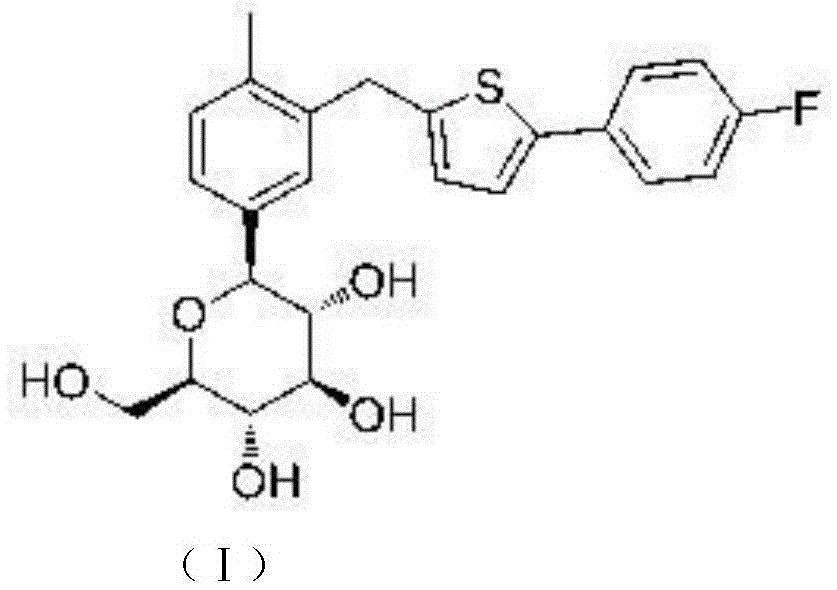
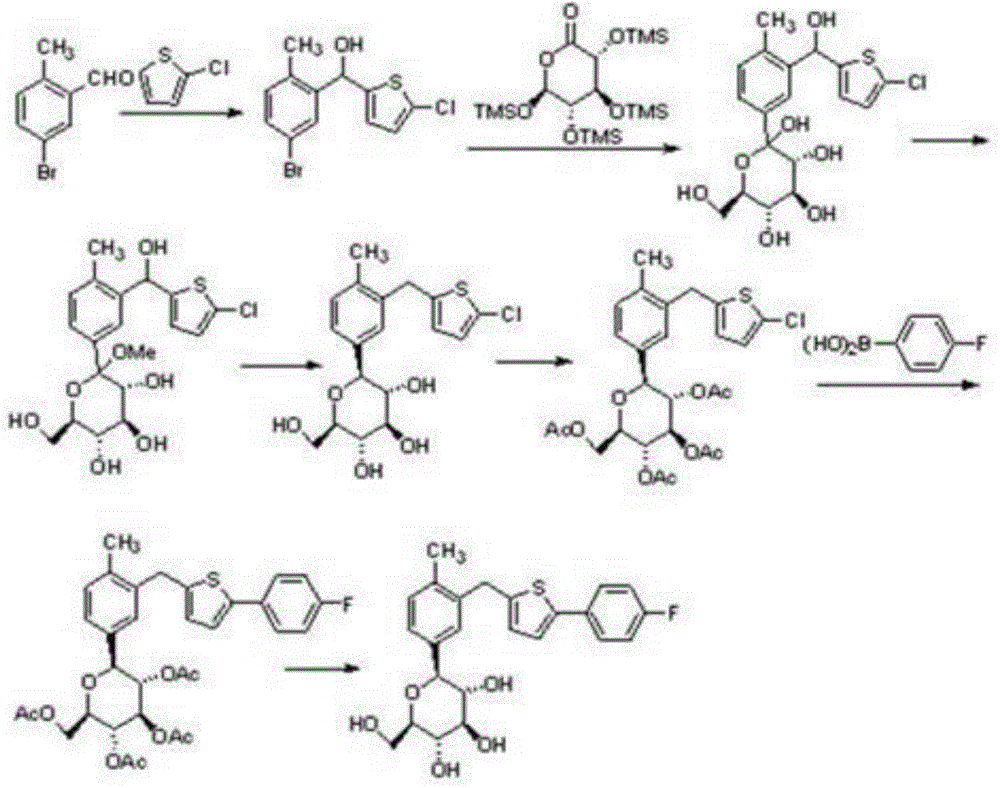
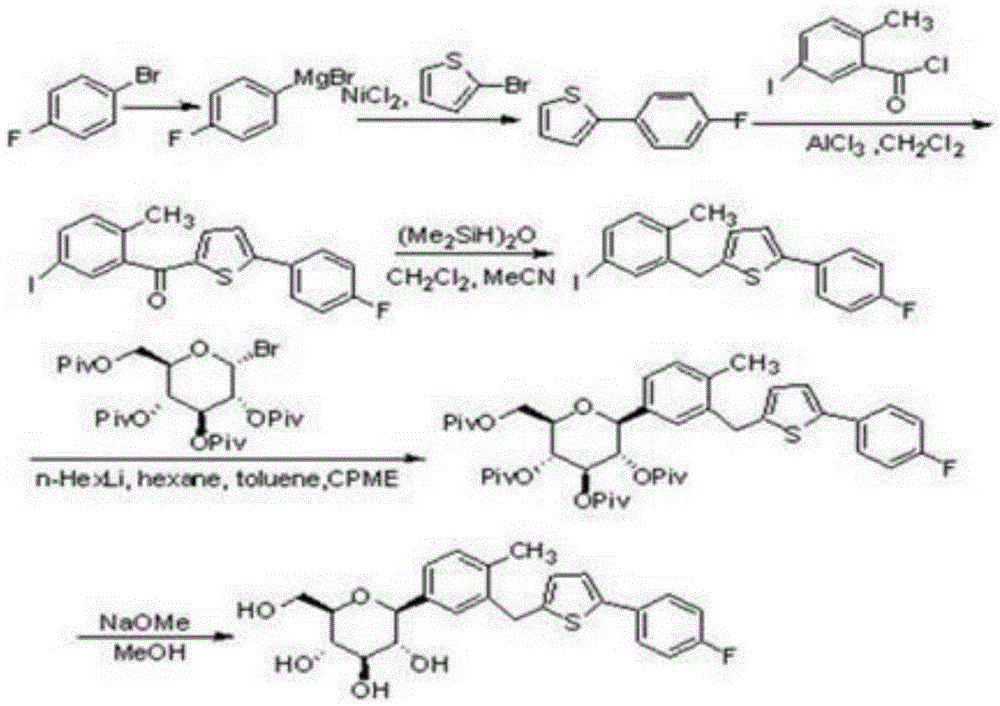
Preparation method for canagliflozin
A synthesis method and compound technology, applied in the field of pharmacy, can solve the problems of low total yield of the route, complex synthesis method, harsh reaction conditions, etc., and achieve the effects of rapid and easy reaction, simple post-processing, and mild reduction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add compound IV (47.5 g, 116.34 mmol), compound V (59.64 g, 127.97 mmol) and tetrahydrofuran (400 ml) into a 1 L three-necked flask. After the resulting mixture was cooled to -20°C, 0.65M trimethylsilylmethyllithium in hexane (268 ml) was slowly added dropwise to the mixture using a dropping funnel, keeping the internal temperature below or equal to -20°C. After the addition was complete, the reaction was quenched with saturated brine and allowed to warm to room temperature. Extract with ethyl acetate, separate the phases and dry (anhydrous sodium sulfate). Filtration and concentration gave intermediate II (82 g, molecular weight 748) as viscous.
[0041] After dissolving the intermediate II obtained in the previous step with 300ml of methanol, add 5g of Pd / C. Start to pass H2, stir at room temperature for 3 h, HPLC detects that the reaction is completed, and recovers the catalyst by suction filtration. The filtrate was concentrated under reduced pressure to obtain v...
Embodiment 2
[0047] Add compound IV (47.5 g, 116.34 mmol), compound V (59.64 g, 127.97 mmol) and tetrahydrofuran (400 ml) into a 1 L three-necked flask. After the resulting mixture was cooled to -20°C, 0.65M trimethylsilylmethyllithium in hexane (268 ml) was slowly added dropwise to the mixture using a dropping funnel, keeping the internal temperature below or equal to -20°C. After the addition was complete, the reaction was quenched with saturated brine and allowed to warm to room temperature. Extract with ethyl acetate, separate the phases and dry (anhydrous sodium sulfate). Filtration and concentration gave intermediate II (83 g, molecular weight 748) as viscous.
[0048] After dissolving the intermediate II obtained in the previous step with 300ml of methanol, add (Example 1) to recover Pd / C. Start to pass H2, stir at room temperature for 3 h, HPLC detects that the reaction is completed, and recovers the catalyst by suction filtration. The filtrate was concentrated under reduced pre...
Embodiment 3
[0051] Add compound IV (47.5 g, 116.34 mmol), compound V (59.64 g, 127.97 mmol) and tetrahydrofuran (400 ml) into a 1 L three-necked flask. After the resulting mixture was cooled to -20°C, 0.65M trimethylsilylmethyllithium in hexane (268 ml) was slowly added dropwise to the mixture using a dropping funnel, keeping the internal temperature below or equal to -20°C. After the addition was complete, the reaction was quenched with saturated brine and allowed to warm to room temperature. Extract with ethyl acetate, separate the phases and dry (anhydrous sodium sulfate). Filtration and concentration gave intermediate II (82.5 g, molecular weight 748) as viscous.
[0052] After dissolving the intermediate II obtained in the previous step with 300ml of methanol, add (Example 1) to recover Pd / C. Start to pass H2, stir at room temperature for 3 h, HPLC detects that the reaction is completed, and recovers the catalyst by suction filtration. The filtrate was concentrated under reduced p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com