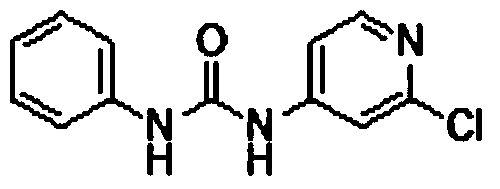
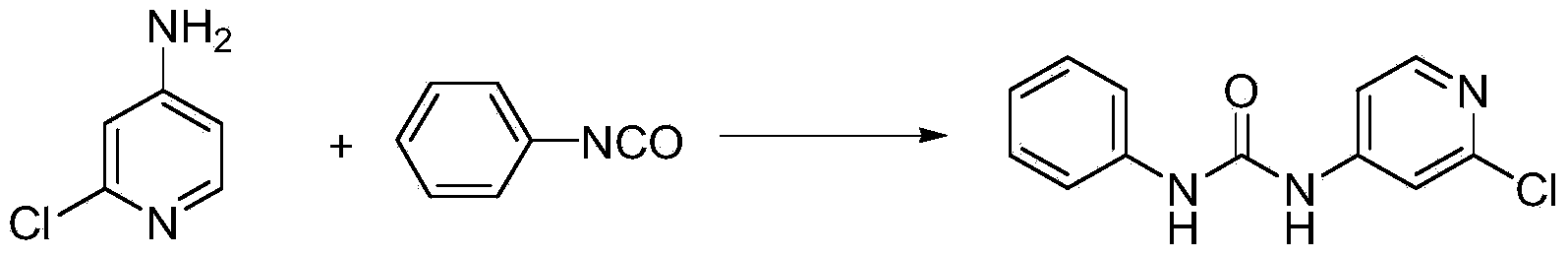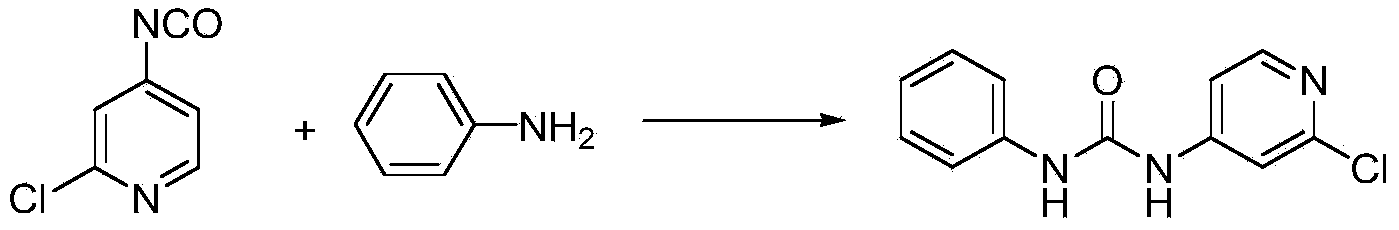Preparation method of 1-(2-chloro-4-pyridyl)-3-phenylurea
A pyridyl phenyl urea and pyridyl technology, applied in the field of preparation of crop growth regulators, can solve the problems of difficult industrialized production, difficult to obtain raw materials, difficult product purity and the like, and achieve easy removal, short preparation period and high product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Its specific preparation method steps are as follows:
[0035] (1) Using aniline and phenyl chloroformate as raw materials, potassium carbonate, sodium carbonate, triethylamine, pyridine, N,N-dimethylaniline, 4-dimethylaminopyridine (DMAP), N,N-di Isopropylethylamine, 1,4-diazabicyclo[2.2.2]octane (DABCO), substituted pyridine and other inorganic bases or organic bases are used as an acid agent, with toluene, xylene, chlorine Benzene, tetrahydrofuran (THF), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), dioxane, acetonitrile, ethylene glycol dimethyl ether, ethylene glycol diethyl ether, acetic acid One of ethyl ester, acetone, methyl ethyl ketone, dichloromethane, and chloroform is used as solvent A; dissolve aniline and acid agent in solvent A, and then add phenyl chloroformate, at -5-80 ° C, preferably Stir the reaction at a temperature of 0-60°C, monitor the completion of the reaction by liquid phase tracking, distill a part of the solvent under reduced pr...
Synthetic example 1
[0039] (1) Dissolve 28.1g of aniline and 33.5g of triethylamine in 400mL of butanone, then add 51.5g of phenyl chloroformate, stir the reaction at 40°C, monitor the completion of the reaction by liquid phase tracking, and distill under reduced pressure Part of the solvent was removed, and a white solid was precipitated, which was filtered to obtain 62.7 g of phenyl aniline carbamate, with a content of 99%.
[0040] (2) Add 25.6g of 2-chloro-4-aminopyridine, 43g of phenyl anilate, and 2.1g of N,N-diisopropylethylamine into 300mL of THF solution, and after the reflux reaction is complete , After THF was distilled under reduced pressure, an appropriate amount of water was added and stirred for 0.5 hours, and filtered under reduced pressure to obtain 47.9 g of white solid CPPU with a content of 98.8%.
Synthetic example 2
[0042] (1) Dissolve 28.1g of aniline and 26.3g of pyridine in 400mL of toluene, then add 51.5g of phenyl chloroformate, stir the reaction at 30°C, monitor the completion of the reaction by liquid phase tracking, and distill a part of the solvent under reduced pressure. A white solid was precipitated and filtered to obtain 62.8 g of phenyl 2-chloro-pyridine-4-carbamate, with a content of 99.1%.
[0043](2) Add 25.6g of 2-chloro-4-amino-pyridine, 43g of phenyl anilate, and 2.4g of DMAP into 300Ml of DMF solution, stir the reaction at 80°C, and add after the reaction is complete Appropriate amount of water precipitated solid, and filtered under reduced pressure to obtain 48 g of white solid CPPU, with a content of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com