Canagliflozin tablet and preparation method thereof
A technology of canagliflozin tablets and canagliflozin, which is applied in the direction of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of unstudied preparation stability, particle fluidity and friability related nature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
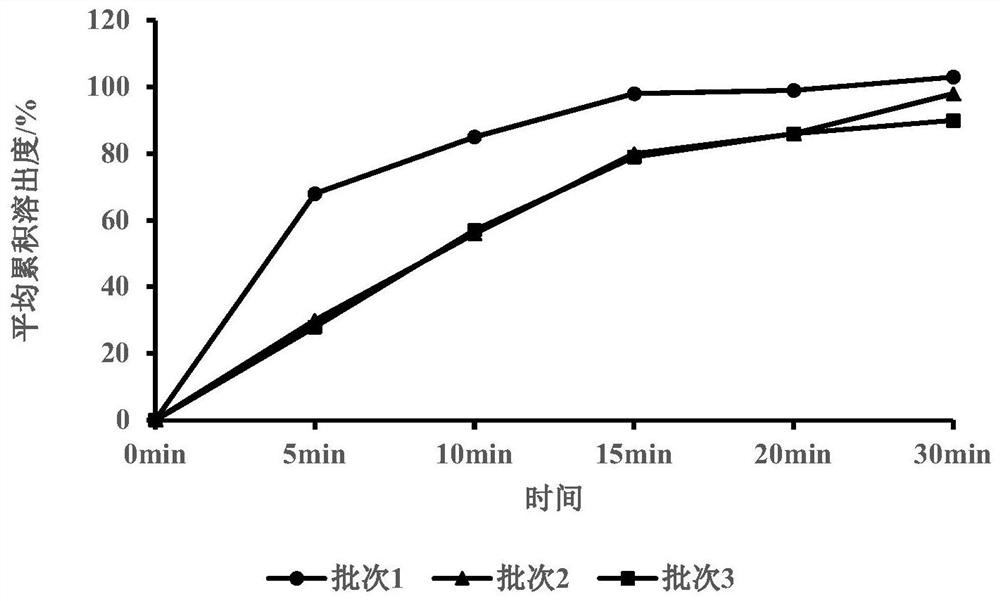
[0060] Embodiment 1 filler ratio investigation
[0061] Table 1
[0062]
[0063]
[0064] in conclusion:
[0065] Investigation of filler ratio: From the data results, it can be known that the increase in the proportion of microcrystalline cellulose will increase the bulk density of the particle intermediate, improve the compressibility of the particle, and have no significant difference between the angle of repose and the brittleness of the plain tablet. However, the dissolution is slow, and when the ratio of anhydrous lactose to microcrystalline cellulose is 1:1, it can dissolve quickly.
Embodiment 2
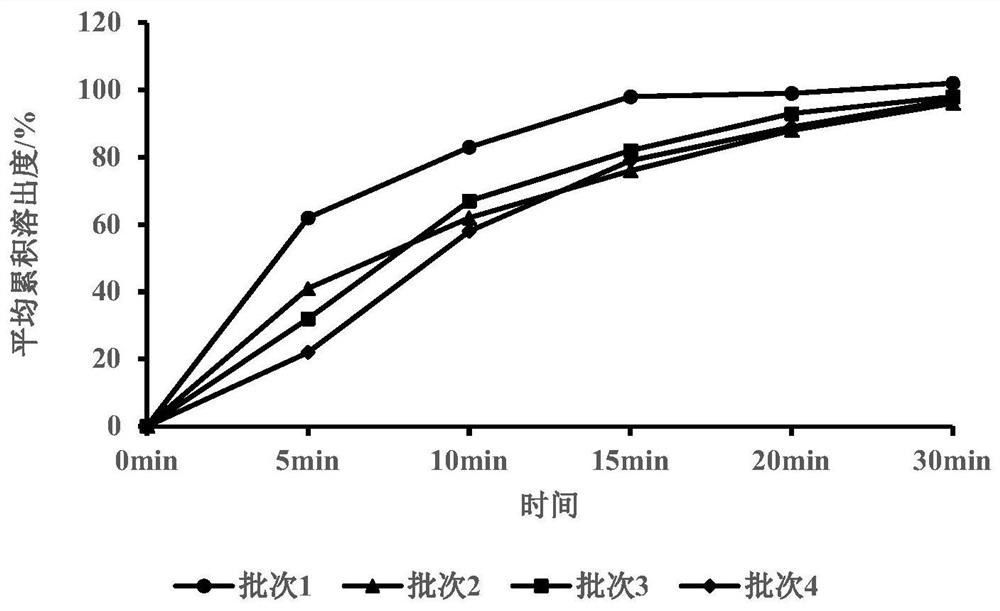
[0066] Embodiment 2 disintegrating agent dosage and adding mode investigation
[0067] Table 2
[0068]
[0069] in conclusion:
[0070] Investigation on the amount of disintegrant and the method of addition: In the prescription research, the cumulative dissolution rate was used as the main index of investigation. The results showed that the amount of disintegrant and the method of addition had an impact on the dissolution of plain tablets, and adding about 6% internally could achieve rapid dissolution. dissolution.
Embodiment 3
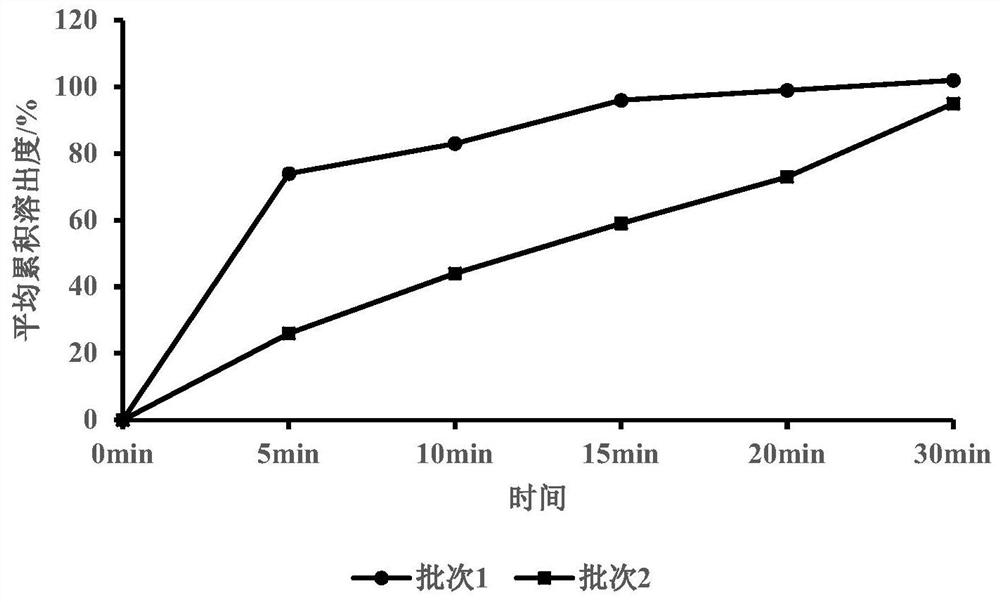
[0071] Embodiment 3 Adhesive type investigation
[0072] table 3
[0073]
[0074] in conclusion:
[0075] Binder type investigation: The effects of two kinds of binders, polyvinylpyrrolidone and hypromellose, on the dissolution, friability, compressibility and fluidity of plain tablets were compared and investigated. The results showed better dissolution using polyvinylpyrrolidone as the binder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com