Preparation method of 2-phenylpropionic acid
A technology of phenylpropionic acid and phenylpropanil, applied in the chemical field, can solve the problems of difficult conditions, complicated preparation steps, high control and high cost, and achieve the effect of simple equipment and simplified process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
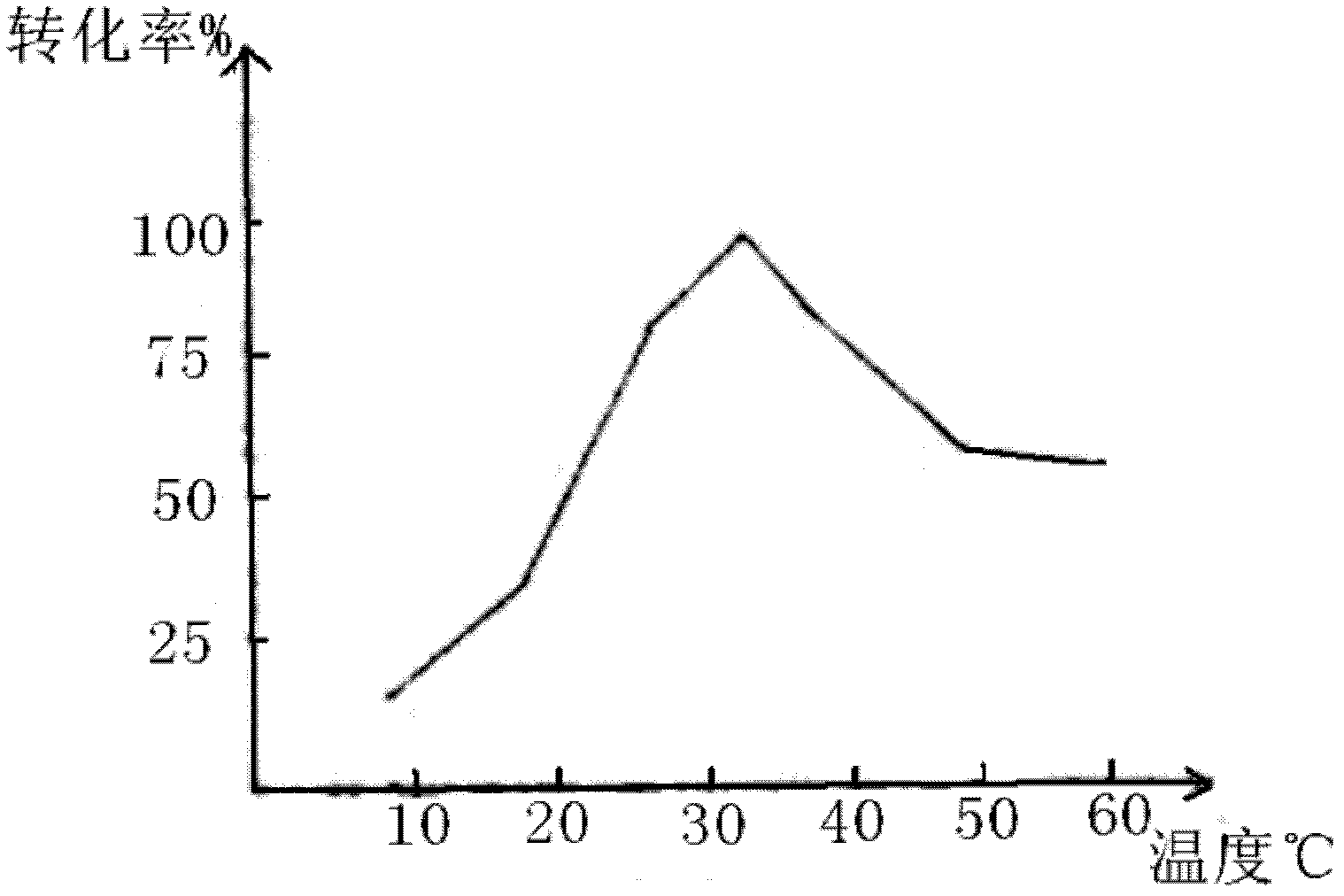
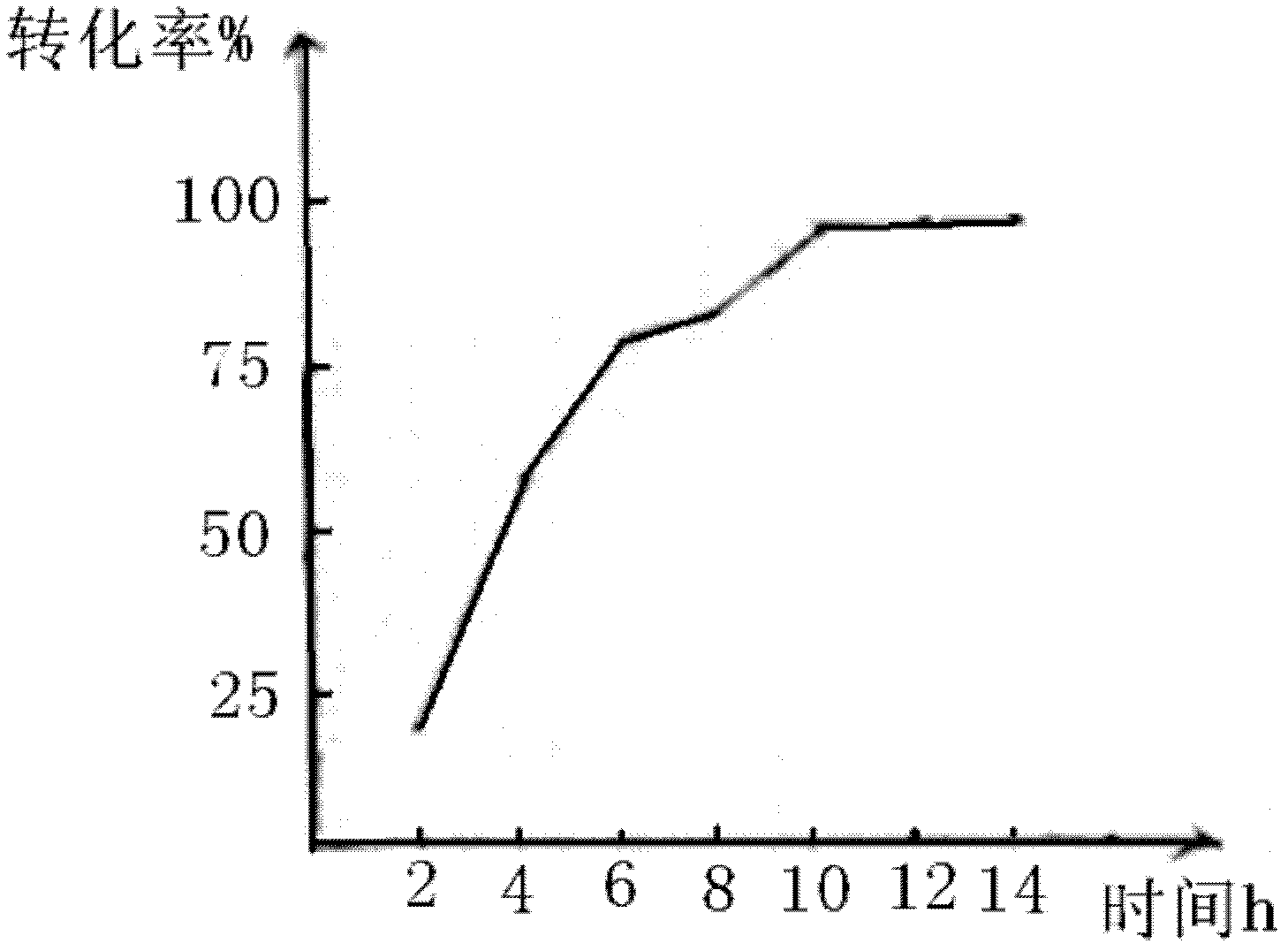
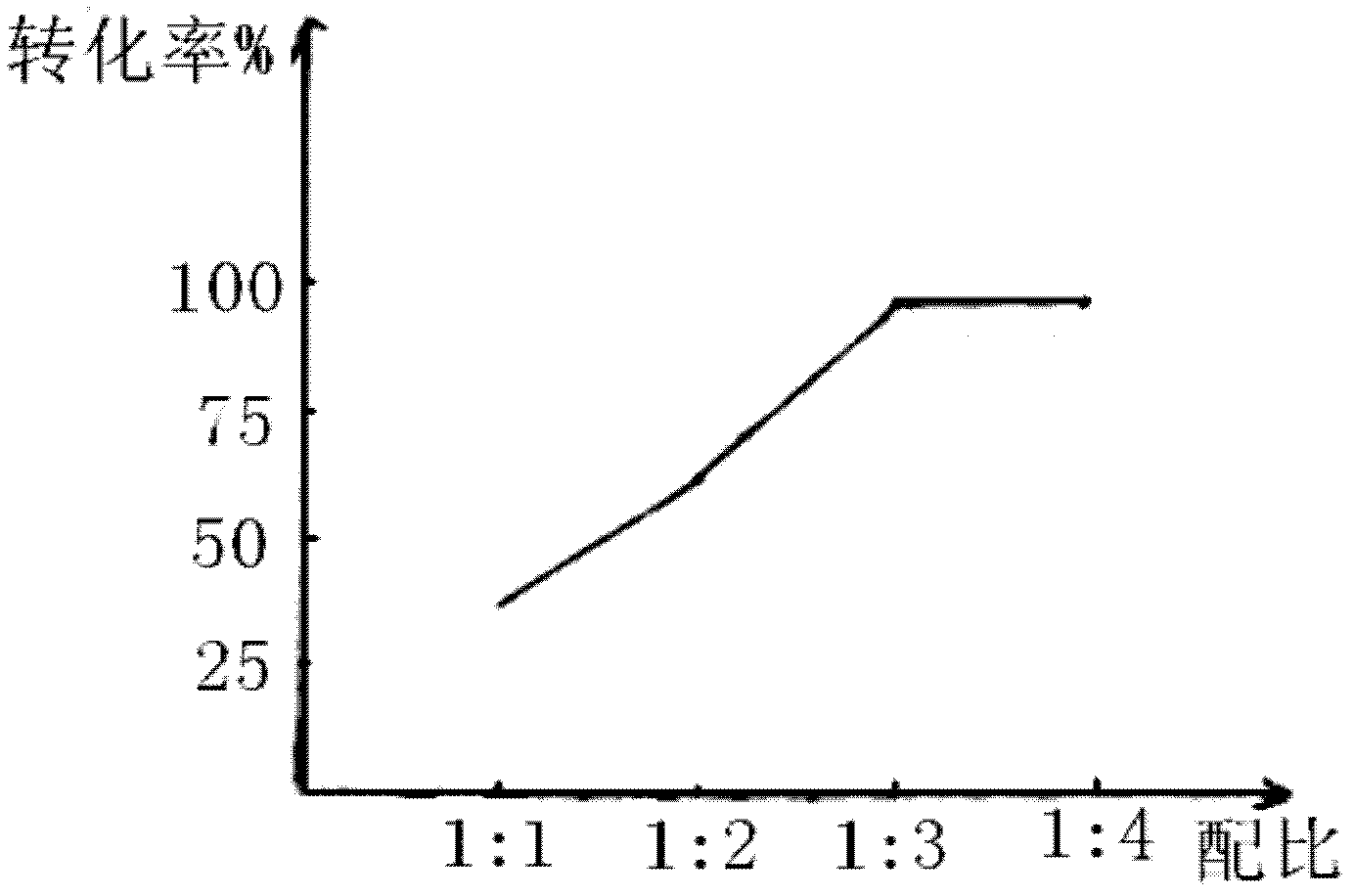
[0026] In a 500ml reaction bottle, put 100g of styrene and 300g of hydrochloric acid solution with a mass concentration of 20%. At a temperature of 35-37°C, stir and react for 10 hours. After drying with sodium thiosulfate for 2 hours, the concentration of the oil layer was weighed to be 97.8%, and the content of 2-chloroethylbenzene analyzed by gas chromatography was 98.67%. Styrene conversion was 95%.
[0027] Gas chromatography: column shape: SE-54; column temperature: 170°C; vaporization chamber: 240°C; detection chamber: 260°C; column front pressure: 0.08MPA.
Embodiment 2
[0029] In a 1000ml reaction bottle, put 150g of styrene and 450g of hydrochloric acid with a mass concentration of 25%, and stir and react at a temperature of 34 to 36°C for 10 hours, then leave to separate layers, wash the oil layer with conventional water, dry and weigh 151.6g, 2 - Chloroethylbenzene content is 98.4%. Styrene conversion was 92%.
[0030] Gas chromatography: column shape: SE-54; column temperature: 170°C; vaporization chamber: 240°C; detection chamber: 260°C; column front pressure: 0.08MPA.
Embodiment 3
[0032] In a 500ml reaction bottle, put 100g of styrene and 400g of hydrochloric acid with a concentration of 30% at a temperature of 35-37°C. After stirring and reacting for 8 hours, the layers were left to stand, the oil layer was washed with conventional water, and the layers were refined. After drying with sodium sulfate for 2 hours, the concentration of the oil layer was weighed to be 97%, and the content of 2-chloroethylbenzene analyzed by gas chromatography was 98%. Styrene conversion was 92%.
[0033] Gas chromatography: column shape: SE-54; column temperature: 170°C; vaporization chamber: 240°C; detection chamber: 260°C; column front pressure: 0.08MPA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com