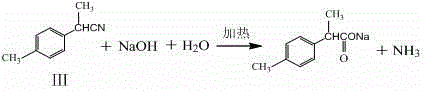
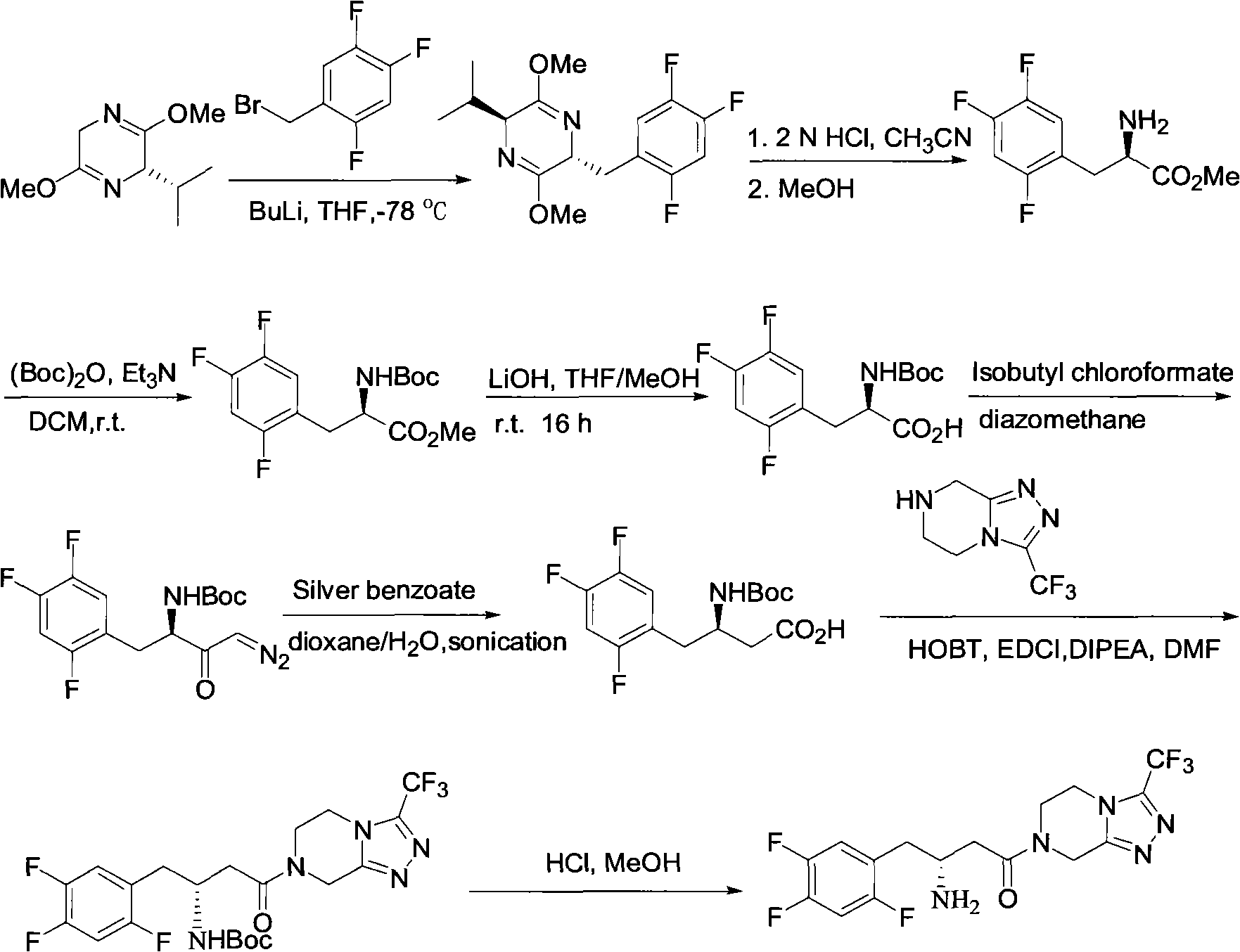
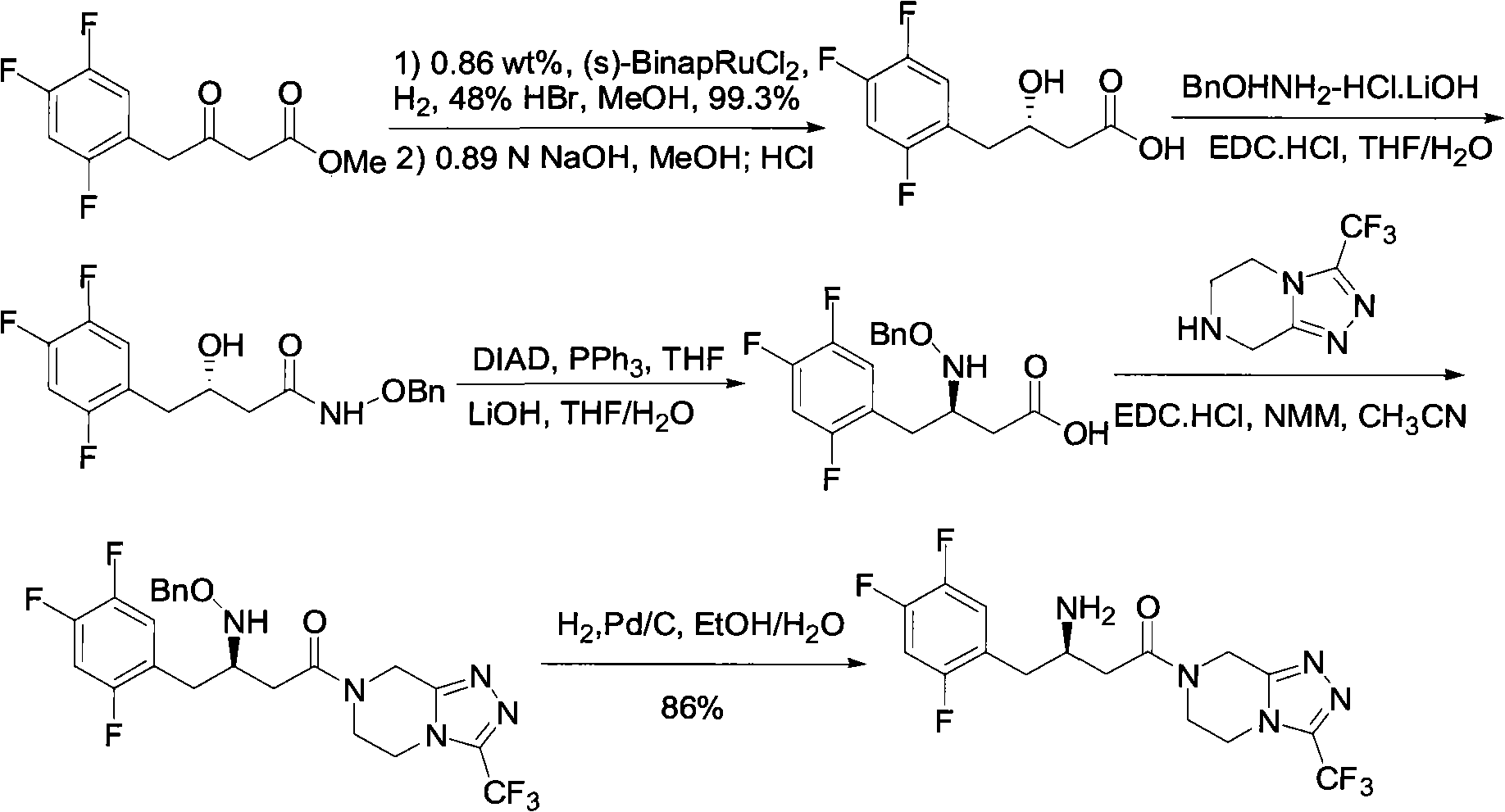
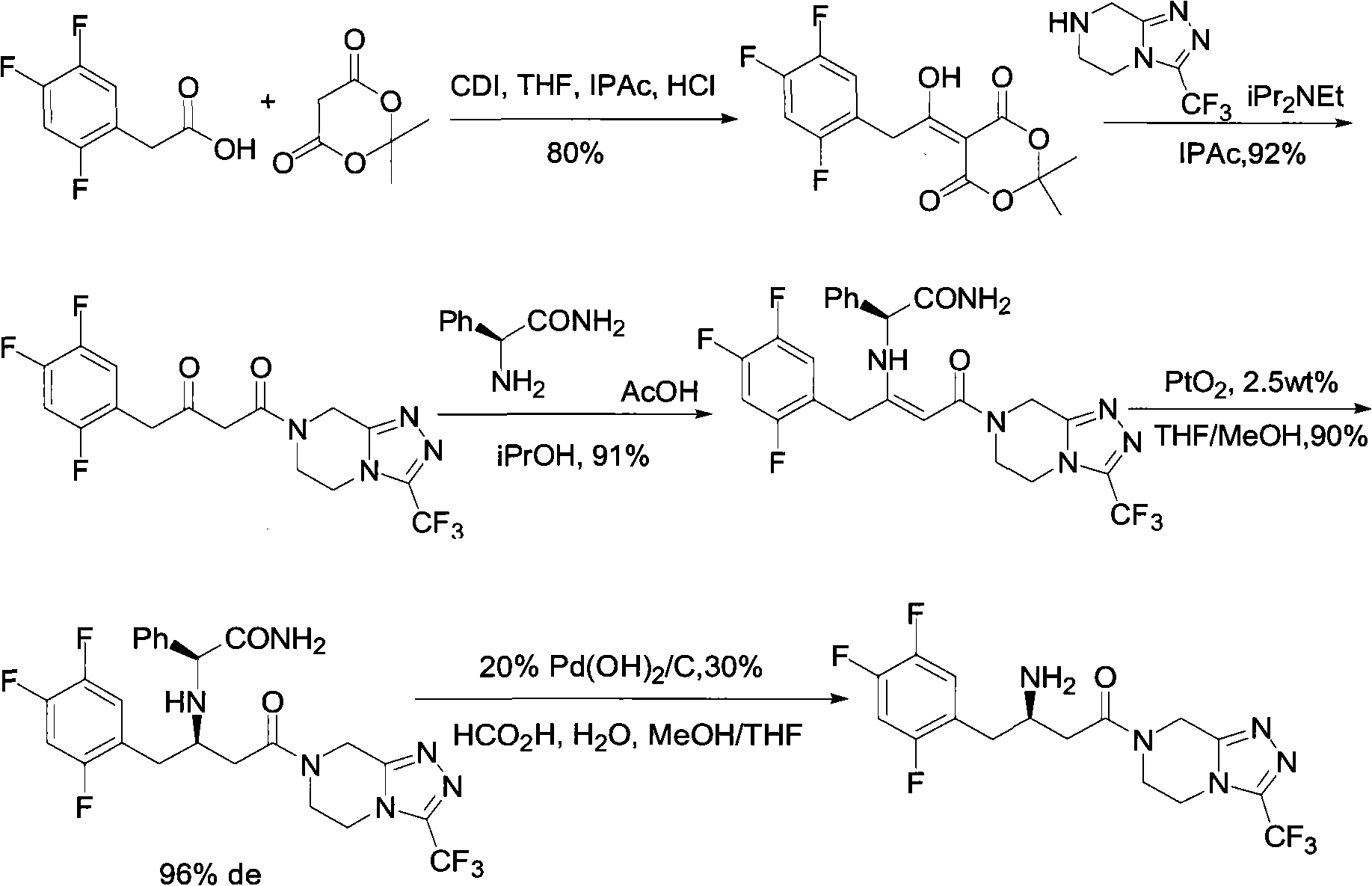
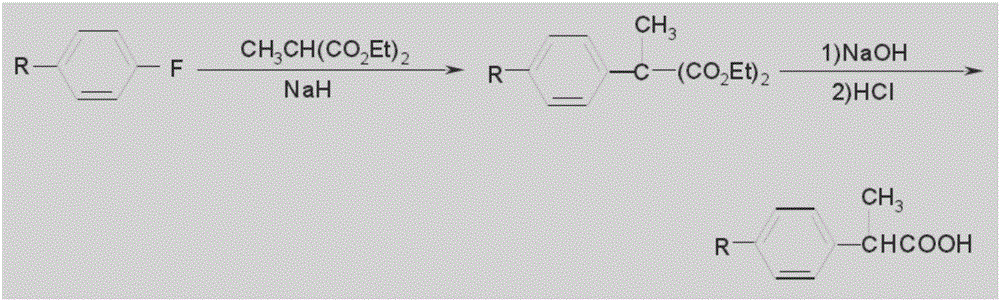
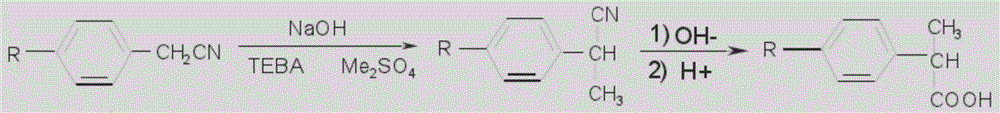
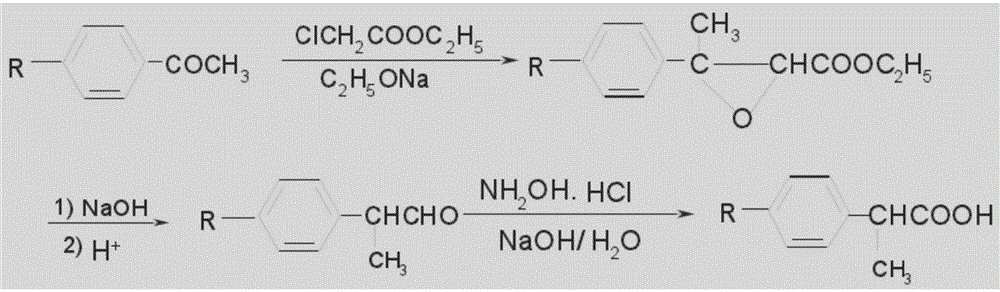
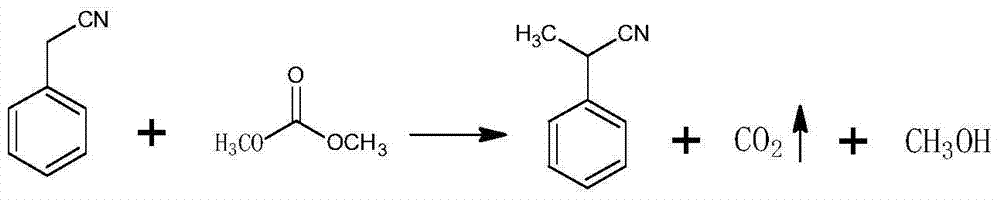
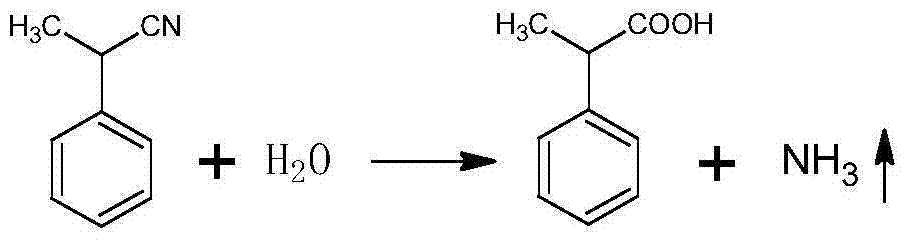
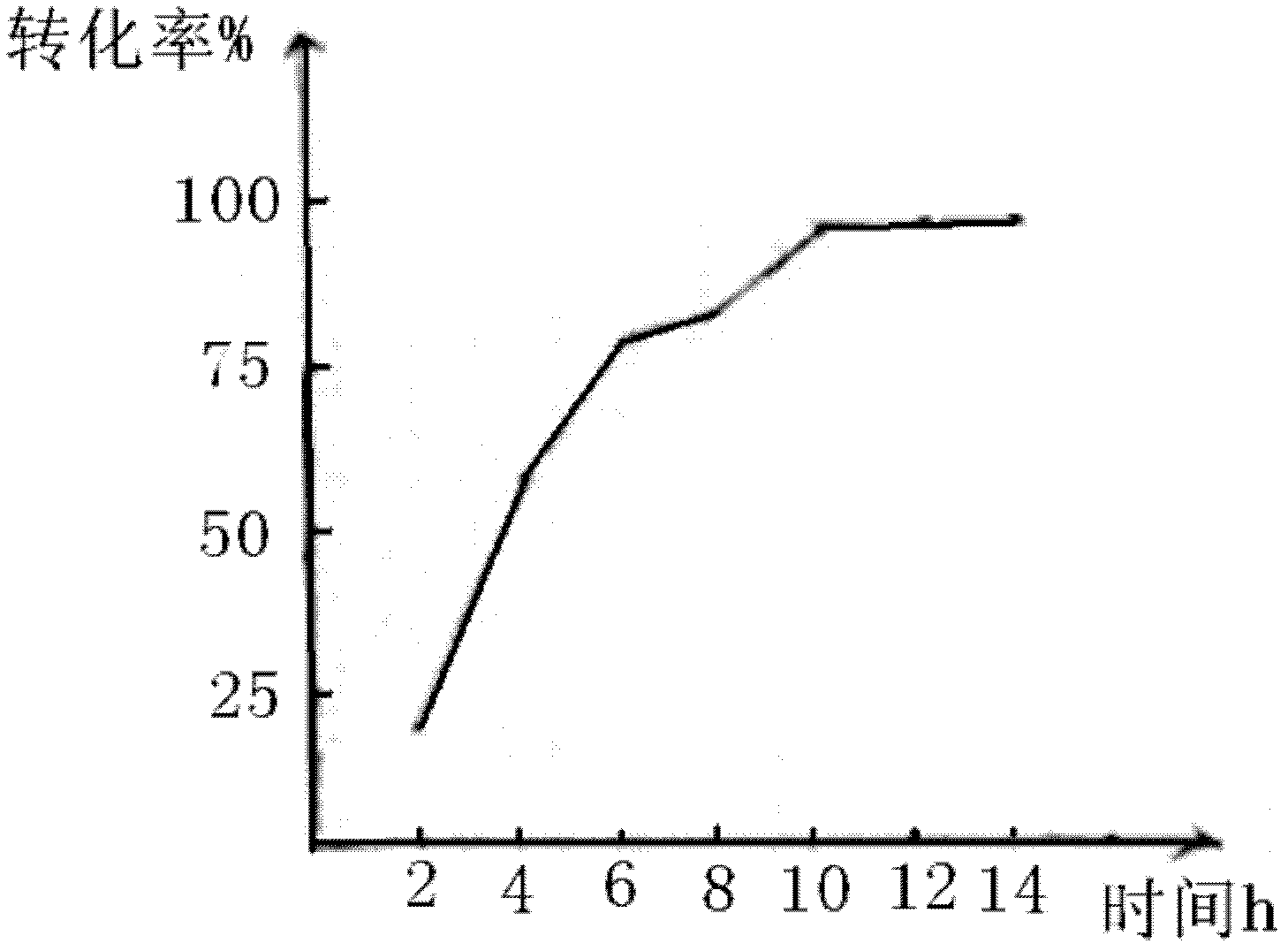
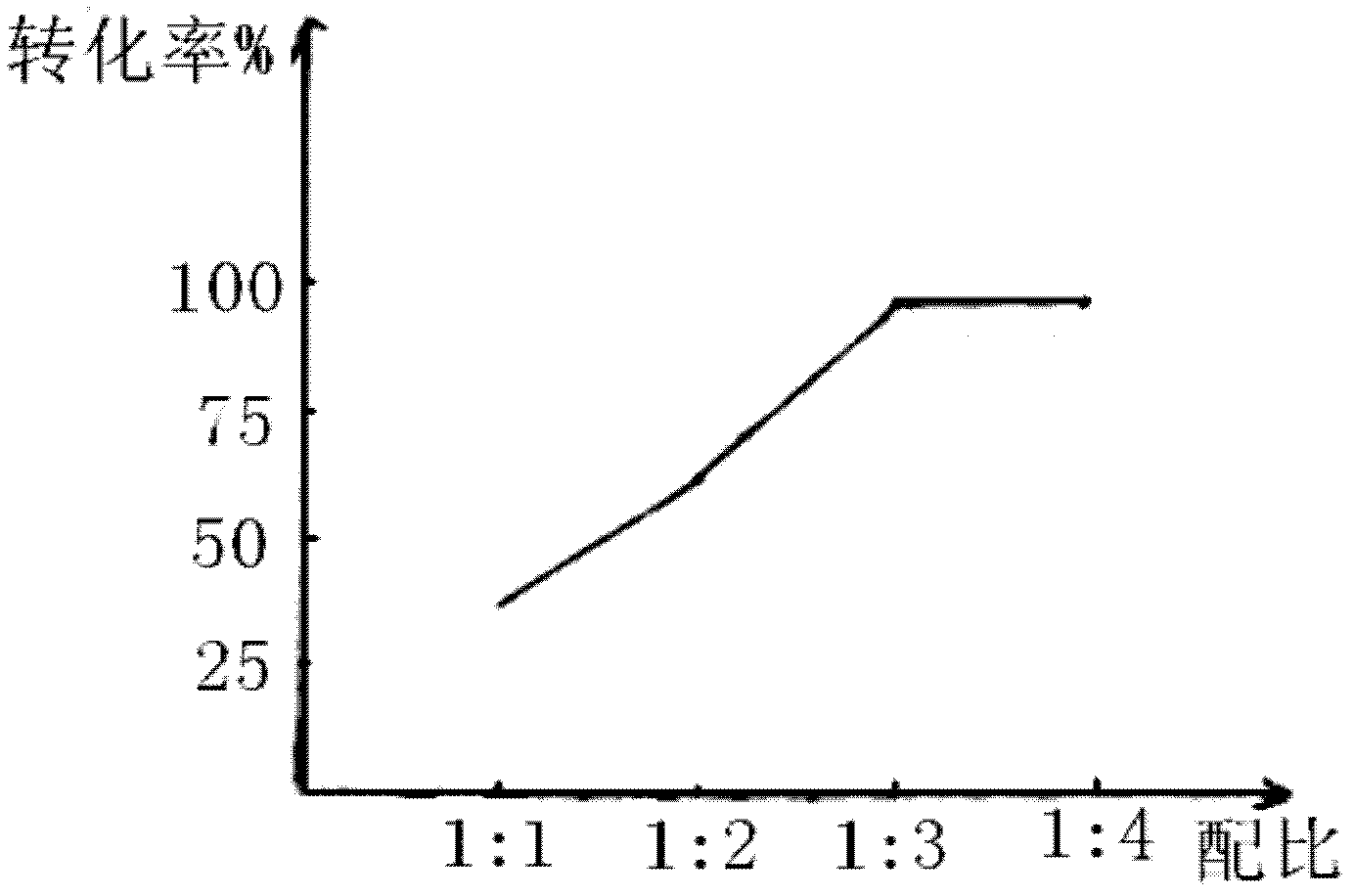
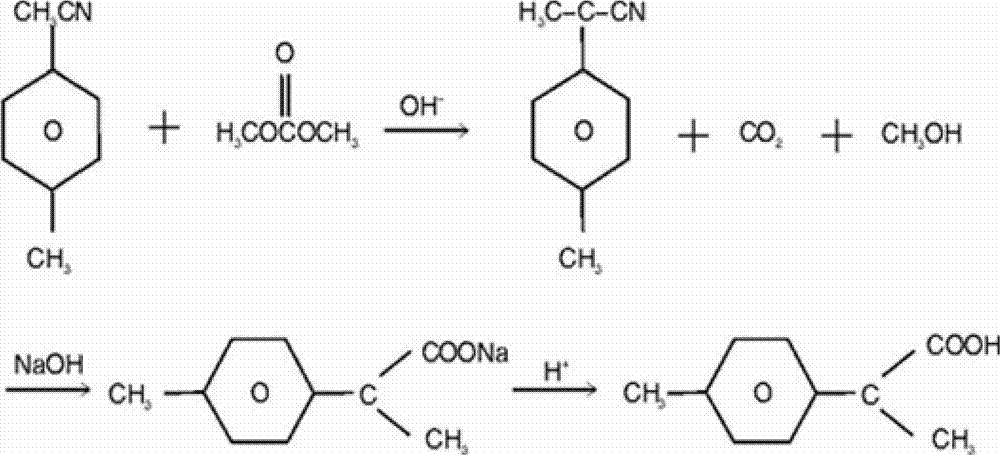
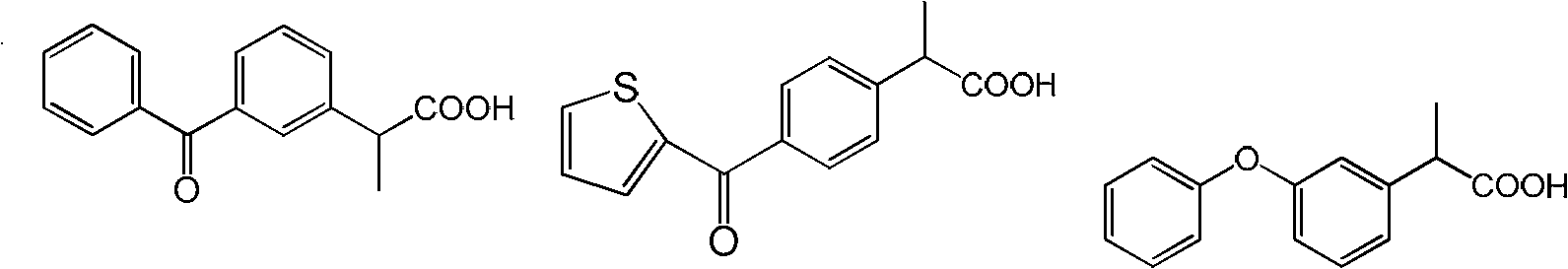
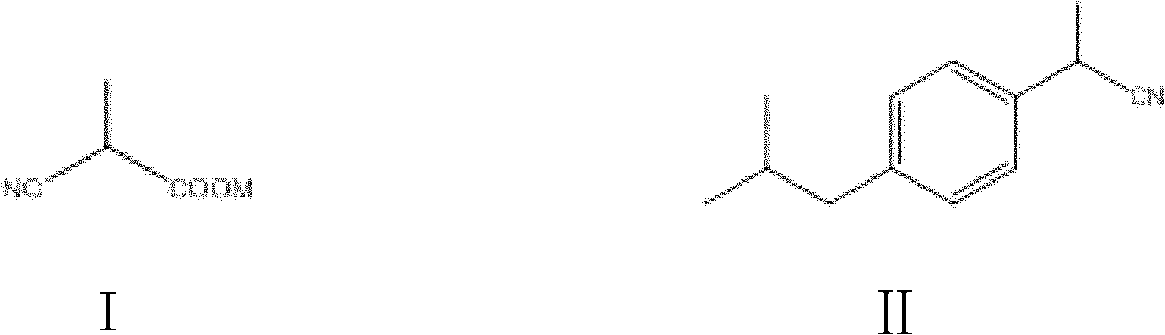
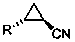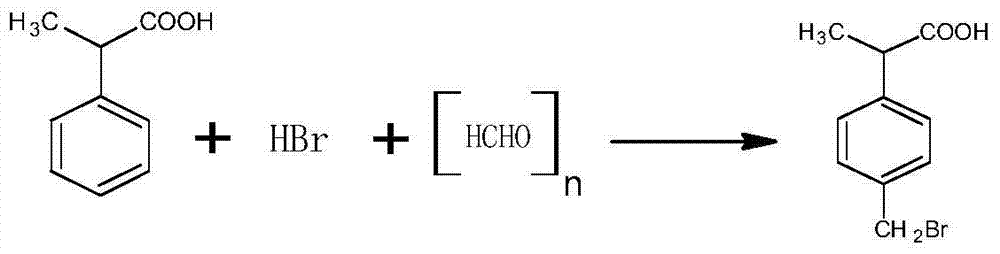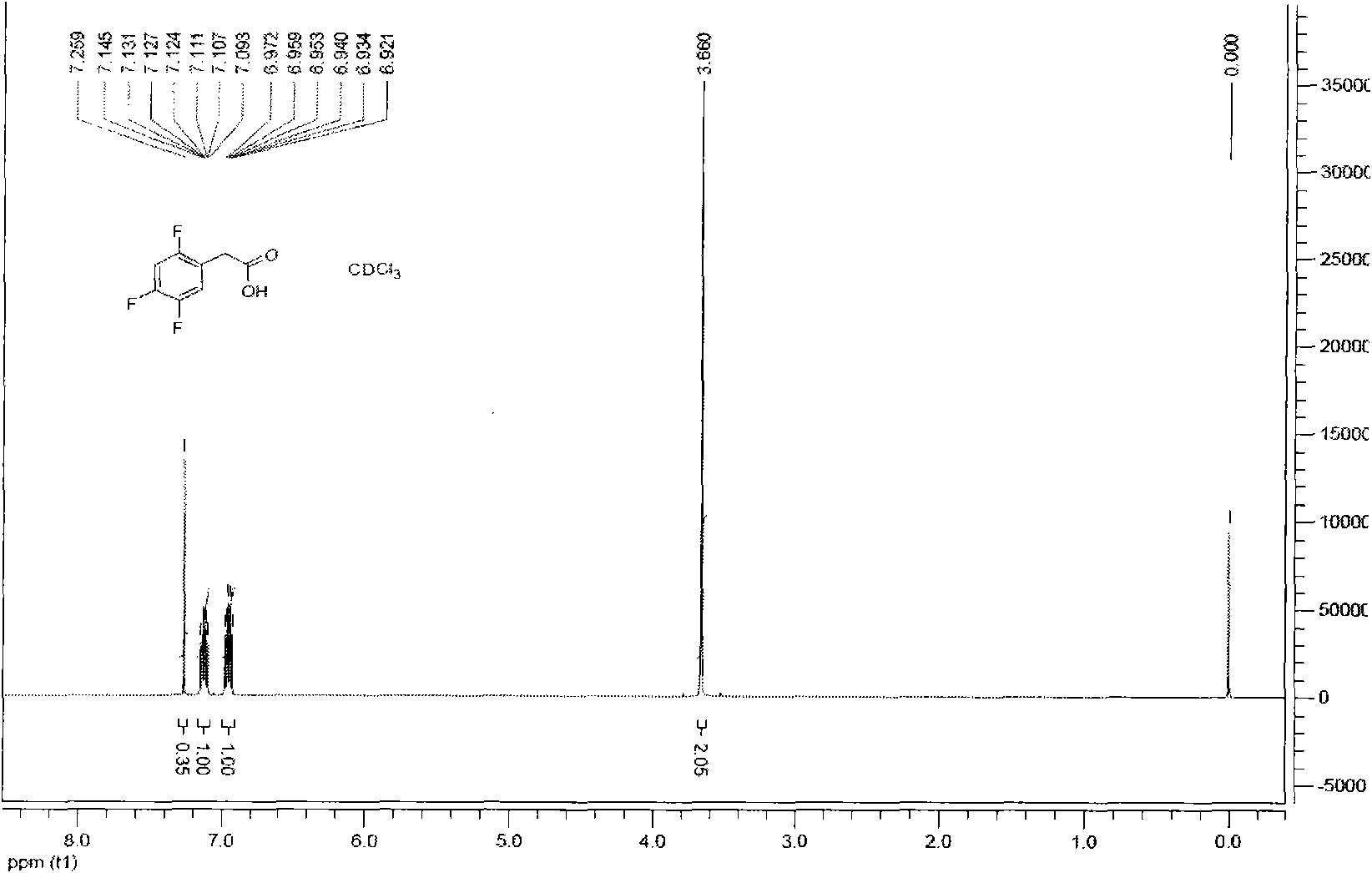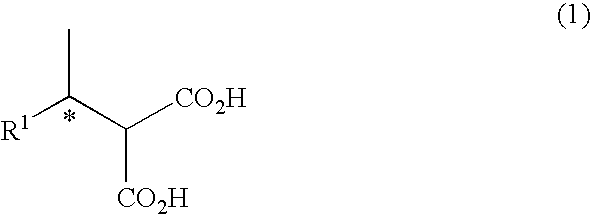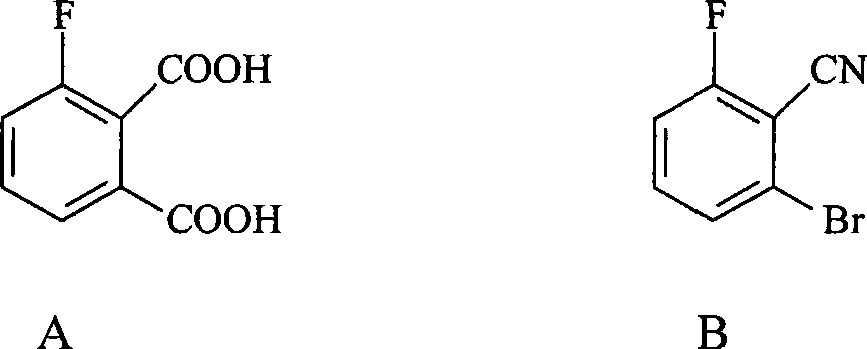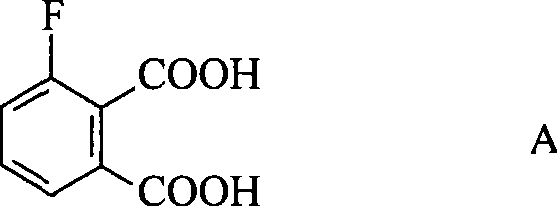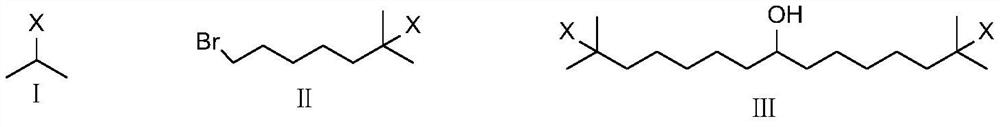Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
335results about "Preparation from nitriles" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
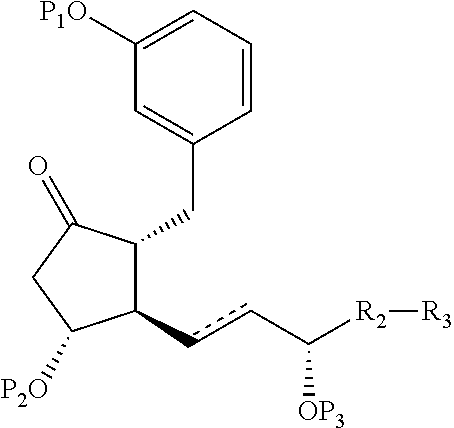
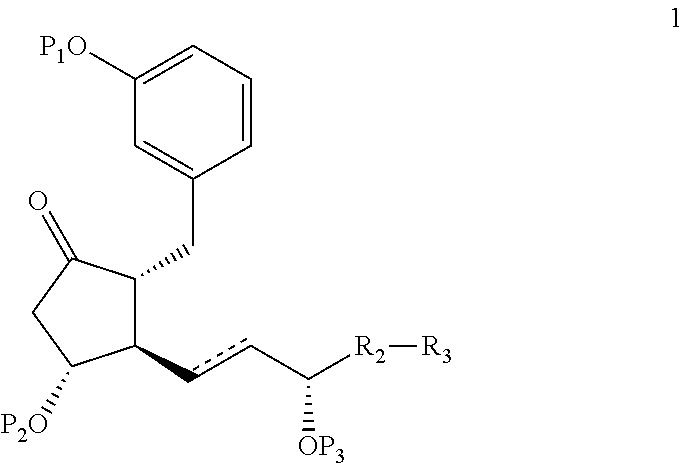
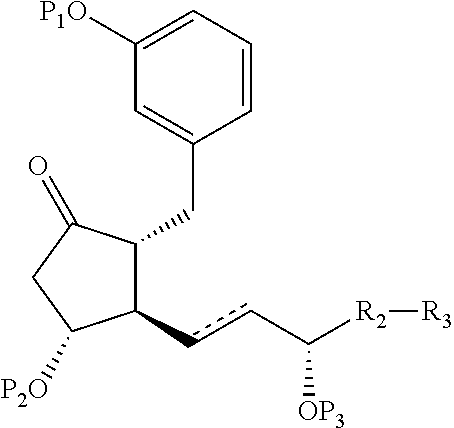
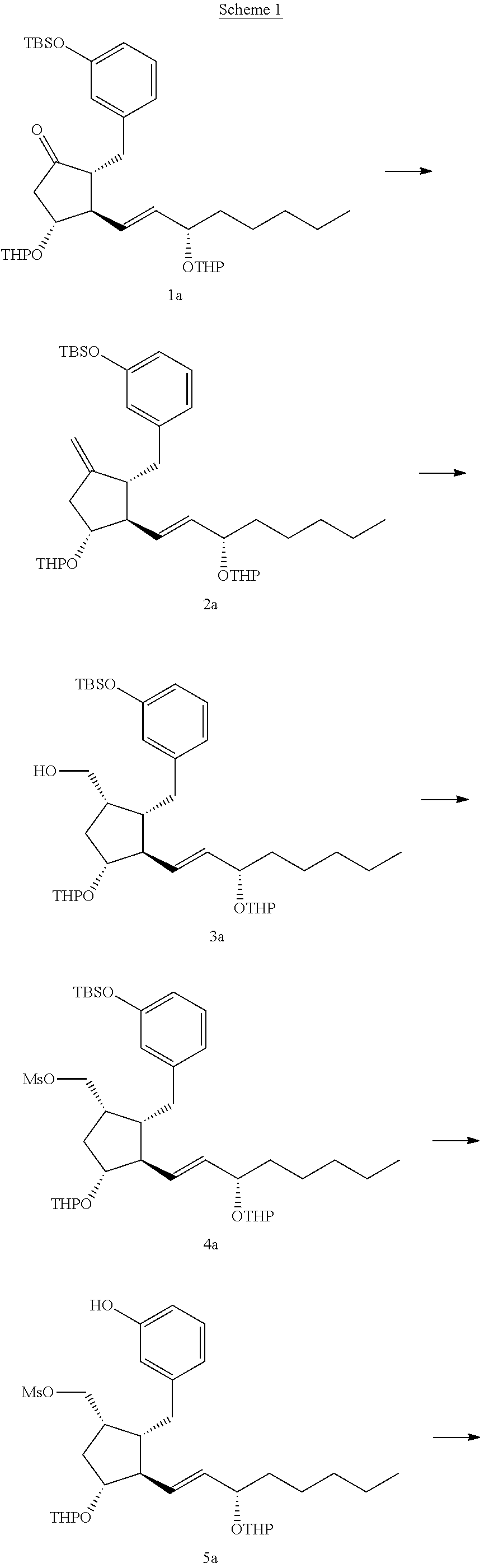
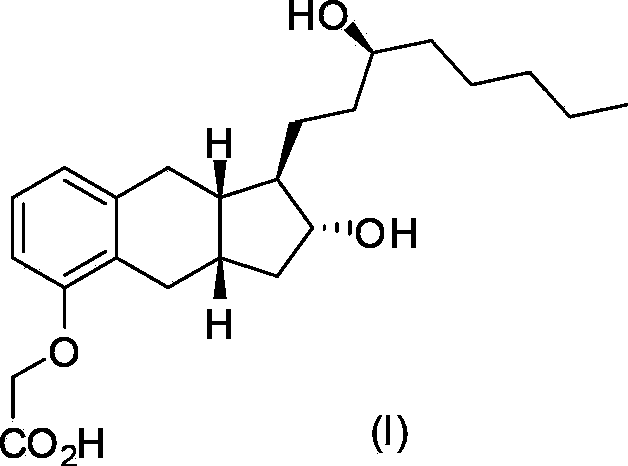
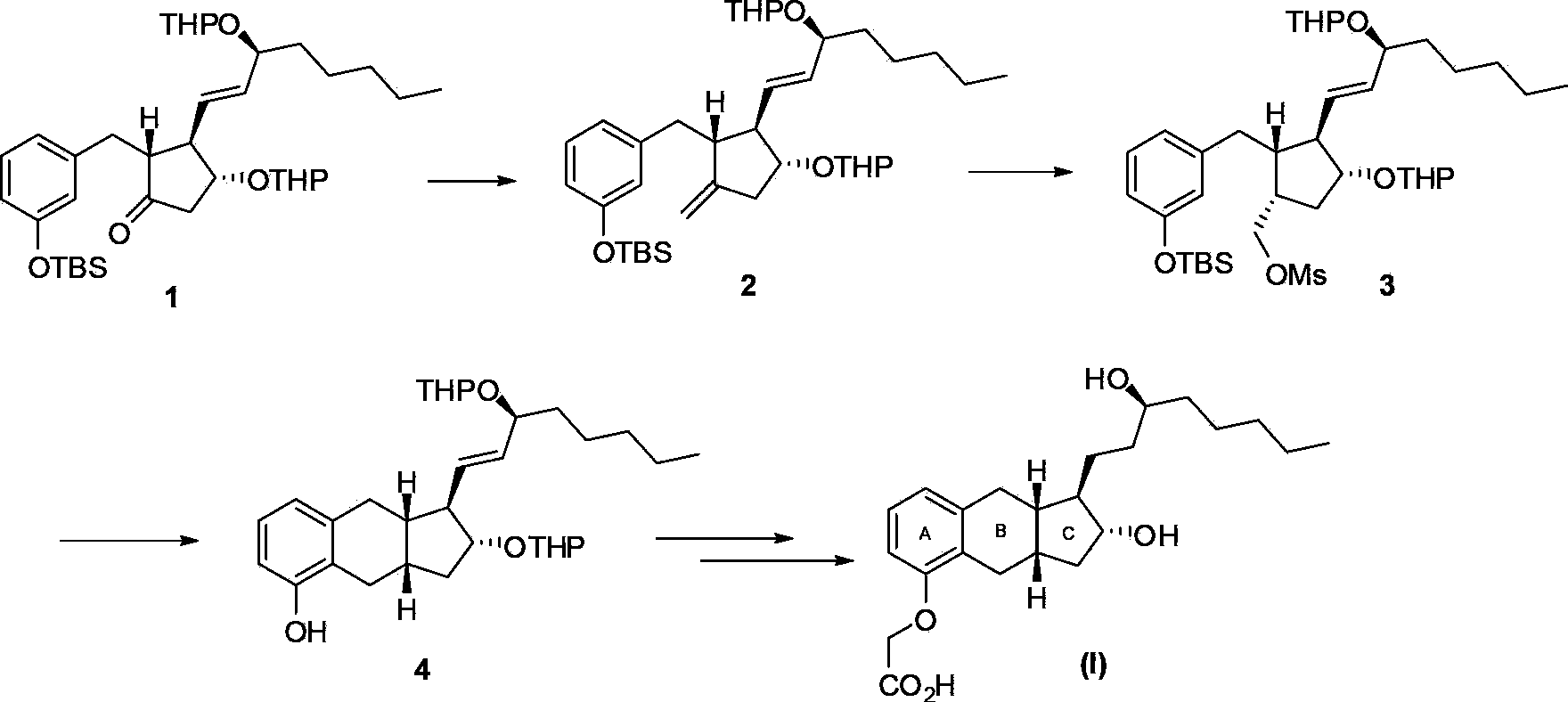
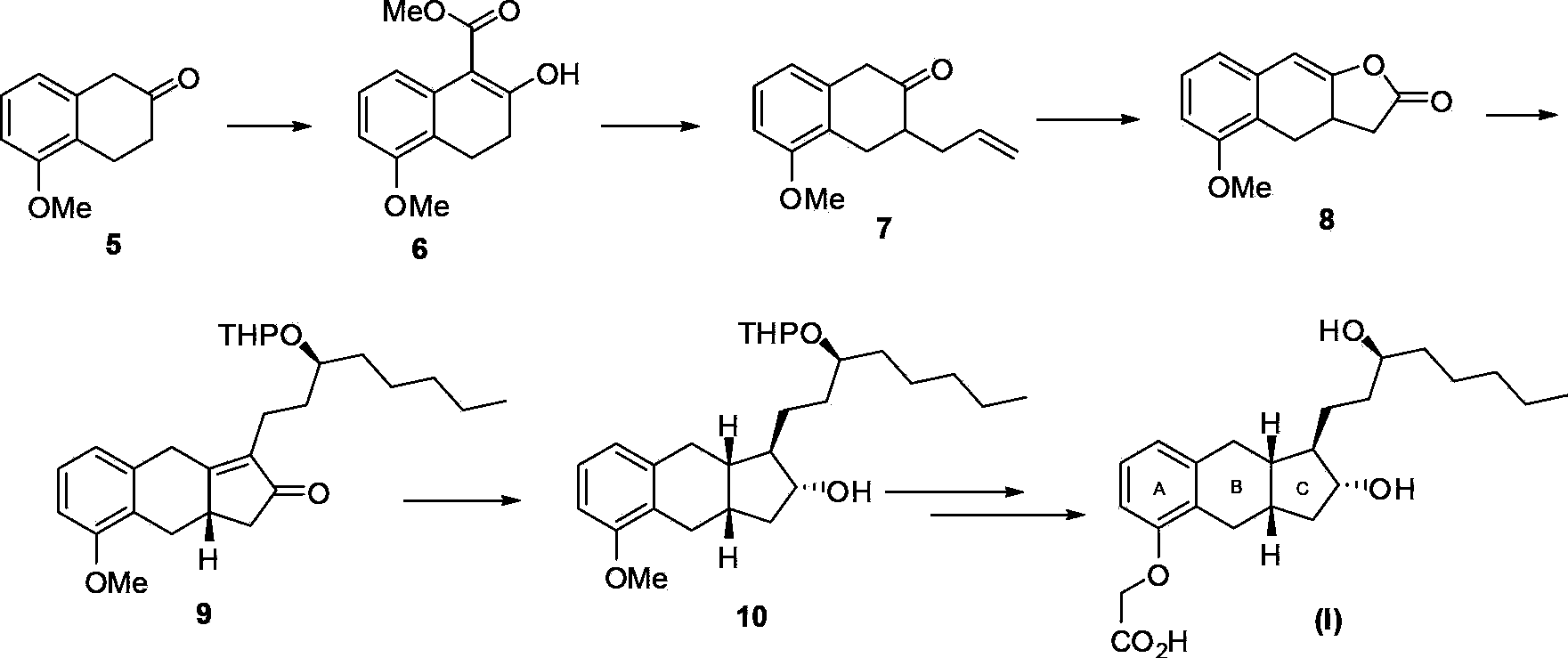
Intermediates for the synthesis of benzindene prostaglandins and preparations thereof
ActiveUS20130053581A1Silicon organic compoundsOrganic compound preparationPhotochemistryProstaglandin
Novel processes for preparing optically active cyclopentanones 1which are useful for the preparation of benzindene Prostaglandins and novel cyclopentanones are provided. The invention also provides novel processes of preparing benzindene Prostaglandins and novel intermediates for benzindene Prostaglandins.
Owner:CHIROGATE INT
Intermediates for the synthesis of benzindene prostaglandins and preparations thereof
Novel processes for preparing optically active cyclopentanones 1which are useful for the preparation of benzindene Prostaglandins and novel cyclopentanones are provided. The invention also provides novel processes of preparing benzindene Prostaglandins and novel intermediates for benzindene Prostaglandins.
Owner:CHIROGATE INT
New compound and preparation method and application thereof
InactiveCN101891596ASimple and fast operationEasy to operateOrganic active ingredientsOrganic compound preparationChemistryCombinatorial chemistry
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
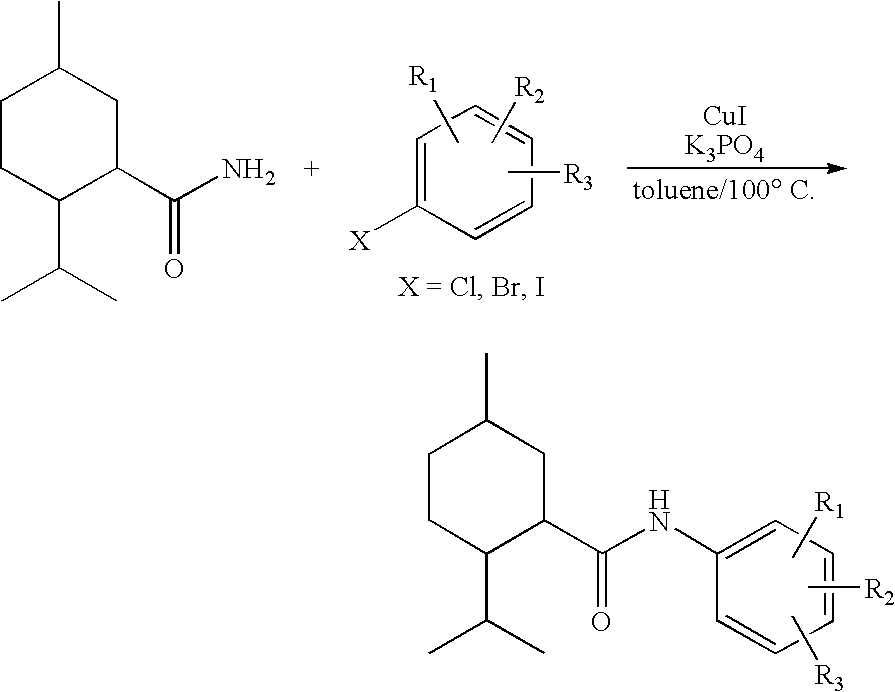
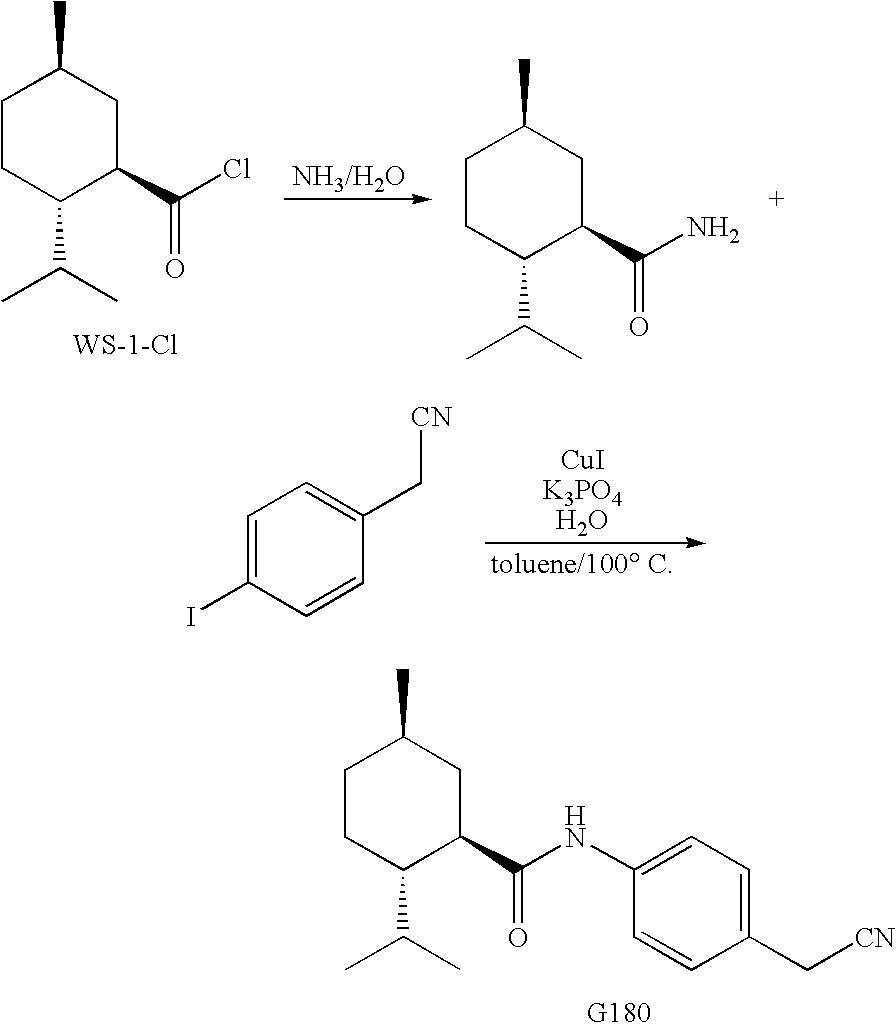
Synthesis of Cyclohexane Derivatives Useful as Sensates in Consumer Products
ActiveUS20100076080A1Improve cooling effectPotent and long lasting cooling effectBiocideCosmetic preparationsCooling effectL menthol
The present invention provides synthetic routes for preparing various isomers of cyclohexane-based coolants, such as menthyl esters and menthanecarboxamide derivatives, in particular those substituted at the amide nitrogen, for example with an aromatic ring or aryl moiety. Such structures have high cooling potency and long lasting sensory effect, which make them useful in a wide variety of consumer products. One synthetic route involves a copper catalyzed coupling of a primary menthanecarboxamide with an aryl halide, such reaction working best in the presence of potassium phosphate and water. Using this synthetic route, specific isomers can be prepared including the menthanecarboxamide isomer having the same configuration as l-menthol and new isomers such as a neoisomer having opposite stereochemistry at the carboxamide (C-1) position. The neoisomer unexpectedly has potent and long lasting cooling effect. Preparation schemes for neoisomers of other menthyl derivatives which are useful as coolants, including esters, ethers, carboxy esters and other N-substituted carboxamides are also provided.
Owner:THE PROCTER & GAMBLE COMPANY
Novel compound and preparation method and use thereof
InactiveCN101891715AEasy to operateMild responseGroup 4/14 element organic compoundsOrganic reductionMedicinal chemistrySilicon based
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Method for the production of 2-hydroxy-4-(methylthio)butyronitrile from 3-(methylthio)propanal and hydrogen cyanide
ActiveUS20120215022A1Increase response rateLow byproduct formationOrganic compound preparationOrganic chemistry methodsCyanidePropynal
A method for the production of 2-hydroxy-4-(methylthio)butyronitrile having good storage stability in a multi-zone reactor, is provided. 3-methylmercaptopropionaldehyde is reacted with hydrogen cyanide in the presence of a base as catalyst in a main reaction zone of the multizone reactor to form a reaction mixture comprising the 2-hydroxy-4-(methylthio)butyronitrile, unreacted 3-methylmercaptopropionaldehyde, the catalyst and residual amounts of gaseous hydrogen cyanide. The residual gaseous hydrogen cyanide is removed from the main reaction zone to an absorption and post-reaction zone of the reactor which comprises a mixture of 3-methylmercaptopropionaldehyde and the catalyst; and the gaseous hydrogen cyanide is further reacted with the 3-methylmercaptopropionaldehyde in the absorption and post reaction zone. A molar ratio of hydrogen cyanide to 3-(methylthio)propanal in the main reaction zone is from 0.98 to 1.03.
Owner:EVONIK OPERATIONS GMBH
Novel hybride materials and related methods and devices
ActiveUS20090171106A1Strong lightHigh tensile strengthMaterial nanotechnologyPigmenting treatmentNanoparticleCarbon nanotube
The invention provides devices and methods for end and side derivatization of carbon nanotubes. Also facile methods to attach moieties and nanoparticles on the side walls and both ends are described. The invention provides hybide materials for analytical, and optoelectronic purposes as well as materials applications. Materials have improved properties in the areas of tensile, electrical and thermal conductivity.
Owner:AMROY EUROPE OY
2,3-diisopropyl-2.3-dicyano-diethyl succinate compound, preparation method and application thereof
ActiveCN101811982AAvoid low temperature operationEasy to industrializeCarboxylic acid nitrile preparationOrganic compound preparationElectron donorPetrochemical
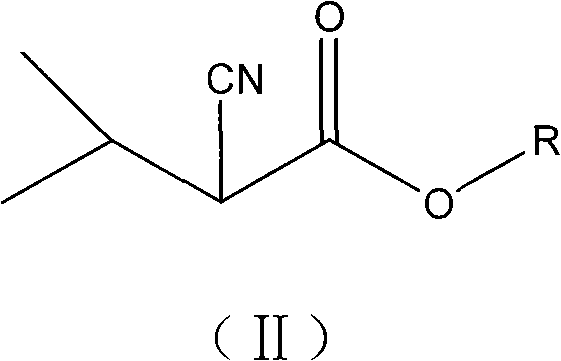
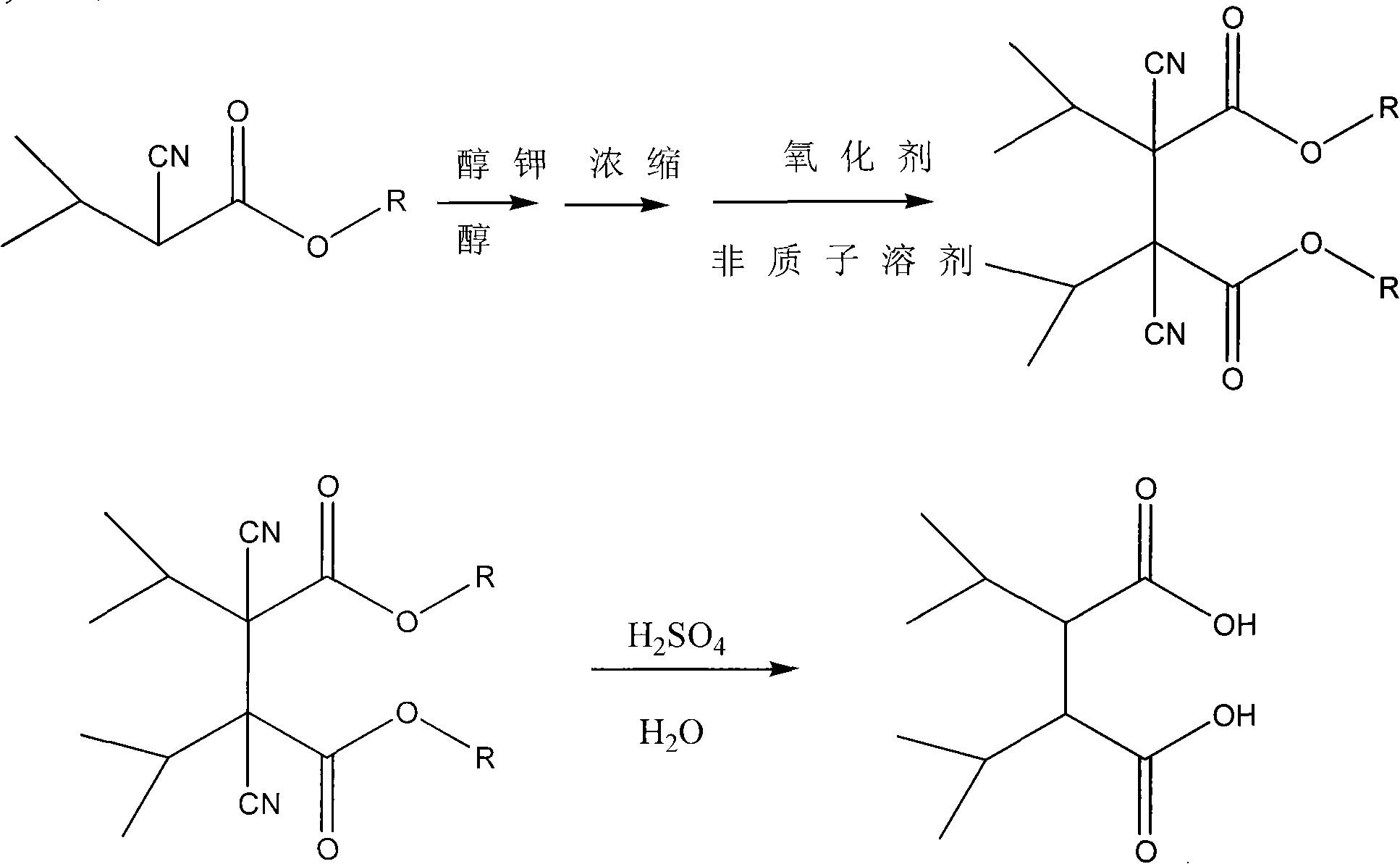
The invention relates to a preparation method of 2,3-diisopropyl succinate and ester compound thereof, which comprises the steps of: carrying out an oxidative coupling reaction of a 3-methyl-2-cyano butyrate compound for preparing a 2,3-diisopropyl-2.3-dicyano-diethyl succinate compound; carrying out a hydrolysis reaction and a decarboxylation reaction for preparing the 2,3-diisopropyl succinate;and further esterifying for preparing the 2,3-diisopropyl diethyl succinate compound. An intermediate of the 2,3-diisopropyl-2.3-dicyano-diethyl succinate compound and the preparation method thereof are beneficial to commercially producing 2,3-diisopropyl succinic ester which is an internal electron donor compound used capable of being used for preparing a Ziegler-Natta polypropylene catalyst. The invention can be used for the technical field of petrochemical polypropylene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Chemical method used for preparing aromatic cyclopropanecarbonitrile and cyclopropylamine
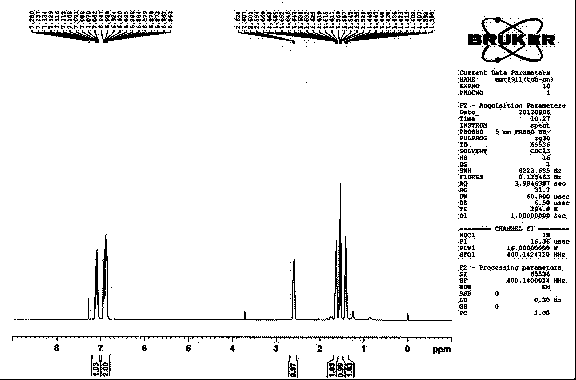
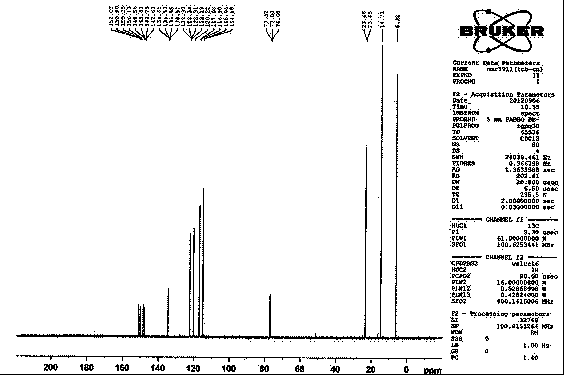
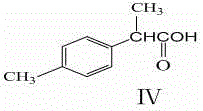
The invention relates to a method for preparing trans-aryl cyclopropanecarbonitrile with a structure shown in a formula (IV) in the specification through reaction between aryl substituted ethylene oxide and cyan substituted phosphate and further relates to a method for preparing cyclopropylamine from trans-aryl cyclopropanecarbonitrile. Trans-aryl cyclopropanecarbonitrile and cyclopropylamine are used for preparing drugs, especially ticagrelor.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
2-(4-methylphenyl)propionic acid syntehsis method
ActiveCN104402698AShort process routeReduce the discharge of three wastesPreparation from carboxylic acid saltsOrganic compound preparationPropanoic acidPropionitrile
The invention relates to a synthesis method of a fine chemical product 2-(4-methylphenyl)propionic acid. According to the method, para-xylene is adopted as a raw material, and a chlorination reaction is carried out, such that a compound I which is p-methylbenzyl chloride is obtained; p-methylbenzyl chloride is subjected to a nitrilation reaction, such that a compound II which is p-methylbenzyl cyanide is adopted; p-methylbenzyl cyanide is subjected to methylation, such that a compound III which is 2-(4-methylphenyl)propionitrile is obtained; and 2-(4-methylphenyl)propionitrile is subjected to hydrolysis acidification, such that a compound IV which is 2-(4-methylphenyl)propionic acid is obtained. The method provided by the invention has the advantages of short process route, low three-waste discharge, low environment pollution, no solid waste production during reaction processes, relatively low production cost, less chemical substances used during reaction processes, less raw material types, easy-to-obtain raw materials, simple operation, high yield, relatively mild process conditions, and the like.
Owner:柳州丰康泰科技有限公司 +2
Sitagliptin intermediates as well as preparation method and application of intermediate
InactiveCN102838511AAvoiding asymmetric catalytic hydrogenation reactionsImprove economyPreparation by cyanide reactionPreparation from nitrilesSitagliptinEpoxy
The invention relates to a Sitagliptin intermediates and a preparation method thereof as well as application of the intermediates in a method for preparing the Sitagliptin. According to the invention, epoxy chloropropane which is low in price and easy to obtain is used as a raw material to synthesize intermediates (S)-(2, 4, 5-trifluorophenyl) epoxy propane and (S)-3-hydroxy-4-(2, 4, 5-trifluorophenyl) butyronitrile, thus a chiral center is introduced, the use of various complex chiral reagents are avoided, and the chiral asymmetric catalytic hydrogenation reaction can be avoided; and the preparation method has the advantages of simple synthetic route, environment conservation, low raw material cost, etc.
Owner:ZHEJIANG HISOAR PHARMA
Process of preparing malonic ester
InactiveCN1834081AReduce consumptionCause a lot of decompositionOrganic compound preparationCarboxylic acid esters preparationAcetic acidMalonic acid
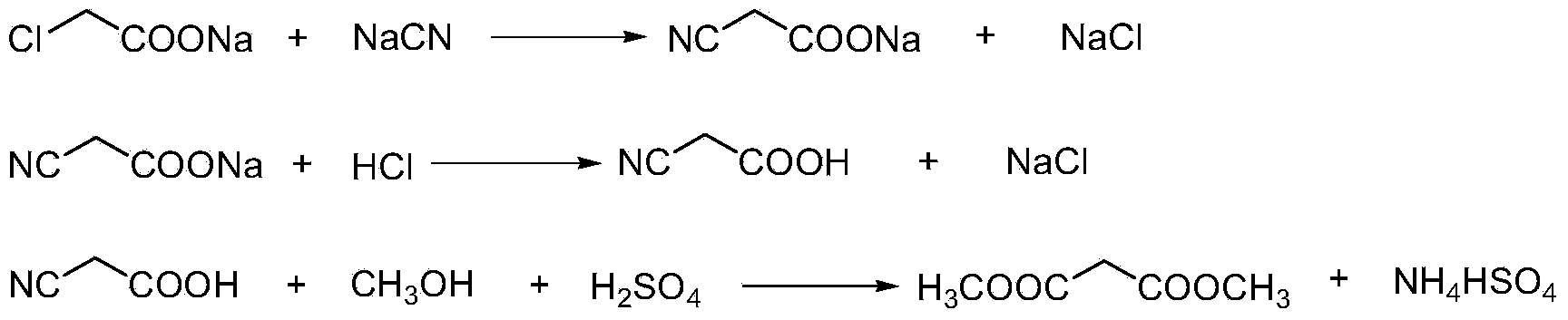
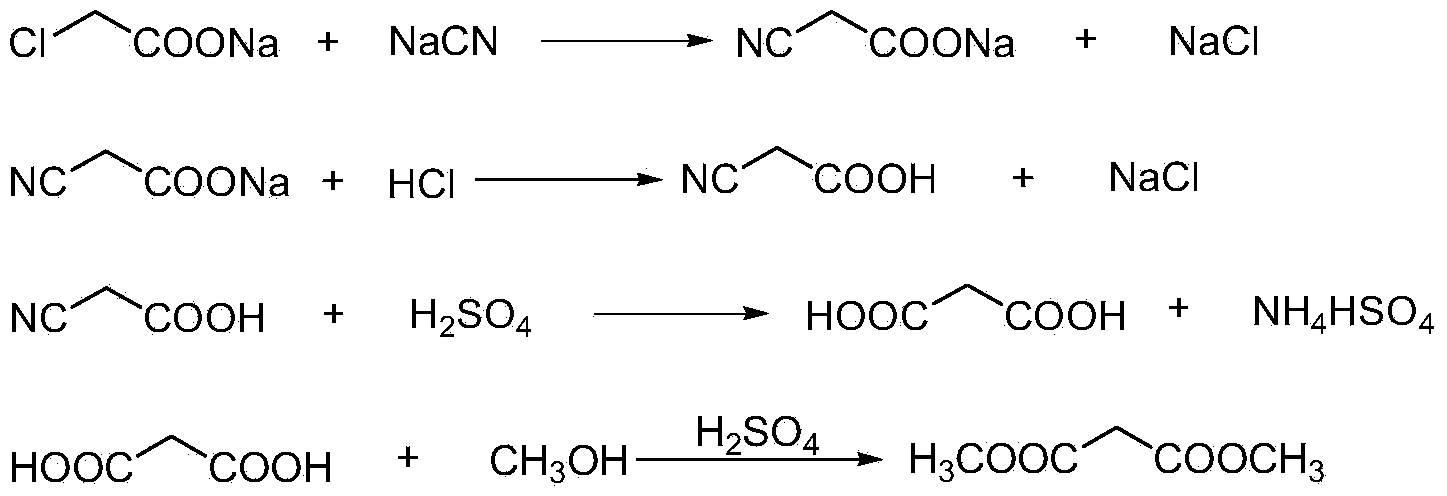
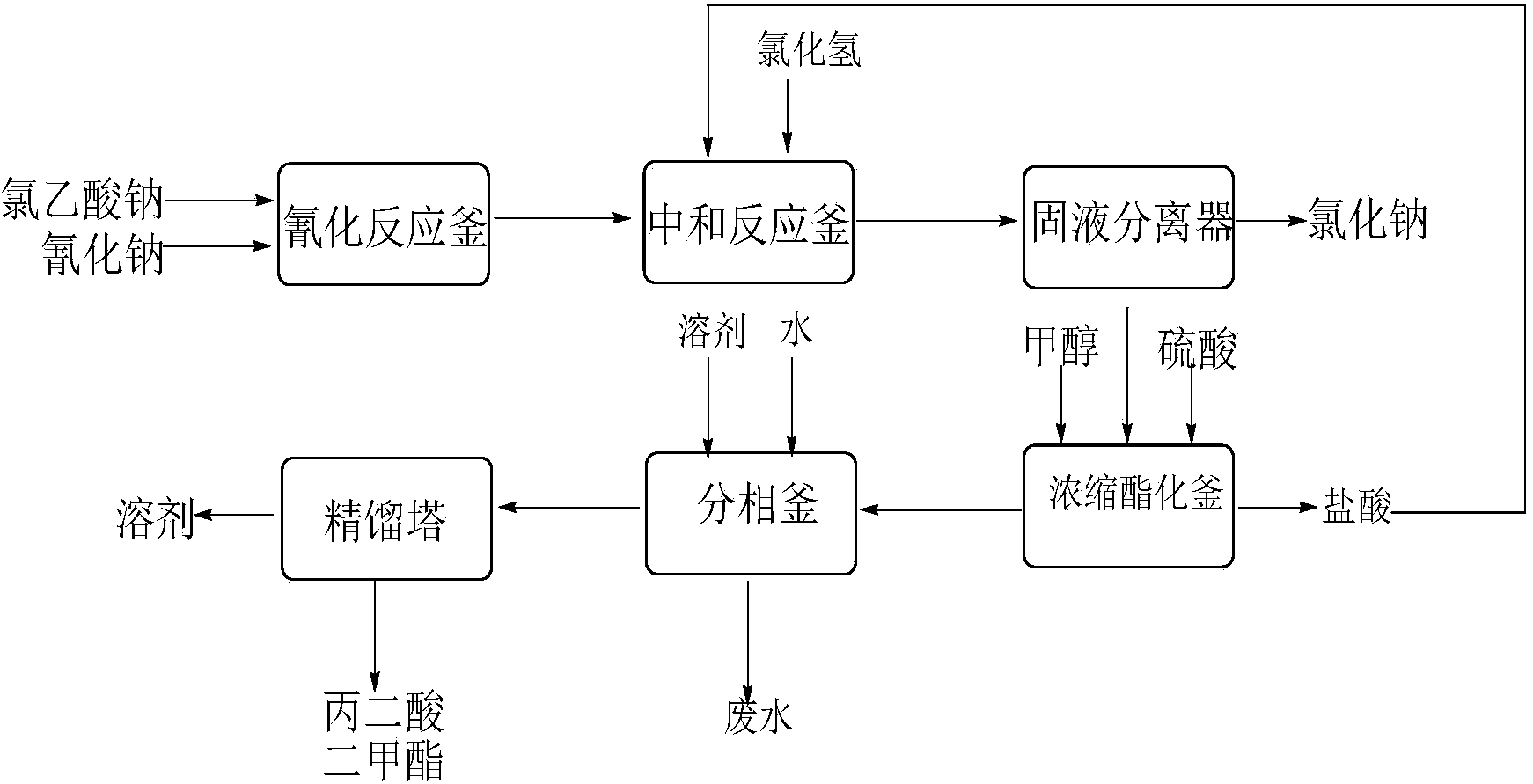
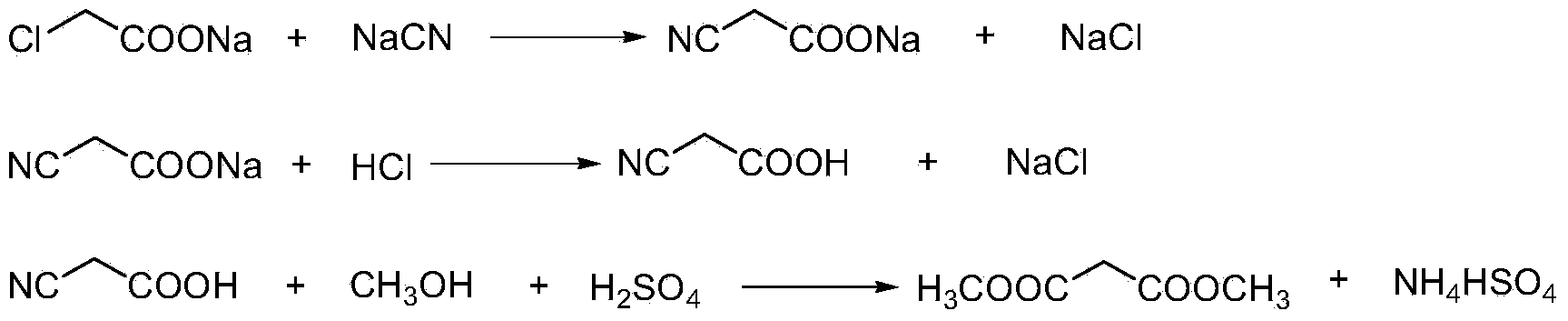
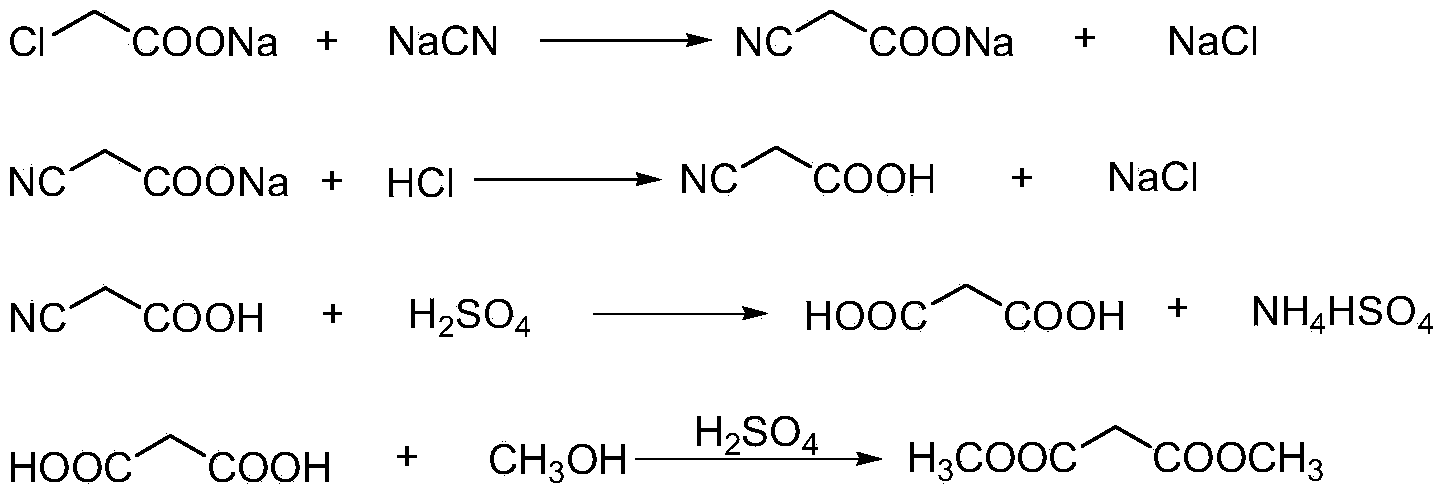
This invention relates to a method to synthesize malonate from chloroacetic acid, which includes neutralization, cyanidation, acidification, dehydration and esterification. It has the characteristics that dilute sulphuric acid is added after dehydration so that cyanoacetic acid is hydrolyzed into malonic acid which is then esterified to obtain malonate. This invention has the advantages of low cost, easily-controlled technique and high yield.
Owner:CHONGQING UNISPLENDOUR CHEM
Method for preparing loxoprofen intermediate
InactiveCN105753685AAvoid bringing inHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidPropionitrile
A method for preparing a loxoprofen intermediate comprises the following steps that 1, on the presence of sodium alkoxide, benzyl cyanide and dimethyl carbonate are subjected to methylation in an organic solvent, and 2-(phenyl cyano) sodium propionate is obtained; 2, 2-(phenyl cyano) sodium propionate and dimethyl sulfate react in an organic solvent to obtain 2-(phenyl cyano) methyl propionate; 4, 2-(phenyl cyano) methyl propionate reacts under the alkaline condition to obtain 2-phenyl propionitrile; 4, 2-phenyl propionitrile is hydrolyzed under the alkaline condition, acid is added for acidizing after the reaction to obtain 2-phenylpropionic acid; 5, 2-phenylpropionic acid, hydrobromic acid and paraformaldehyde are mixed and subjected to a bromine methylation reaction under the acidic condition, and 2-(4-tribromomethyl phenyl) propionic acid is obtained.According to the method, a new synthesis route is designed, product selectivity is good, the purity is high, the conversion rate is high, and few by-products are generated; the raw materials are simple and easy to obtain, the production conditions are mild, the process is simple, production cost is low, and pollution is small.
Owner:UPCHEM CHINA
Preparation method of 2-(4-bromomethylphenyl) propionic acid
ActiveCN104744237AHigh purityHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidMethyl carbonate
The invention discloses a preparation method of 2-(4-bromomethylphenyl) propionic acid. The preparation method is characterized by comprising a preparation stage of 2-phenyl propionitrile, a preparation stage of 2-phenyl propionic acid and a preparation stage of 2-(4-bromomethylphenyl) propionic acid. The preparation stage of 2-phenyl propionitrile comprises the following steps: mixing benzyl cyanide, dimethyl carbonate and potassium carbonate; heating to 100-300 DEG C and carrying out thermal reaction for 5-50h under the pressure of 0.5-6MPa; and by the end of the reaction, carrying out filter pressing and rinsing to be neutral; distilling to removal excessive dimethyl carbonate; and carrying out high-vacuum rectification to obtain 2-phenyl propionitrile. Compared with the prior art, the preparation method disclosed by the invention has the following advantages and effects that the main raw material is benzyl cyanide which is subjected to methylation, hydrolysis reaction and bromomethylation to finally obtain the finished product; the purity of the finished product is high and the yield is up to 90% or above; and in addition, the preparation method has the advantages of simple production process, low production cost and low environment pollution.
Owner:ZHEJIANG BOJU NEW MATERIALS CO LTD
Method for preparing 2, 4, 5-trifluoro-phenylacetic-acid
ActiveCN101659611AEasy to separateMeet the requirementsOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from nitrilesAcetic acidPhenylacetic acid
The invention provides a method for preparing 2, 4, 5-trifluoro-phenylacetic-acid. The method adopts 1, 2, 4-trifluoro-benzene to react with polyformaldehyde and chlorinating agent to obtain 2, 4, 5-trifluoro-benzyl chloride, and adopts the 2, 4, 5-trifluoro-benzyl chloride to react with cyaniding agent in ionic liquid to obtain 2, 4, 5-trifluoro-benzyl cyanide, and then the 2, 4, 5-trifluoro-benzyl cyanide is hydrolyzed in acid or alkalic conditions to obtain the 2, 4, 5-trifluoro-phenylacetic-acid. The preparation method for preparing 2, 4, 5-trifluoro-phenylacetic-acid requires cheap and available materials, and has moderate reaction conditions, less three-wastes and better safety; industrialized production can be easily realized, the product purity is high, and quality is stable, thuscompletely meeting the using requirement of 2, 4, 5-trifluoro-phenylacetic-acid as a pharmaceutical intermediate.
Owner:ZHEJIANG YONGTAI TECH CO LTD
Functionalized column aromatic hydrocarbon derivative and preparation method thereof
ActiveCN109400501AThe means of expanding functionalityAddresses less functional flawsOrganic compound preparationSulfonic acid esters preparationAromatic hydrocarbonOxime
The invention discloses a functionalized column aromatic hydrocarbon derivative and a preparation method thereof. The functionalized column aromatic hydrocarbon derivative comprises an X unit, a Y unit and a Z unit, wherein the X unit is shown in a formula (I), the Y unit is shown in a formula (II), the Z unit is shown in a formula (III), and the six-membered rings of the X unit, the Y unit and the Z unit all have two para-position substituents and four unsubstituted positions; the formulas are as shown in the specification, each unit is connected through CH2 groups to form a ring-shaped structure, and each unit is connected with the other two adjacent units in a para-position manner at an unsubstituted position; and the formed ring-shaped structure is the functionalized column aromatic hydrocarbon derivative. Cyano groups, carboxyl groups and oxime are directly connected to the benzene ring monomer of the column aromatic hydrocarbon, so that the method for functionalizing the column aromatic hydrocarbon is expanded; and the connected groups can effectively change the electronic environment of the column aromatic hydrocarbon rings, so that the functionalized column aromatic hydrocarbon derivative has wide application in the fields of supramolecular host-guest identification and the like.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Preparation method of 2-phenylpropionic acid
ActiveCN102351684AAvoid Explosion HazardsSimple processPreparation from nitrilesHydrolysisReaction step
The invention discloses a preparation method of 2-phenylpropionic acid. The method comprises the following steps: stirring and reacting styrene and hydrochloric acid solution with the mass concentration of 20-30% used as raw materials for 8-14 hours at the temperature of 30-40 DEG C, standing and layering, water-washing an oil layer to obtain alpha-chloro-ethylbenzene, and further sequentially performing cyanidation and hydrolysis on alpha-chloro-ethylbenzene so as to prepare 2-phenylpropionic acid. The method adopts styrene as the raw material for direct chlorination, thereby reducing the reaction steps, being easy to control the conditions and being simple in equipment; and furthermore, the obtained product is stable in quality and high in purity, and the benefits are significantly improved.
Owner:CHANGZHOU TIANHUA PHARMA
Polycarboxylic acid mixture
InactiveUS7262256B2High levelMaintain good propertiesElectrolysis componentsOrganic compound preparationNitrogenCarboxylic acid
A polycarboxylic acid mixture comprising 80% by weight or more of 1,3,6-hexanetricarboxylic acid, wherein the polycarboxylic acid mixture has a psychometric lightness L-value of 98 or more, a psychometric chroma a-value of from −2.0 to 2.0 and a psychometric chroma b-value of from −2.0 to 3.0, and has a nitrogen content of 5,000 ppm by weight or less.
Owner:ASAHI KASEI CHEM CORP
Method For Producing Alcohol and Carboxylic Acid Having Optical Activity
InactiveUS20080233621A1High optical purityProduced inexpensively and efficientlyOrganic compound preparationOrganic chemistry methodsCarboxylic acidMethyl group
It is an object of the present invention to provide an inexpensive and efficient industrial method for obtaining (S)-2-pentanol, (S)-2-hexanol, 1-methylalkyl malonic acid and 3-methyl carboxylic acid at a high optical purity. The present invention provides a method of producing (S)-2-pentanol or (S)-2-hexanol which comprises allowing certain types of microorganisms or transformed cells, a product obtained by treating said microorganisms or cells, a culture solution of said microorganisms or cells, and / or a crude purified product or purified product of a carbonyl reductase fraction obtained from said microorganisms or cells, to act on 2-pentanone or 2-hexanone.
Owner:API CORP (JP)
Method for preparing carboxylic acid compound by promoting rapid hydrolysis of cyan
InactiveCN102093149ASimple processLow reaction temperatureOrganic compound preparationPreparation from nitrilesIodideAcid hydrolysis
The invention discloses a method for preparing a carboxylic acid compound by promoting rapid hydrolysis of cyan. Under the alkaline condition, cyan undergoes a hydrolysis reaction under the catalytic action of an iodine ion-containing compound, so that the carboxylic acid compound is generated. The method comprises the following steps of: 1) dissolving an alkaline compound or carbonate in water to obtain an alkaline solution; 2) adding an iodide into the alkaline solution, stirring for dissolving, and adding a cyan compound to perform a hydrolysis reaction; 3) cooling to the room temperature, adding sulfuric acid, and filtering after crystals are fully separated out to obtain a solid; 4) dissolving the solid in deionized water, and extracting with absolute ether; and 5) drying extract liquor, and removing diethyl ether by distilling to obtain the carboxylic acid compound. The invention has the advantages of simple process, low reaction temperature, reaction in the conventional heating device, easiness in implementing, short required reaction time, yield of up to 60 to 99 percent, and great improvement on production efficiency.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Novel synthetic method of key intermediate2-anisacetone of loxoprofen
The invention relates to a synthetic method for loxoprofen, in particular to a novel synthetic method of key intermediate2-anisacetone of loxoprofen. The method comprises the following steps: taking methylbenzyl cyanide as an initial material to generate alpha-methylation with dimethyl carbonate in an alkaline environment so as to generate alpha-methyl-methyl phenylacetonitrile, and hydrolyzing the alpha-methyl-methyl phenylacetonitrile in alkaline water to obtain the 2-anisacetone. The method provided by the invention has the advantages of simplicity, easiness in control and small waste water amount. Moreover, the synthesized 2-anisacetone is high in purity and stable in quality, which lays a good foundation for synthesizing the high-purity loxoprofen.
Owner:ANHUI HERYI CHEM
Preparation methods of 2, 4, 5-trifluoro-benzyl chloride and 2, 4, 5-trifluoro-phenylacetic acid
InactiveCN102690166AEmission reductionReduce manufacturing costPreparation from nitrilesHalogenated hydrocarbon preparationBenzeneAlkaline water
The invention provides a preparation method of 2, 4, 5-trifluoro-benzyl chloride. The preparation method of the 2, 4, 5-trifluoro-benzyl chloride comprises the following steps of: adding sulfuric acid solution with hydrogen chloride gas and sulphuric acid concentration being 80-95 percent into 1, 2, 4-trifluoro-benzene and paraformaldehyde so as to be used as a chlorinating agent, and carrying out chloromethylation reaction to obtain the 2, 4, 5-trifluoro-benzyl chloride. The invention also provides a preparation method of 2, 4, 5-trifluoro-phenylacetic acid. The preparation method of the 2, 4, 5-trifluoro-phenylacetic acid comprises the following steps of: adding the sulfuric acid solution with the hydrogen chloride gas and the sulphuric acid concentration being 80-95 percent into the 1, 2, 4-trifluoro-benzene and the paraformaldehyde so as to be used as the chlorinating agent, carrying out chloromethylation reaction to obtain the 2, 4, 5-trifluoro-benzyl chloride, carrying out cyaniding reaction in organic solvent of cyaniding reagent to obtain the 2, 4, 5-trifluoro-benzyl chloride, heating and hydrolyzing the 2, 4, 5-trifluoro-benzyl chloride in acidic or alkaline aqueous solution, after the reaction is finished, and processing to obtain the 2, 4, 5-trifluoro-phenylacetic acid. According to the preparation methods, the discharging of waste liquor in a production process can be reduced, the production cost is reduced, the environmental and economic benefits are achieved, the product purity is good, and the yield is high.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Preparation method of 3-fluorophthalic acid
InactiveCN101381303AHigh purityStarting materials are cheap and readily availablePreparation from nitrilesSolventPhthalic acid
The invention relates to a preparation method for 3-fluorophthalic acid. The preparation method comprises the following steps that: (I) 2,6-difluorobenzonitrile which is used as a starting material is subjected to an ammoximation reaction in an aprotic solvent to obtain 2-amino,6 -fluorobenzonitrile; (II) the 2-amino,6 -fluorobenzonitrile is subjected to a diazotization reaction and then reacts with liquid HBr to form 2- bromo,6 -fluorobenzonitrile; (III) the 2- bromo,6 -fluorobenzonitrile is subjected to a cyanation reaction in an aprotic solvent to form 3-fluorophthalic benzenedicarbonitrile; (IV) the 3-fluorophthalic benzenedicarbonitrile is decomposed in an aqueous solution of inorganic acid to obtain a target substance. The preparation method overcomes the defects of difficult preparation of raw materials, low yield of the target substance, long preparation time or / and low purity and so on in the prior art of preparing the 3-fluorophthalic acid and is capable of meeting commercial preparation requirements.
Owner:EAST CHINA UNIV OF SCI & TECH
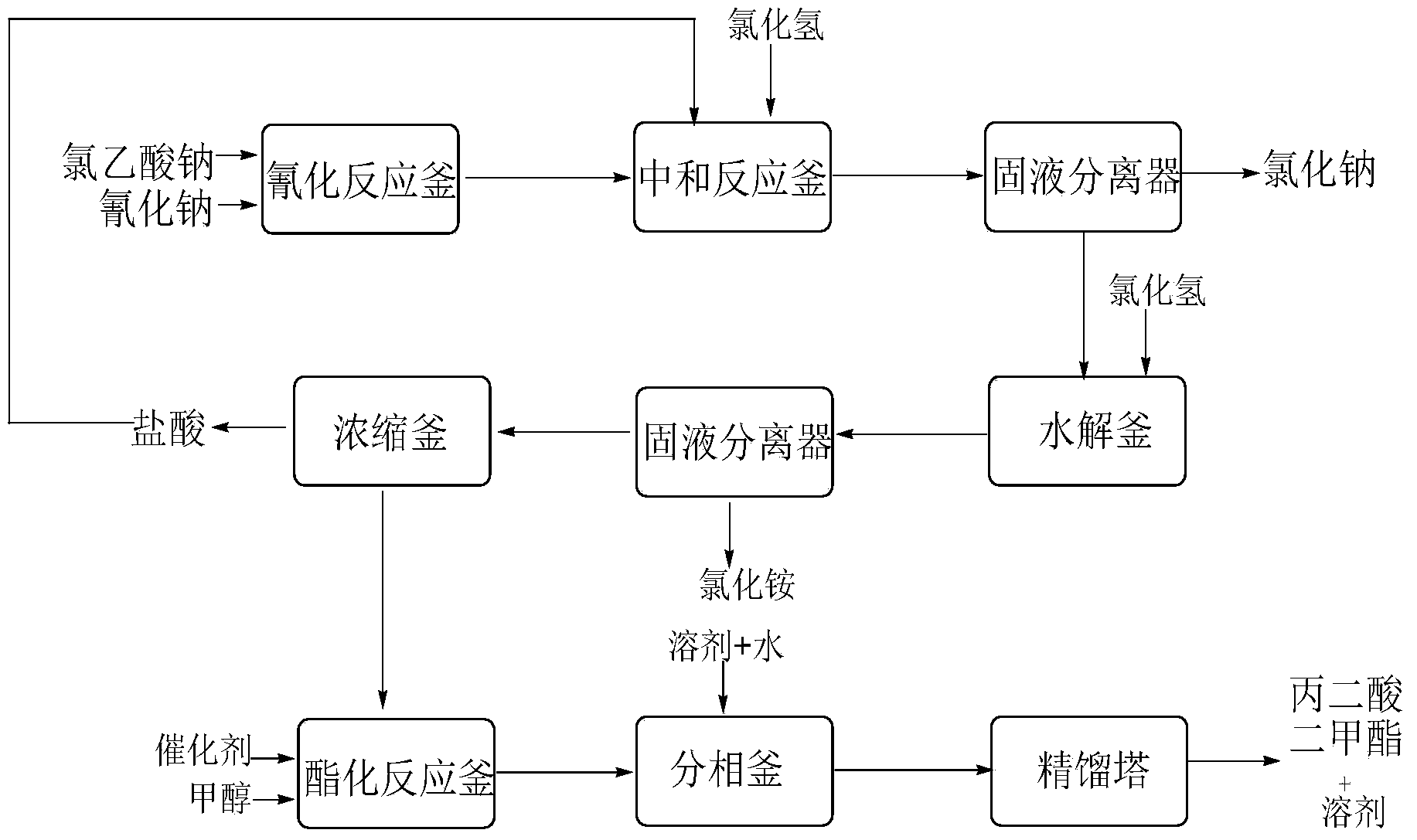
Dimethyl malonate preparation method
InactiveCN103724191AReduce productionReduce dosageOrganic compound preparationCarboxylic acid esters preparationCyanoacetic acidDecomposition
The invention discloses a dimethyl malonate preparation method. The shortcomings of mass decomposition of cyanoacetic acid and low yield caused by the traditional distillation concentration desalination are avoided by introducing hydrogen chloride into cyanoacetic acid reaction liquid to separate out sodium chloride; meanwhile, malonic acid and ammonium chloride are obtained by hydrolysis in a closed system, and hydrogen chloride gas is introduced to separate out ammonium chloride; after desalination, an esterification reaction can reduce the catalyst dosage and wastewater generation while a byproduct ammonium chloride is obtained. The method disclosed by the invention is simple to operate, low in production cost and high in product yield and generates a small quantity of three wastes, thereby being an environment-friendly and clean production method.
Owner:CHONGQING UNISPLENDOUR CHEM
Method of making fluorinated alkoxy carboxylic acids and precursors thereof
ActiveUS20110245520A1Organic compound preparationCarboxylic acid esters preparationOrganic solventCarboxylic acid
A method for preparing saturated partially fluorinated alkoxy carboxylic acids or salts thereof by treating a compound: (II), where Rf represents a fluorinated, linear or branched alkyl residue interruptible by one or more oxygen atoms, n is 0 or 1, with a Z-anion in a reaction medium comprising water and an organic solvent, where the Z-anion is selected from CN—, SCN— and OCN— or combinations thereof. A method of making partially fluorinated ethers of the general formula (I) wherein Rf is defined as above, n is 0 or 1, and Z is nitrile (—CN), azide (—N3), thiocyanate (—SCN) or cyanate (—OCN) group, said method comprising treating a fluorinated olefin of the general formula (II) wherein the Z-anion is CN—, OCN—, SCN— or N3−. A compound of the general formula (I) as previously described where Z is selected from SCN, OCN and N3.
Owner:3M INNOVATIVE PROPERTIES CO
Technology of preparing hdyroxy acetic acid
The invention provides a process for preparing hydroxy-acetic acid which comprises, hydrolyzing hydroxyl acetonitrile aqueous solution and sulfuric acid to obtain hydroxy-acetic acid and the aqueous solution of the acidic salts of ammonium sulfate, then charging methanol into the aqueous solution for esterification, and distilling the mixture of methanol, water and hydroxy-acetic acid methyl ester, remaining the acidic salts of ammonium sulfate, then charging water into the distilled mixture for the hydrolysis of hydroxy-acetic acid methyl ester, separating methanol and obtaining crystallized hydroxy-acetic acid.
Owner:XINMAO DACRON CHEM GENERAL PLANT TAICANG CITY
Dimethyl malonate preparation method
InactiveCN103724196AReduce dosageReduce productionOrganic compound preparationCarboxylic acid esters preparationDecompositionCyanoacetic acid
The invention discloses an environment-friendly and clean dimethyl malonate preparation method. Hydrogen chloride gas is introduced into the mixed liquid of cyanoacetic acid and sodium chloride, and then methanol and sulfuric acid are added for an esterification reaction to obtain dimethyl malonate. The shortcomings of mass decomposition of cyanoacetic acid and low yield caused by the traditional distillation concentration desalination are avoided; meanwhile, after desalination, the esterification reaction can reduce the dosage of sulfuric acid and wastewater generation. The method is simple to operate, low in production cost, high in product yield and suitable for large-scale industrial production.
Owner:CHONGQING UNISPLENDOUR CHEM
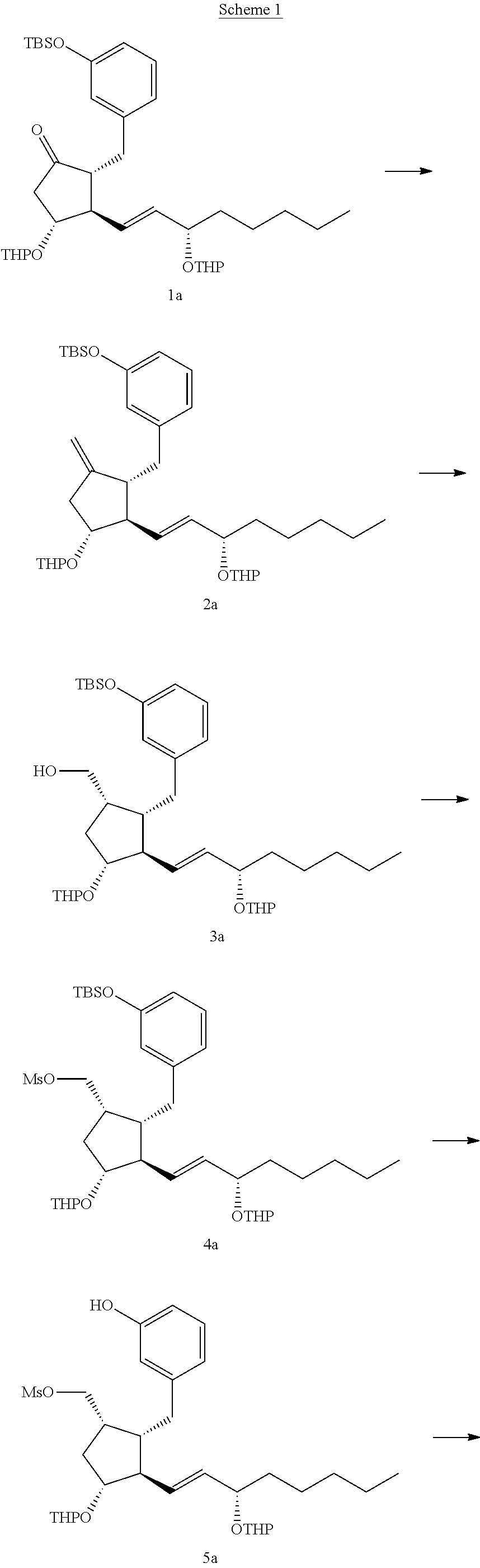
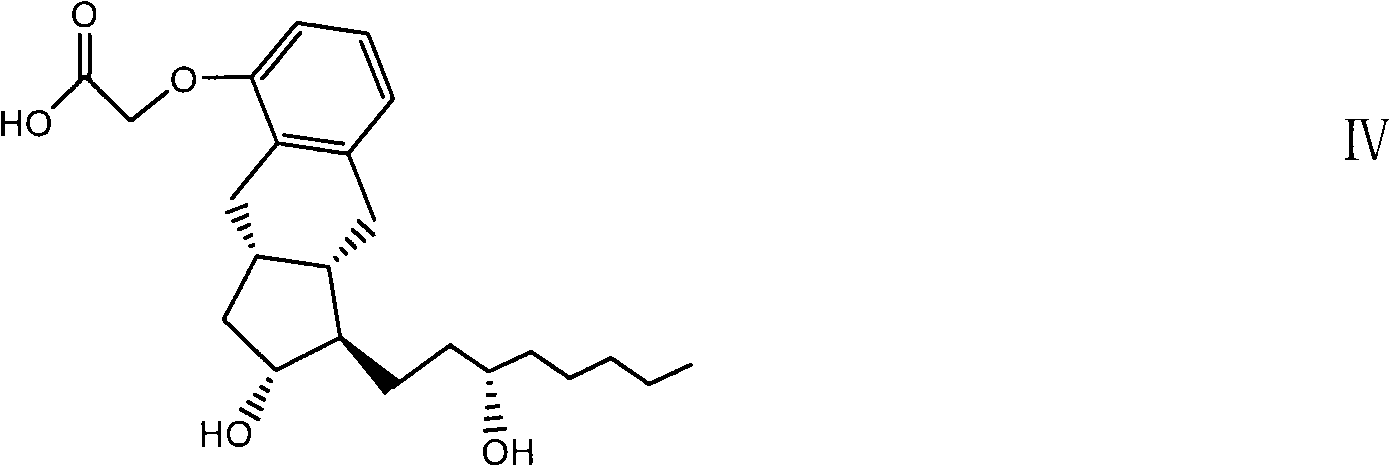
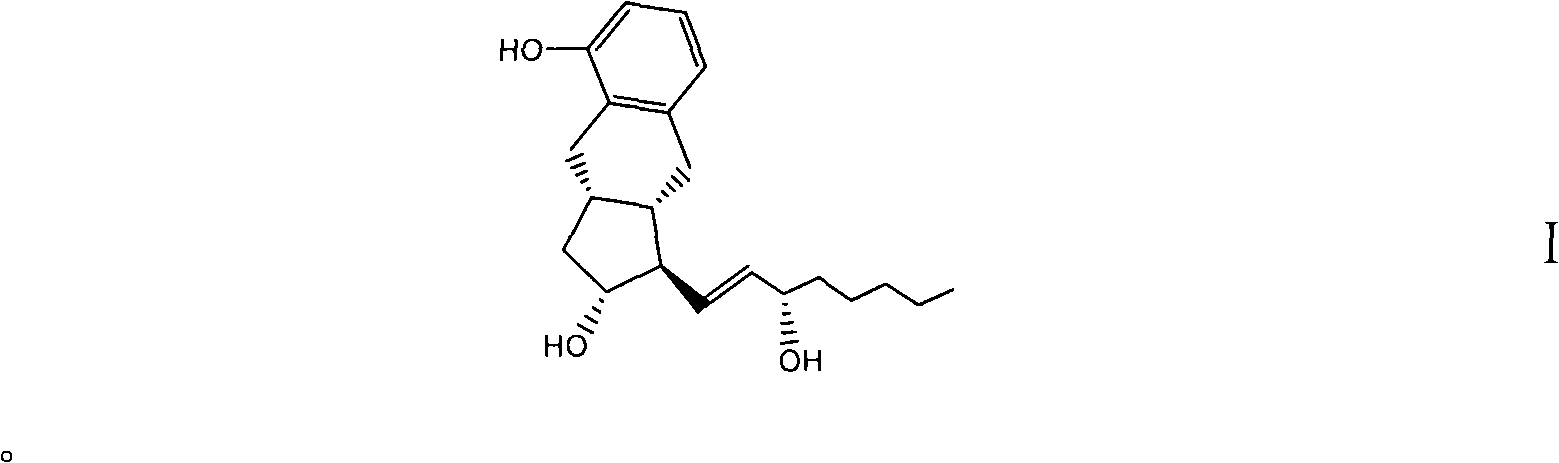
Intermediates for synthesizing treprostinil and preparation method thereof as well as the preparation method of treprostinil thereby
ActiveCN103880801ASilicon organic compoundsCarboxylic acid nitrile preparationPresent methodTreprostinil
The present invention relates to intermediates for preparing treprostinil, a preparation method for same, and a preparation of treprostinil thereby. Specifically, the invention relates to a compound of formula (VI), the preparation thereof and the application of said compound for the synthesis of treprostinil (as represented by formula (I)). The method for the preparation of treprostinil comprises: obtaining the compound of formula (III) by means of reducing the compound of formula (VI) and deprotecting the hydroxy group thereof; reacting said compound of formula (III) with chloroacetonitrile followed by hydrolysis to obtain the treprostinil of formula (I). The present method has simple operation and high synthesis yields, and is suitable for large-scale production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Synthetic method of bempedoic acid
PendingCN111825546AShort reaction stepsHigh yieldOxygen-containing compound preparationCarboxylic acid nitrile preparationBempedoic acidGrignard reagent
The invention discloses a synthetic method of bempedoic acid. The method comprises the following steps of: taking isobutyronitrile (ester) as a starting raw material, carrying out reaction with 2, 5-dibromopentane under the catalysis of alkali to generate 7-bromo-2, 2-dimethylheptonitrile (ester), then forming a Grignard reagent with magnesium, carrying out reaction with formate to generate 8-hydroxy-2, 2, 14, 14-tetramethylpentadecane dinitrile (ester), and finally carrying out alkaline hydrolysis and acidification to obtain bempedoic acid. The synthetic route is short, all the used raw materials are easy to obtain, the cost is low, the yield of each step of reaction is high, and the purity is high, therefore the method is suitable for industrial production.
Owner:合肥市梓熤科技贸易有限公司
Method for synthesizing ibuprofen and analogues thereof
The invention provides a method for synthesizing ibuprofen and analogues thereof. In the method, 1-halogenated-4-isobutylbenzene and cyanogen acetate derivatives are taken as raw materials for synthesizing the ibuprofen; halogenated benzene derivatives and the cyanogen acetate derivatives are taken as raw materials for synthesizing the analogues of the ibuprofen; and the raw materials for synthesizing the ibuprofen and the raw materials for synthesizing the analogues of the ibuprofen are subjected to a palladium catalyzed decarboxylation coupling reaction, a methylation reaction and a hydrolysis reaction to synthesize the ibuprofen and the analogues thereof respectively. The synthesizing method is simple, and easy to operate and has wide raw material sources and low cost, and only inorganic salt and carbon dioxide are produced in the synthesizing process, so that the method is safe and environmentally-friendly, meets the requirement of green chemistry, and is suitable for industrialized production.
Owner:UNIV OF SCI & TECH OF CHINA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com