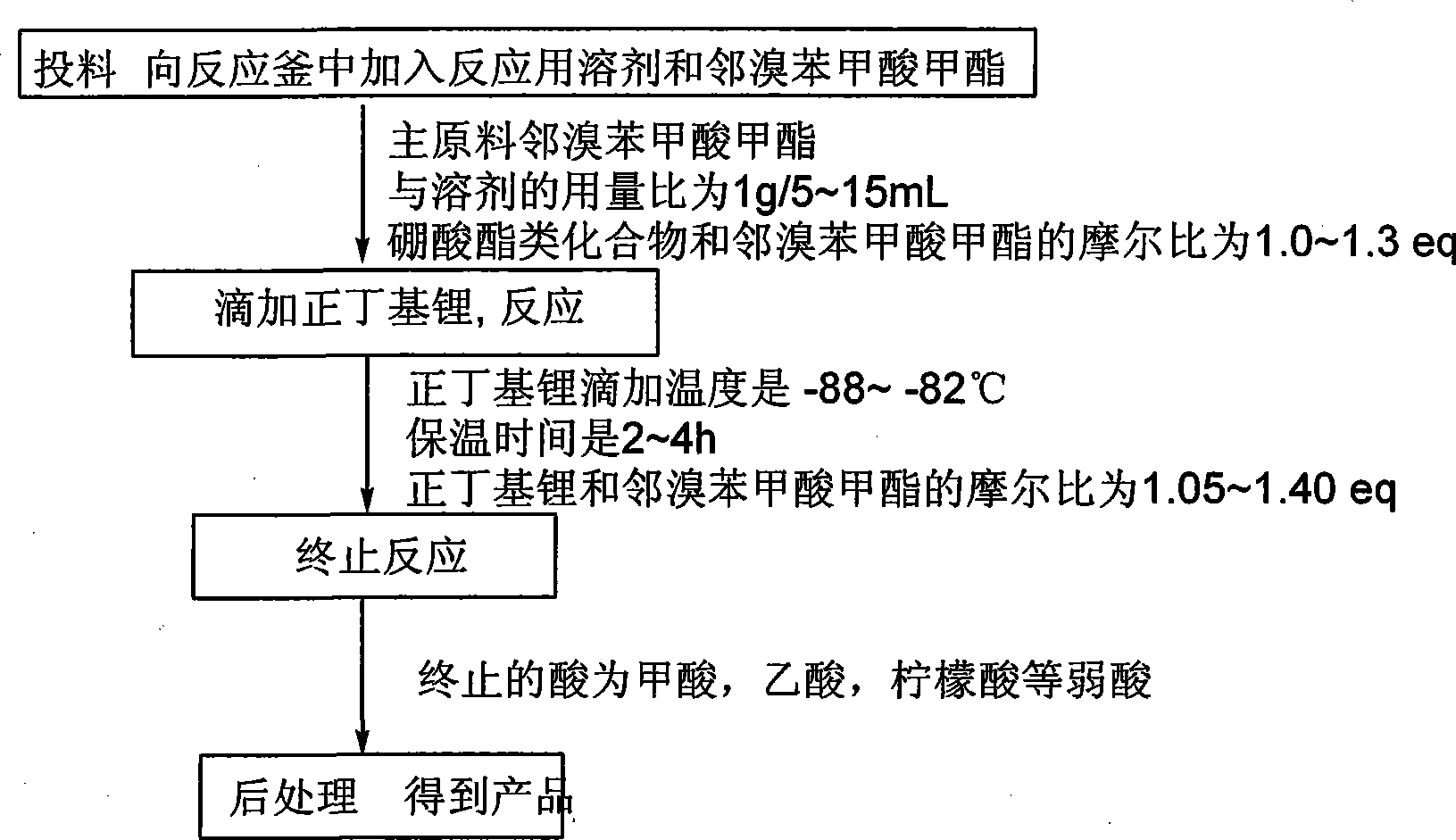
[0027] In the 72L reaction flask, add 19.9kg tetrahydrofuran (1g / 7ml), 3.2kg methyl o-bromobenzoate (1.0eq) and 3.36kg isopropyl borate (1.2eq) at one time, after the addition is complete, start stirring, and the system Cool down to -88~-82°C, and add 4.6kg (1.1eq) of 2.5M n-butyllithium dropwise at -88~-82°C. After the drop is complete, the system is kept at -88~-82°C for 3 hours. The response is complete. The system was terminated with acetic acid. After the termination, the system was warmed up to -35~-30°C, and the pH of the system was adjusted to 1~2 with 18% dilute hydrochloric acid, and then the pH was adjusted to 5~6 with saturated sodium bicarbonate solution. Separate the liquid, extract the aqueous phase with isopropyl acetate, combine the organic phases, wash with brine, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to a certain extent, add petroleum ether to the system, and then cool down the system for -2 to 2 ℃, stirred and crystallized...
Embodiment 2
[0029] In a 20L reaction flask, add 8.17kg methyl tert-butyl ether (1g / 5ml), add 2.15kg methyl o-bromobenzoate (1.0eq) and 1.90kg triethyl borate (1.3eq) at one time, and the addition is complete , the system was cooled to -88~-82°C, and 2.5M n-butyllithium 3.36kg (1.2eq) was added dropwise at -88~-82°C. After the addition was complete, the reaction was complete after 2 hours. After completion, return the system to -35~-30℃, adjust the pH of the system to 1~2 with dilute hydrochloric acid, then adjust the pH of the system to 5~6 with saturated sodium bicarbonate solution, let stand to separate the liquids, and wash the organic phase with brine , dried over anhydrous sodium sulfate, filtered, concentrated, cooled to crystallize, filtered with suction, dried at 40-45°C to obtain 0.94kg of the product. Yield 52.2%, liquid chromatography purity (HPLC): 98.0%.
Embodiment 3
[0031] Into the 350L reactor, pump 101.48kg of 2-methyltetrahydrofuran (1g / 10ml) into the system at one time, add 11.8kg of methyl o-bromobenzoate (1.0eq) and 5.7kg of trimethyl borate (1.0eq) After the addition, start stirring, cool down the system to -88~-82°C, and add 16.2kg (1.05eq) of 2.5M n-butyllithium dropwise at -88~-82°C. (3h), terminate the reaction with citric acid, after the termination, return the system to -35~-30°C, adjust the pH of the system to 1~2 with dilute hydrochloric acid, and then continue to adjust the pH of the system to 5~5 with saturated sodium bicarbonate solution 6. Stand still for liquid separation, wash the organic phase with brine, dry over anhydrous sodium sulfate, filter, concentrate, cool down to crystallize, filter with suction, and dry at 40-45°C to obtain 5.76kg of the product. Yield 58.2%, liquid chromatography purity (HPLC): 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com