Method for preparation of Efinaconazole
A compound and iodide technology, applied in the field of preparation of efluconazole, can solve the problems of consuming manpower and material resources, increasing operating procedures, and long reaction time, and achieve the effects of promoting technological progress, simplifying process operation, and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 prepares 4-methylene piperidine hydrochloride
[0039]
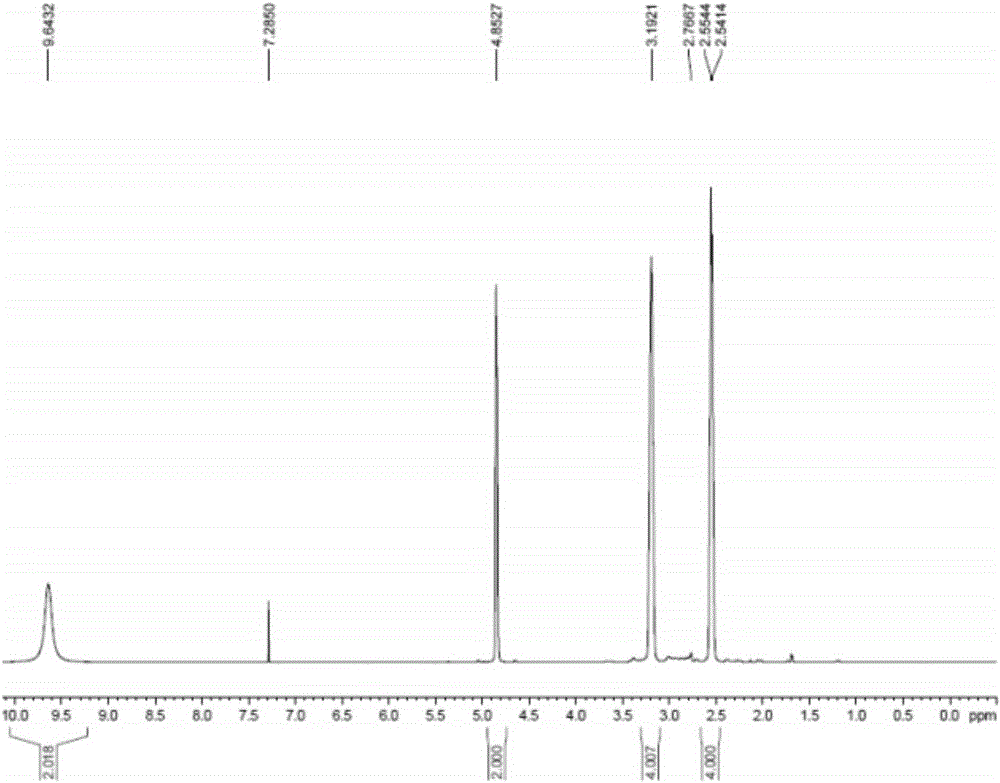
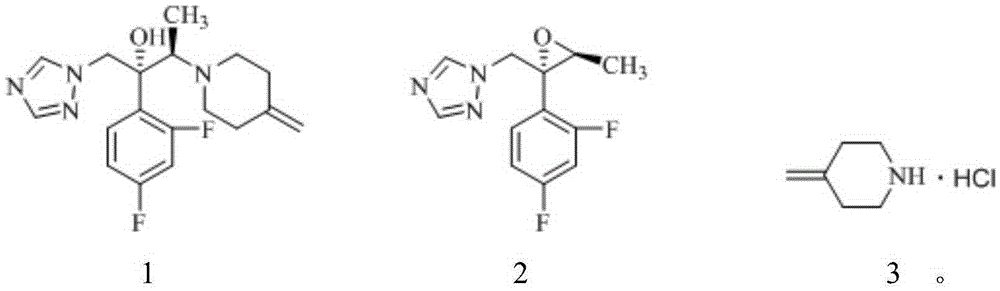
[0040] Add 600g (3.03mol) of N-Boc-4-methylenepiperidine and 3L methanol into a 5L reaction flask, add 768g (7.56mmol) of concentrated hydrochloric acid with a content of 36%, the reaction is complete, and after concentration under reduced pressure, Add 2L of ethyl acetate for slurry filtration, and dry under reduced pressure at room temperature to obtain 338.4 g of 4-methylene piperidine hydrochloride (91.0% yield, 99.9% purity), which 1 H-NMR spectrum such as figure 1 as shown,
[0041] 1 H-NMR (400MHz, CDCl 3 ): δ: 2.55 (4H, t, J = 6.09Hz), 3.19 (4H, t, J = 6.09Hz), 4.85 (2H, s), 9.64 (2H, br).
Embodiment 2
[0042] Embodiment 2 prepares efluconazole
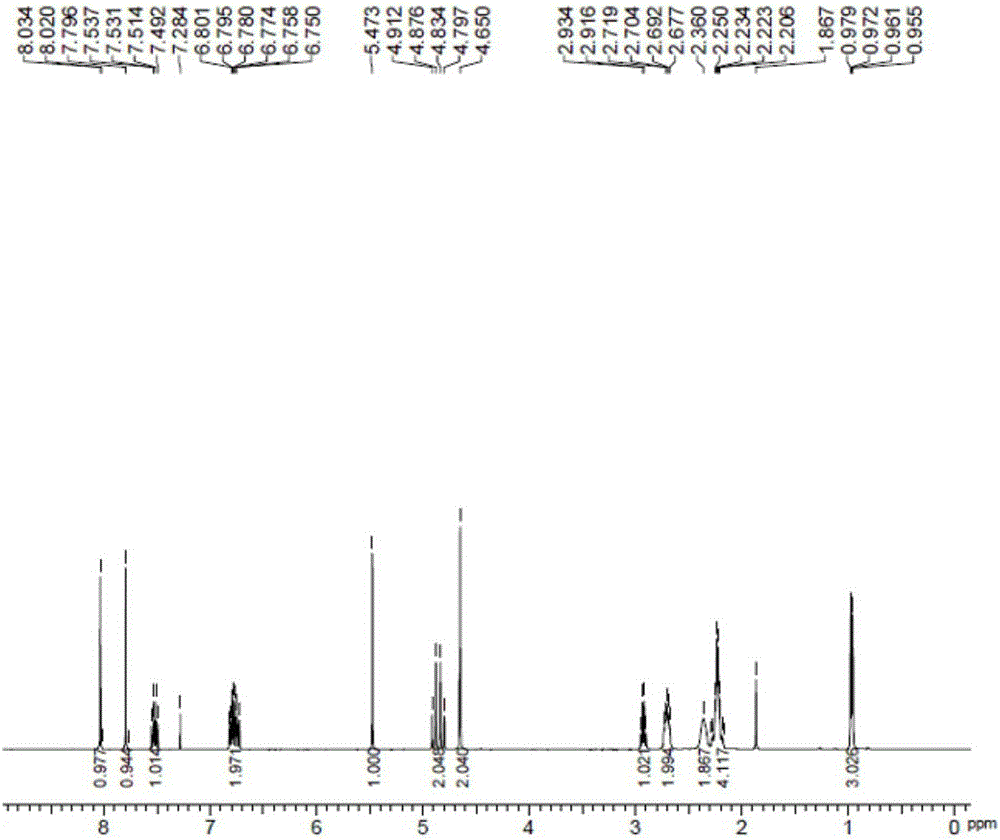
[0043] 4-methylenepiperidine hydrochloride, 3.2g (134.0mmol) lithium hydroxide and 14.8g (89.3mmol) potassium iodide obtained in 17.9g (134.0mmol) embodiment 1 were added in 67.2g acetonitrile, after stirring , add 22.4g (89.3mmol) of the compound shown in formula 2, heat and reflux in an oil bath (external temperature 100°C) for 8 hours, after the reaction is over, add 44ml of ethanol and 100ml of water to the reaction solution, stir for 1 hour and then filter , dried under reduced pressure at 50°C to obtain 27.3g white solid (87.8% yield, 99.6% purity as detected by HPLC), which 1 H-NMR spectrum such as figure 2 as shown,
[0044] 1 H-NMR (400MHz, CDCl 3 ): δ:0.97(3H,dd,J=2.6,7.0Hz),2.21-2.25(4H,m),2.30(2H,br),2.68~2.72(2H,m),2.92-2.93(1H,m ),4.65(2H,s),4.86(1H,d,J=14.8Hz),4.87(1H,dd,J=14.4Hz),5.47(1H,s),6.75-6.80(2H,m),7.49 ~7.54 (1H, m), 7.79 (1H, s), 8.03 (1H, s).
Embodiment 3
[0045] Embodiment 3 prepares efluconazole
[0046] 17.9g (134.0mmol) of 4-methylenepiperidine hydrochloride obtained in Example 1, 3.2g (134.0mmol) lithium hydroxide and 1.48g (8.93mmol) potassium iodide were added to 67.2g cyclopentyl methyl ether After stirring, add 22.4g (89.3mmol) of the compound shown in formula 2, and heat to reflux in an oil bath (external temperature 100°C) for 10 hours. After the reaction is completed, add 44ml of ethanol and 100ml of water to the reaction solution, and stir After 1 hour, it was filtered and dried under reduced pressure at 50° C. to obtain 26.5 g of white solid (85.2% yield, 99.1% purity by HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com