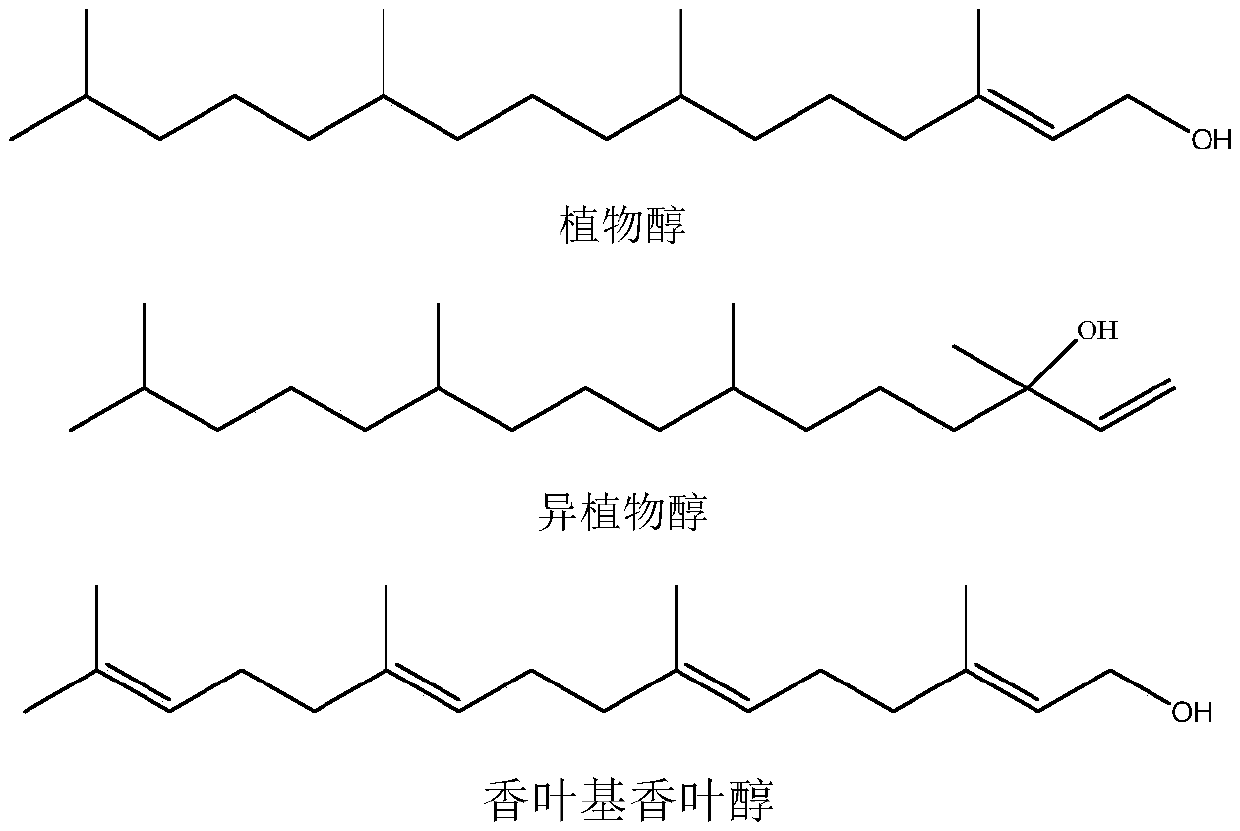
Synthesis method of intermediate farnesyl acetone and method for synthesizing phytol, isophytol and geranyl geraniol by using intermediate farnesyl acetone
A technology of farnesyl acetone and synthesis method, which is applied in the direction of carbon-based compound preparation, hydroxyl compound preparation, magnesium organic compound, etc., and can solve the problems of many synthesis steps, reduction of total reaction and hydrogenation reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
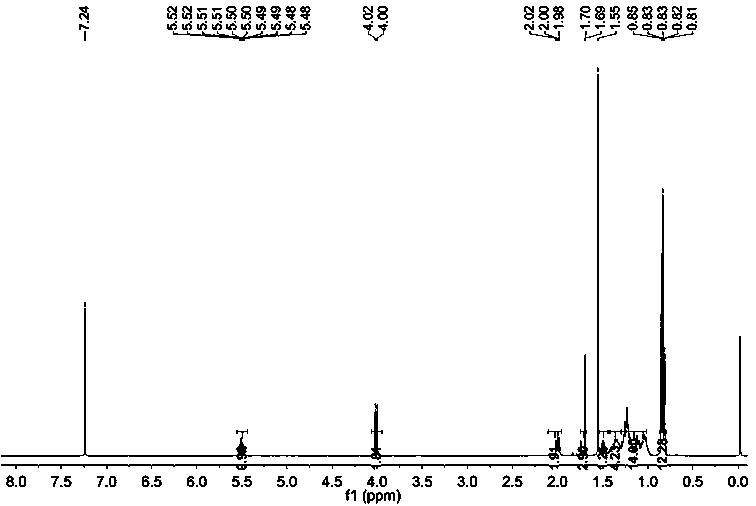
[0072] Embodiment 1 synthetic farnesyl acetone
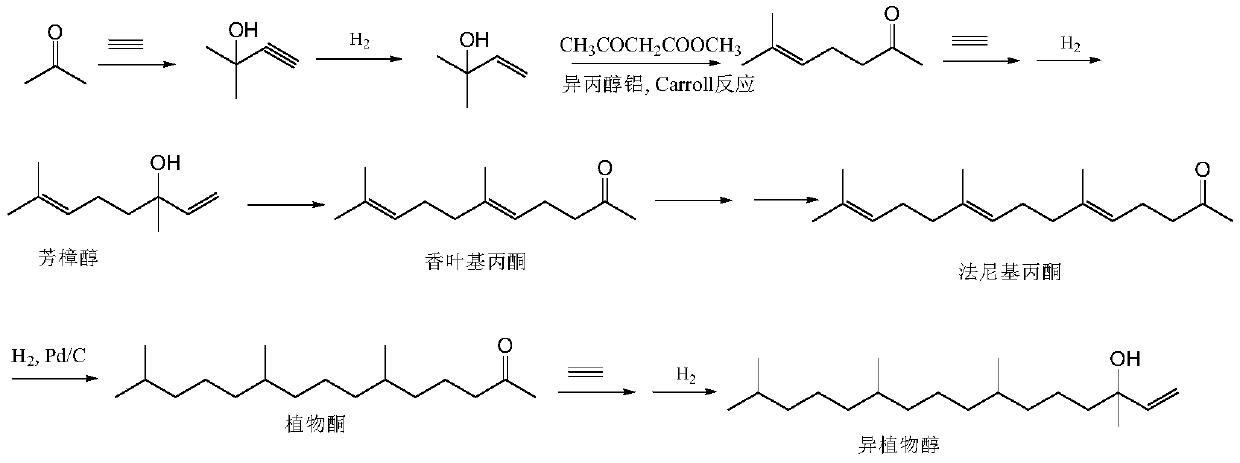
[0073] A kind of synthetic method of intermediate farnesyl acetone, it is raw material with acetone, 5-chloro-2-pentanone, obtains key intermediate farnesyl acetone through three Grignard reactions, specifically comprises the following steps:
[0074] 1) Synthesis of 5-chloro-2-pentanone vinyl ketal:
[0075] Put 600ml of n-heptane, 120.6g (1.00mol) of 5-chloro-2-pentanone, 74.4g (1.20mol) of ethylene glycol and 1.2g (6.3mmol) of p-toluenesulfonic acid into the reaction bottle, install a thermometer and water tank, reflux and water separation reaction for 6 hours. After the reaction was completed, it was lowered to room temperature, and the reaction solution was washed with 100ml of saturated sodium bicarbonate solution, and then washed twice with 100ml of water, and allowed to stand for stratification. The organic layer was dried with anhydrous sodium sulfate, and n-heptane was recovered under reduced pressure. Distillation, ...
Embodiment 2
[0084] Embodiment 2 Phytoketone
[0085] Add 500 ml of ethanol, 100 g (0.38 mol) of farnesyl acetone, 10 g (10%) of Pd / C into the hydrogenation kettle, replace with nitrogen and pass through hydrogen, and stir the reaction at room temperature (25-30°C) at 2 atmospheres until no hydrogen is absorbed (about 24 hours), the reactor was opened after nitrogen replacement, Pd / C was removed by rapid filtration, ethanol was recovered under reduced pressure, and a light yellow transparent liquid was obtained, which was phytoketone (92 g, yield 90%).
Embodiment 3
[0086] Embodiment 3 vinyl chloride Grignard solution
[0087] Add 24.3g (1.0mol) of magnesium powder into a reaction flask equipped with stirring, a thermometer and a dry reflux tube, add 500ml of dry methyl tert-butyl ether, stir, replace the reaction system with dry nitrogen, and then heat up to 50°C, slowly Slowly feed vinyl chloride, and add 1~2 iodine particles at the same time. After the reaction is triggered, the temperature of the system rises, and the color of iodine disappears. and stirring, the reaction solution was cooled to room temperature, and allowed to stand, the supernatant was the vinyl chloride Grignard solution, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com