Method for separating canagliflozin five-membered-ring impurity enantiomer through high performance liquid chromatography
A high-performance liquid chromatography, five-membered ring technology, applied in the direction of material separation, analysis materials, measuring devices, etc., can solve problems such as difficult separation, inability to analyze structures and accurate quantitative research, and achieve high preparation efficiency, high degree of automation, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
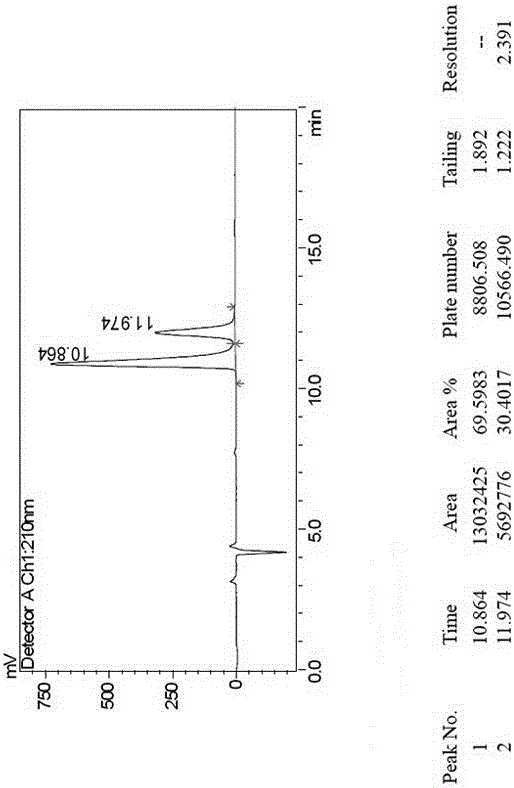
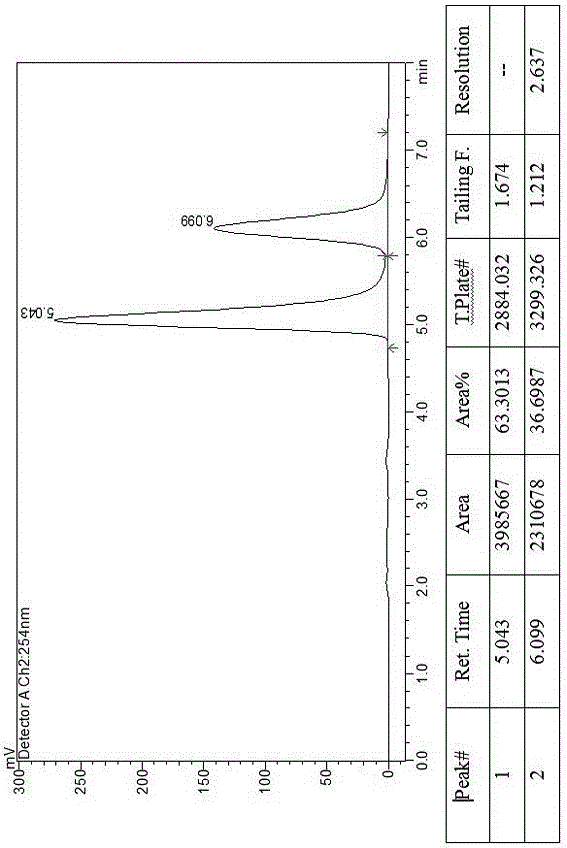
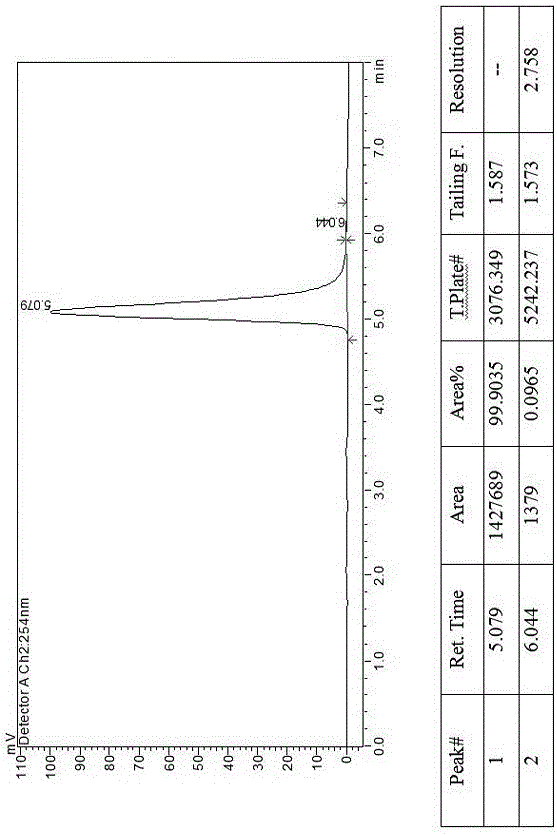
[0018] Example 1, a method for splitting canagliflozin five-membered ring impurity enantiomers by high performance liquid chromatography: dissolving the raw material five-membered ring impurity enantiomer mixture in an organic solvent to a concentration of 0.1 mg / ml; using high-efficiency liquid A phase chromatograph uses an amylose-type chiral column as a chromatographic column, and uses a mixture of n-hexane and ethanol as a mobile phase for resolution and preparation. The amylose-type chiral column is a bonded chiral chromatography column CHIRALPAK IE. The organic solvent is selected from one of absolute ethanol, n-hexane, ethanol, and methanol; the flow rate of the mobile phase is 1.0 mL / min, the temperature of the chromatographic column is 25° C., and the detection wavelength is 220 nm. The injection volume is 2 μL. The mobile phase is calculated by volume percentage, n-hexane: absolute ethanol is 40:60.
Embodiment 2
[0019] Example 2, a method for splitting canagliflozin five-membered ring impurity enantiomers by high-performance liquid chromatography: dissolving the raw material five-membered ring impurity enantiomer mixture in an organic solvent to a concentration of 25 mg / ml; using high-performance liquid phase The chromatograph uses an amylose-type chiral column as a chromatographic column and uses a mixture of n-hexane and ethanol as a mobile phase for resolution and preparation. The amylose-type chiral column is a bonded chiral chromatography column CHIRALPAK IE. The filler is preferably amylose-tris(3,5-dichlorophenylcarbamate) covalently bonded to the surface of the silica gel.
[0020] The organic solvent is selected from the mixed solvent of absolute ethanol, n-hexane, ethanol and methanol. The flow rate of the mobile phase is 70mL / min, the temperature of the chromatographic column is 40°C, and the detection wavelength is 290 nm. The injection volume is 10mL. The mobile phase ...
Embodiment 3
[0021] Example 3, a method for splitting canagliflozin five-membered ring impurity enantiomers by high-performance liquid chromatography: dissolving the raw material five-membered ring impurity enantiomer mixture in an organic solvent to a concentration of 5 mg / ml; using high-performance liquid phase The chromatograph uses an amylose-type chiral column as a chromatographic column and uses a mixture of n-hexane and ethanol as a mobile phase for resolution and preparation. The amylose-type chiral column is a bonded chiral chromatography column CHIRALPAK IE.
[0022] Described organic solvent is selected from three kinds of mixed solvents in absolute ethanol, normal hexane, ethanol, methanol.
[0023] The flow rate of the mobile phase is 10 mL / min, the temperature of the chromatographic column is 30° C., and the detection wavelength is 280 nm. The injection volume is 1 mL. The mobile phase is calculated by volume percentage, n-hexane: absolute ethanol is 50:50.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com