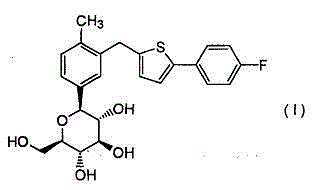
Canagliflozin compound
A technology of canagliflozin and hydrate, which is applied in the field of medicine and can solve problems such as crystallization difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]In a 500ml reaction bottle equipped with stirring, thermometer and condenser, add 60 grams of canagliflozin and 400 milliliters of water, add 0.5 grams of dimethylformamide (DMF) to the above aqueous solution, stir for 30 minutes, filter, and the filtrate Cool to 12°C and set aside.
[0045] Cool 800ml of acetonitrile-methyl ethyl ketone = 5:5 mixture to 13°C, add the above standby solution while stirring, keep it warm for 18 hours, crystals precipitate, filter, and dry to obtain 53.3 grams of white crystals. Purity 99.9% (HPLC normalization method), optical purity 99.96% ee (chiral HPLC).
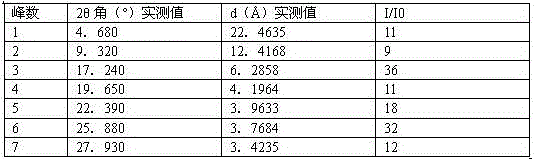
[0046] Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa, 40Kv, 100mA; 2θ scanning range: 0-50 ° .
[0047]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com