Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81 results about "Thiazepine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
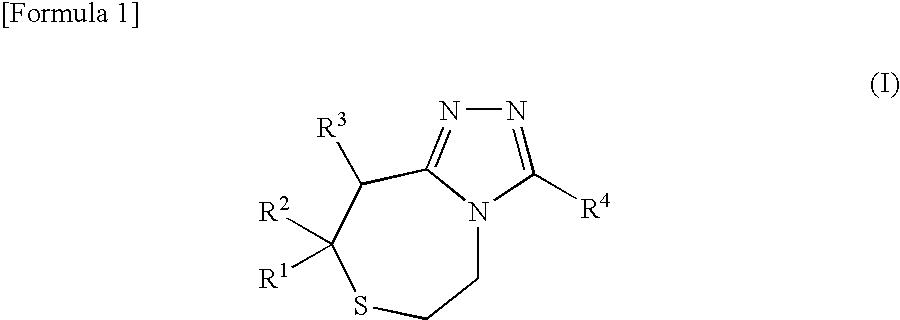
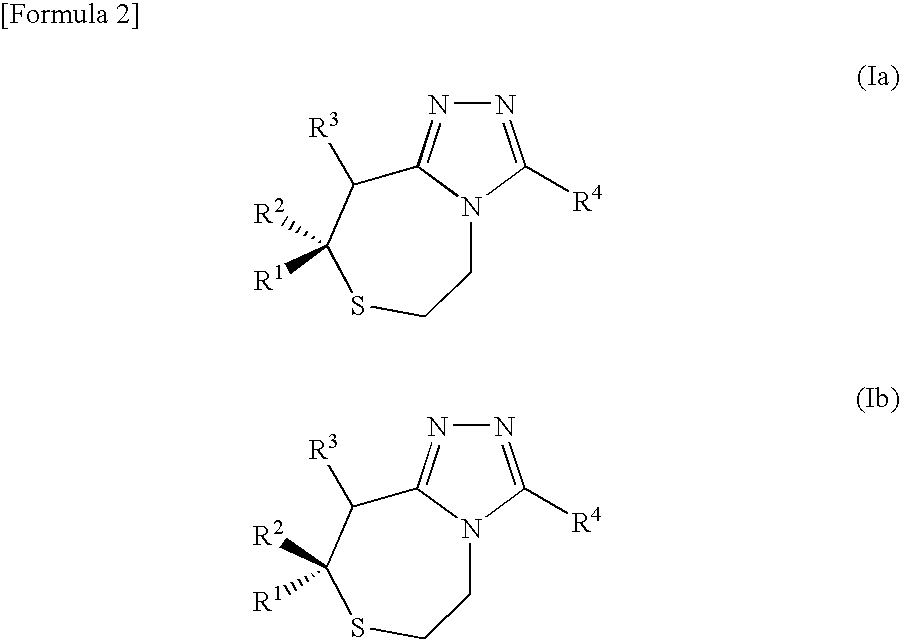
Thiazepines are substituted thiepins, with a nitrogen replacing a carbon in the seven-membered heterocyclic compound. Depending on the location of the nitrogen, one distinguishes 1,3-thiazepine and 1,4-thiazepine.
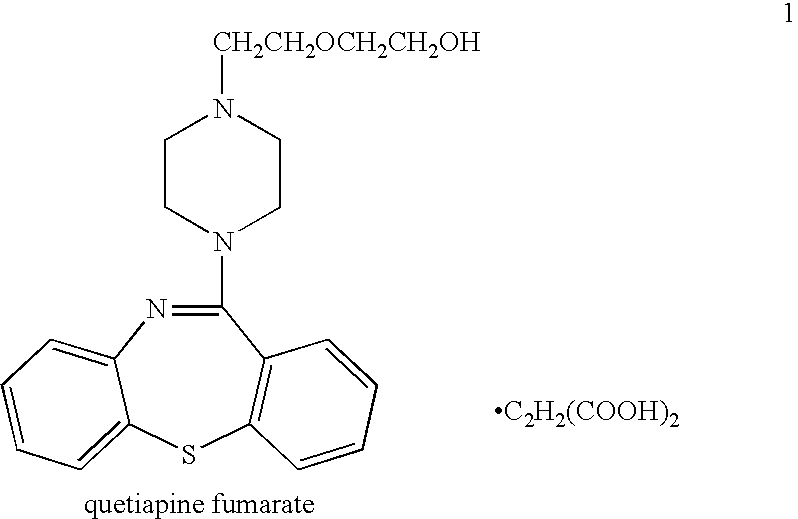
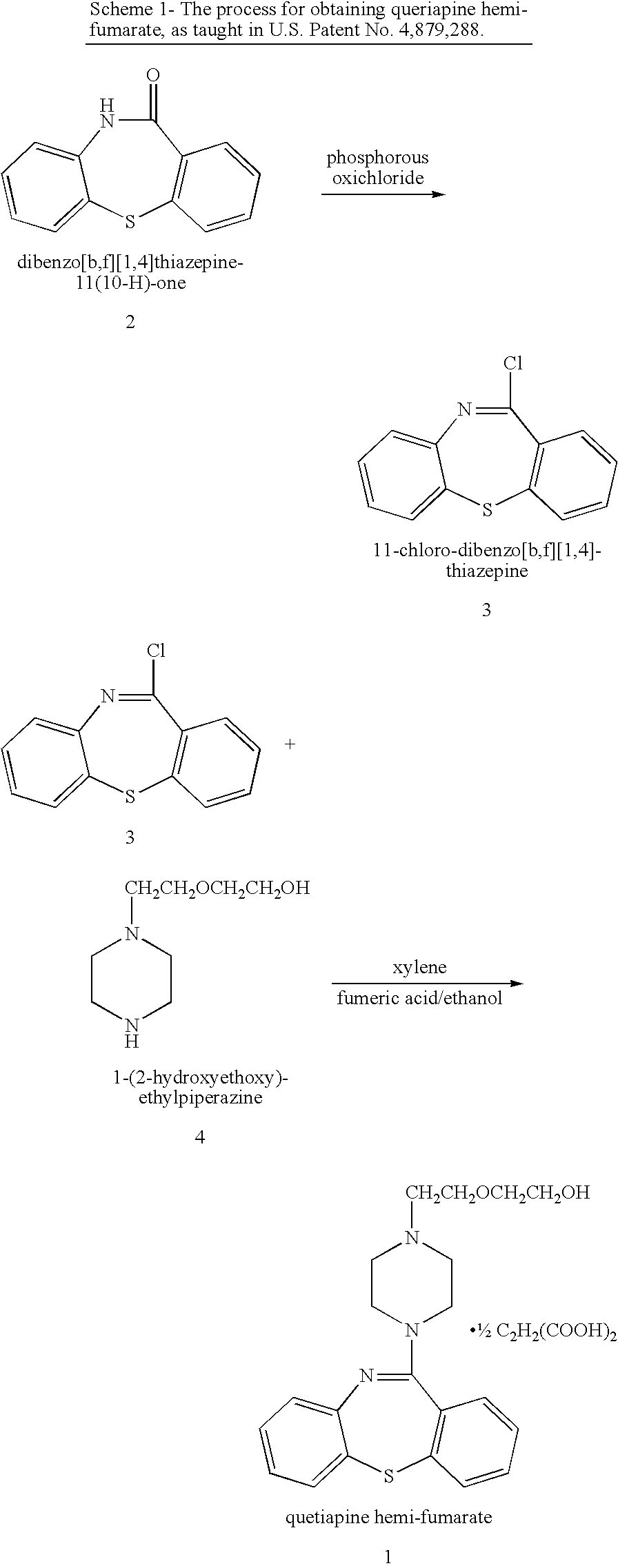
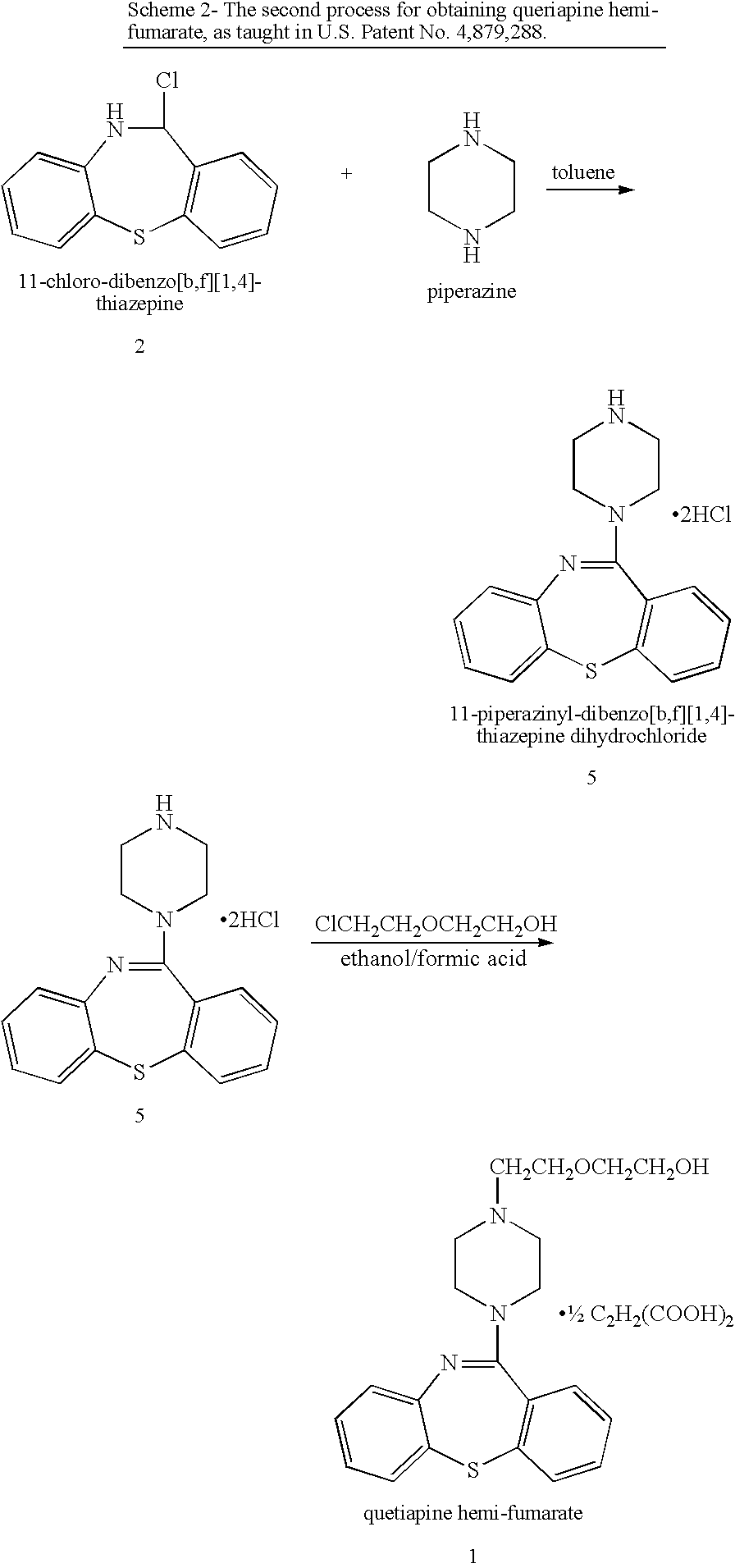
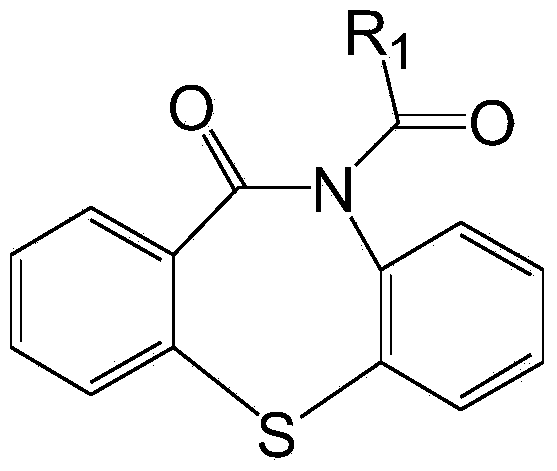
Processes for preparing quetiapine and salts thereof
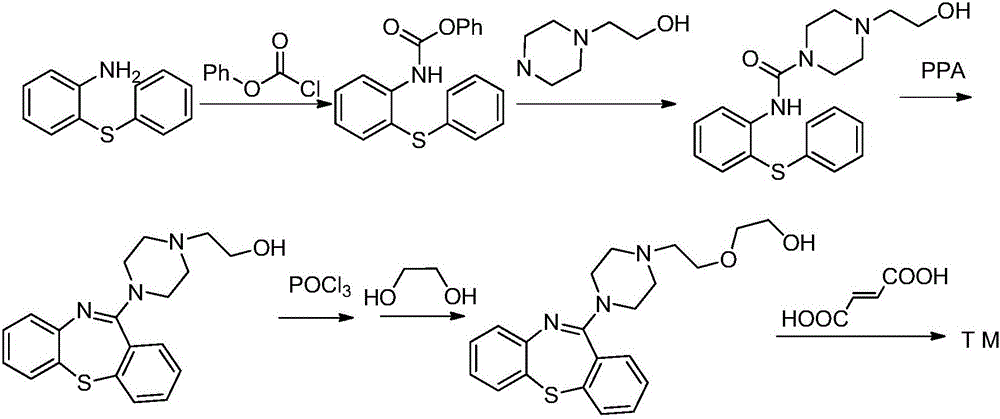
The present invention provides herein a two-step process for preparing pharmaceutically pure quetiapine and salts thereof by obtaining the starting material 11-chloro-dibenzo-thiazepine followed by reacting the 11-chloro-dibenzo-thiazepine with 1-(2-hydroxyethoxy)ethylpiperazine, or its salt, in the presence of an inorganic or organic base in an organic solvent or in a two-phase solvent system. The present invention provides also a novel, one-pot reaction process for preparing pharmaceutically pure quetiapine and salts thereof. The two processes provided herein can be easily, conveniently and inexpensively scaled-up.
Owner:CHEMAGIS
Process for the preparation of 11-amino-3-chloro-6, 11-dihydro-5, 5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine
Owner:LES LAB SERVIER
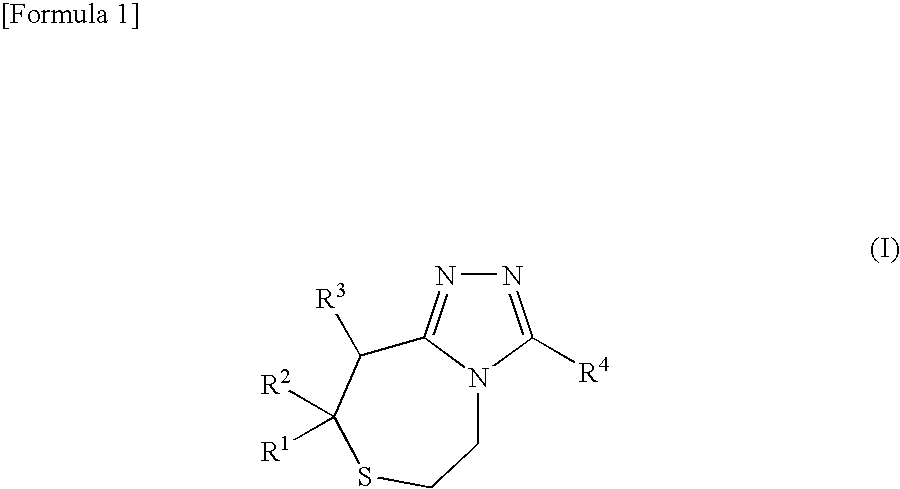
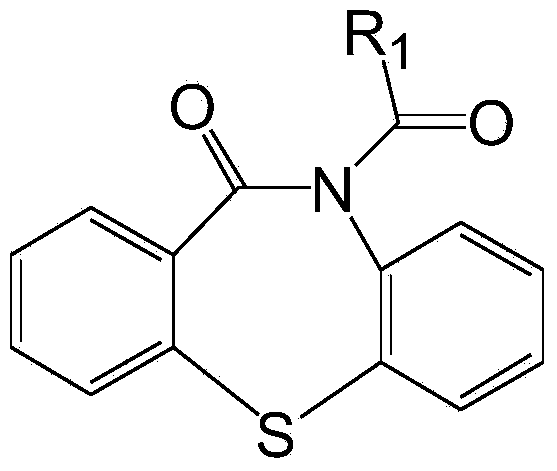
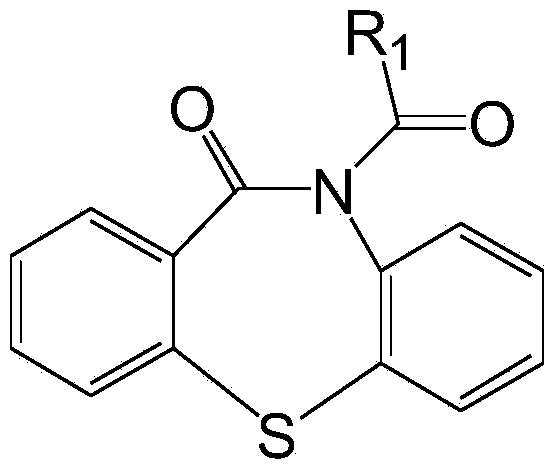
Substituted 1, 4-thiazepine and analogs as activators of caspases and inducers of apoptosis and the use thereof
The present invention is directed to substituted 1,4-thiazepine and analogs thereof, represented by the general Formula I: wherein the dashed lines, A1, A2, A3, X1 and R1 are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of capases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CELERA CORPORATION +2
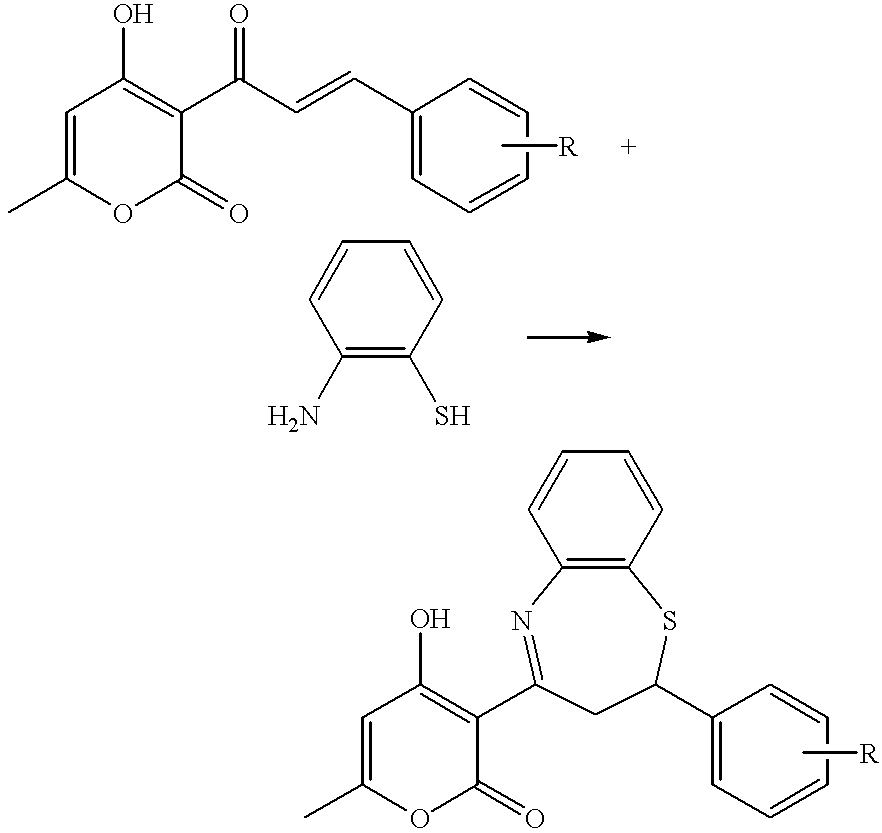
3,4-dihydrobenzo[f][1,4]thiazepine compound or salts of same and medical use of same
InactiveCN101891706AInhibitory activityOrganic active ingredientsNervous disorderDiseaseDiabetes mellitus
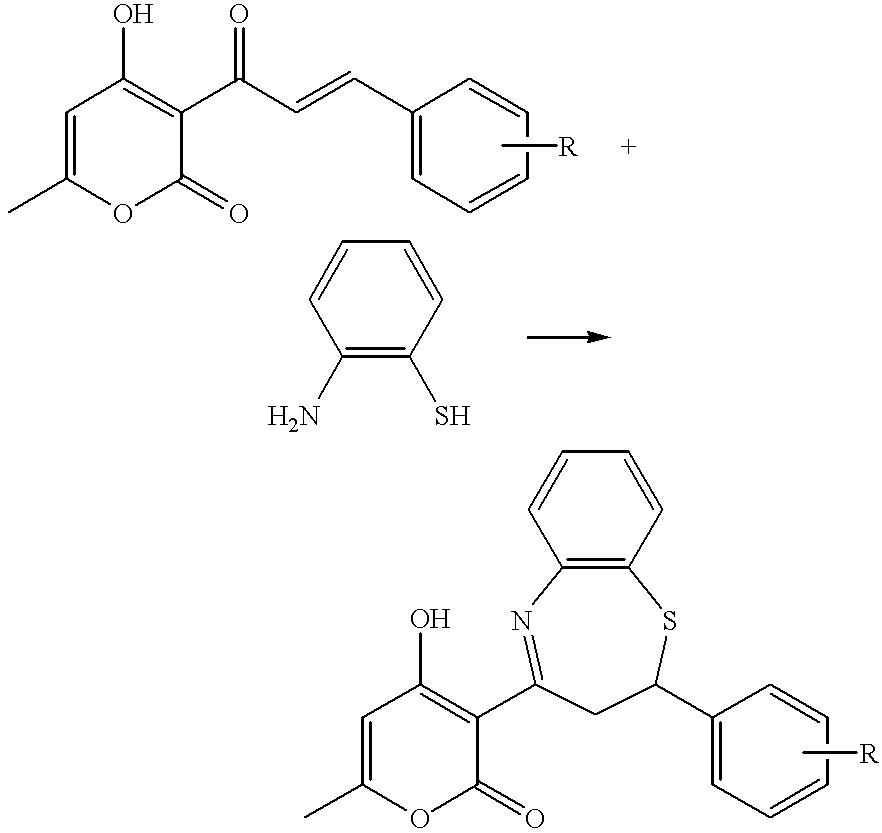
The invention relates to 3,4-dihydrobenzo[f][1,4]thiazepine compound of the general formula I or salts of the same and medicinal use of the same, belonging to the field of pharmaceutical chemistry. The 3,4-dihydrobenzo[f][1,4]thiazepine compound can inhibit glycogen synthase kinase-3beta (GSK-3beta) and be used as the non-ATP competitive micromolecular inhibitor of the GSK-3beta to prepare medicaments used for preventing or treating diseases related to the GSK-3beta. The invention further discloses the application of a medical combination formed by the compound and pharmaceutically acceptablesalts of the same to preparation of medicaments for preventing or treating the diseases related to the GSK-3beta, such as diabetes, Alzheimer's diseases and tumors.
Owner:FUDAN UNIV
Thiazepine derivative
InactiveUS20100004221A1Inhibition effectInhibitionBiocideOrganic active ingredientsArylHydrogen atom
Thiazepine derivative or a pharmacologically acceptable salt thereof having an effect of inhibiting 11β-hydroxysteroid dehydrogenase type 1 and having a formula (1):wherein in one embodiment,R1 represents a hydrogen atom, a C1-C6 alkyl group; R2 represents a C1-C6 alkyl group, a C1-C6 halogenated alkyl group, a C1-C6 hydroxyalkyl group; R1 represents a hydrogen atom or a C1-C6 alkyl group; R4 represents a C6-C10 aryl group that may be substituted with 1 to 5 group(s) independently selected from Substituent Group a or a heterocyclic group that may be substituted with 1 to 3 group(s) independently selected from Substituent Group a; Substituent Group a consists of a halogen atom, a C1-C6 alkyl group, a C6-C10 aryl group that may be substituted with 1 to 5 group(s) independently selected from Substituent Group b; Substituent Group b consists of a halogen atom, a C1-C6 alkyl group, and a C1-C6 halogenated alkyl group.
Owner:DAIICHI SANKYO CO LTD
Method for preparing pharmaceutical pure quetiapine fumarate
ActiveCN101190902ASolve technical problems with strong side reactionsSolve technical problems of side reactionsOrganic chemistryDimethylaniline N-oxideThiazepine
The invention relates to a preparation method of pure quetiapine fumarate and the steps of the process thereof are that: (1)chlorination: four materials of dibenzo(b,f)(1,4)thiazepine-11-(10H)-ketone, chlorinated oxidant, N,N-Dimethylaniline and toluol react to obtain toluol solution of chloride; (2) addition and salification: the toluol solution of chloride reacts to obtain quetiapine according to the ratio of 1:0.5-1:0.5-0.8 of cyclic product (kg) to N-(2-(2-Hydroxyethoxy)ethyl)piperazine (kg) to anhydrous sodium carbonate; the mixture of the quetiapine (kg) and fumaric acid (kg) and ethanol (kg) is then salified to obtain the quetiapine fumarate according to the respective ratio 1:0.1-0.3:2-4. The invention effectively controls the impurity content of the quetiapine fumarate below 0.1 percent, improves the quality of products and achieves the standard of pharmaceutical purity and causes little side effects to patients. In addition, the invention also solves the problems of recovery of phosphorus oxychloride and environment pollution, shortens the cycle of reaction, improves the yield and reduces the cost.
Owner:HUNAN DONGTING PHARMA
Synthesis method of tianeptine sodium
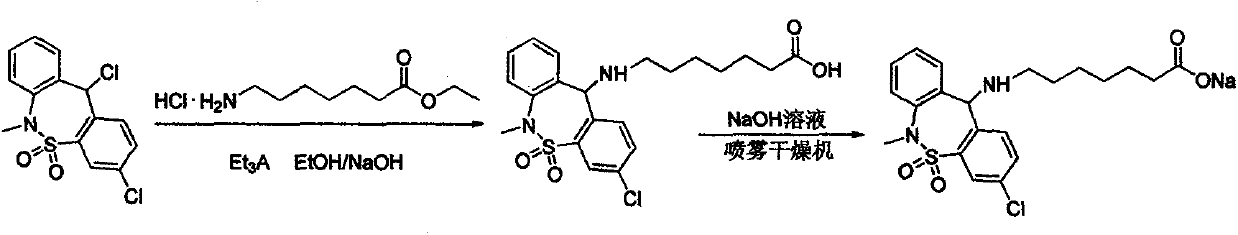
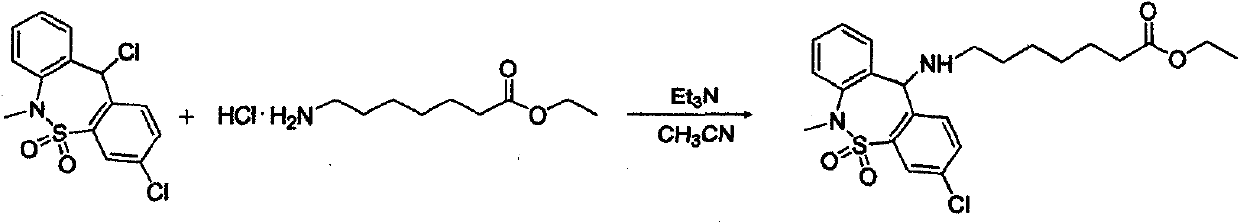
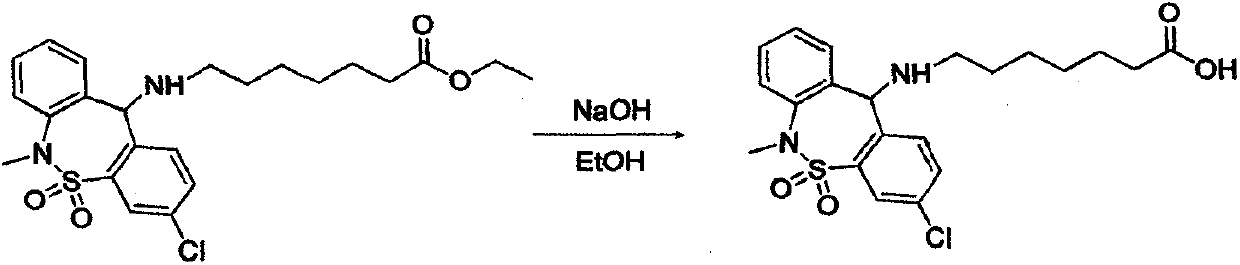
The invention relates to a synthesis method of the medicine of tianeptine sodium. The conventional salifying method comprises the following steps: hydrolyzed tianeptine acid and sodium hydroxide aqueous solution are dissolved and then subjected to freeze drying to form salt. According to the conventional salifying method, a product with higher purity and yield can be obtained, but the cost of the product is higher. The invention aims to develop a novel synthesis method of tianeptine sodium. The novel synthesis method comprises the following steps: step 1, 3,11-dichloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2]thiazepine-5,5-dioxide is taken as starting material, subjected to an condensation reaction, hydration and spray drying to form salt so as to synthesize the tianeptine sodium, which is the preparation process of 7-[(3-chloro-6,11-dihydro-5,5-dioxo-6-methyldibenzo(c, f) (1,2) thiazole-11 group) amino] ethyl enanthate; step 2, tianeptine acid is prepared; step 3, the tianeptine sodium is prepared. The synthesis method has the advantages that the technological process is simplified, the salifying process is efficient and environment-friendly, and the product yield and purity are improved.
Owner:陕西方舟制药有限公司
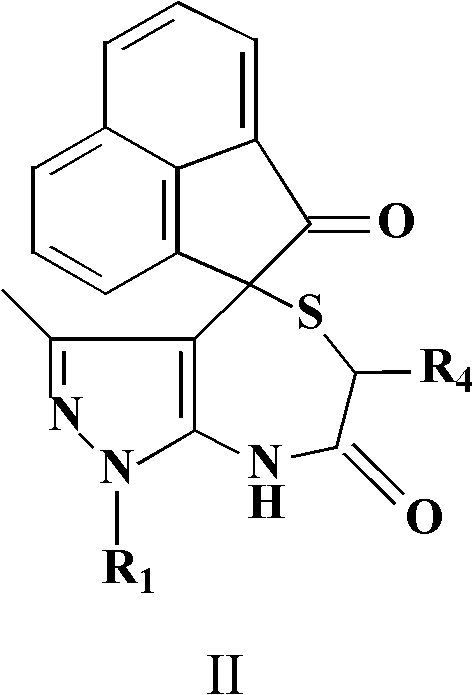
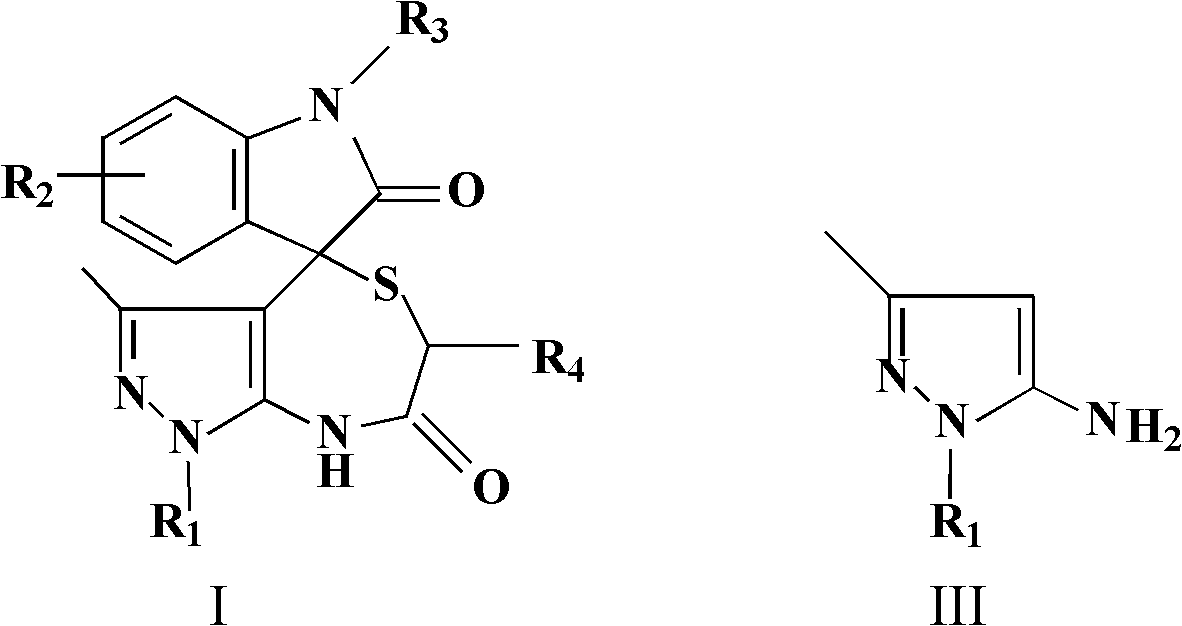
Spiro-heterocyclic compound containing indole structures and preparation method of spiro-heterocyclic compound
InactiveCN102584860ABiologically activePharmacologically activeOrganic chemistryAcenaphthyleneOrganic acid
The invention relates to the field of pharmaceutical chemistry, and discloses a spiro-heterocyclic compound containing indole structures and a preparation method of the spiro-heterocyclic compound. The spiro-heterocyclic compound containing the indole structures comprises a dihydro-spiro[indole-3,4'-pyrazolo[3,4-e][1,4]thiazepine] diketones compound and a dihydro-spiro[acenaphthylene-1,4'-pyrazolo[3,4-e][1,4]thiazepine] diketones compound, and adopts the structure which is shown in a formula I and a formula II, wherein R1 is -CH3 or -Ph, R2 is -H, -CH3, -F, -Cl or -Br, R3 is -H or -CH3, and R4 is -H or -CH3; in addition, the preparation method comprises the steps as follows: isatin or acenaphthylenedione, a 5-aminopyrazole compound and mercapto carboxylic acid are dissolved in a solvent, organic acid or mineral acid is taken as catalytic agents, and reaction is performed for 8 to 24 hours at the temperature ranging from 65 to 95 DEG C. The spiro-heterocyclic compound has stable properties, the preparation method is simple to operate, the productivity of reaction products is high, and the post-processing is simple.
Owner:SUZHOU UNIV
Oral modified-release formulations containing thiazepines
InactiveUS20110052648A1High feasibilitySimple processBiocideOrganic active ingredientsControlled releaseThiazepine
An oral modified-release formulation using Quetiapine or pharmaceutically acceptable salts thereof as an active ingredient, while avoiding the use of a gelling material. As used herein, the term “modified release” includes but is not limited to one or more of controlled release, sustained release, prolonged release and extended release.
Owner:AVRAMOFF AVI +3
Substituted 1, 4-thiazepine and analogs as activators of caspases and inducers of apoptosis and the use thereof
The present invention is directed to substituted 1,4-thiazepine and analogs thereof, represented by the general Formula I: wherein the dashed lines, A1, A2, A3, X1 and R1 are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of capases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CELERA CORPORATION +2
Process for the preparation of 11-amino-3-chloro-6,11-dihydro-5,5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine
Owner:LES LAB SERVIER
Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine
InactiveUS20070225494A1MinimizationImprove productivityNervous disorderOrganic chemistryOrganic solventThiazepine
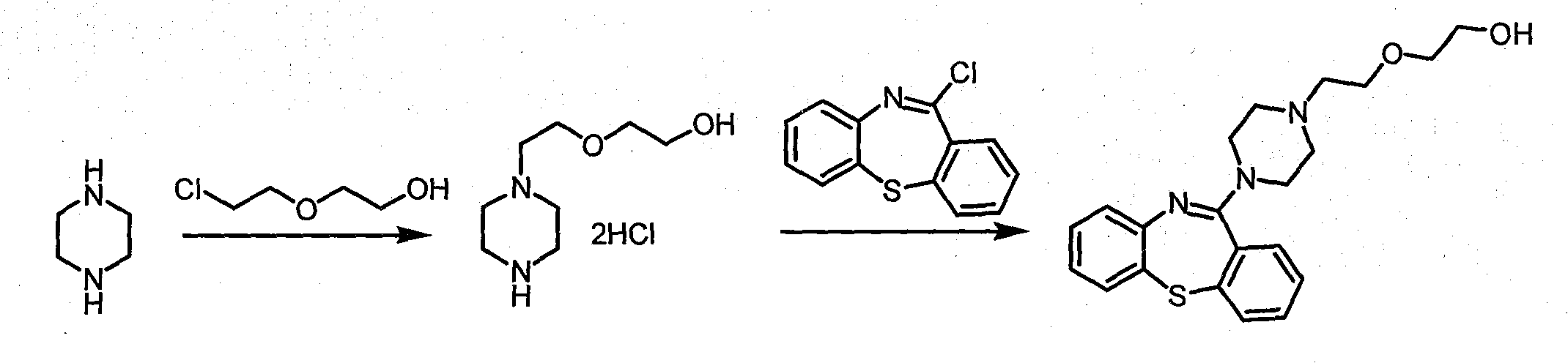
Disclosed is a process for the preparation of 11-(4-[2-(2-hydroxyethoxy)ethyl]-1-piperazinyl)-dibenzo[b,f][1,4]thiazepine. In the process, low-priced 2,2′-dithiosalicylic acid as starting material is subjected to bond formation reaction with 1-chloro-2-nitrobenzene in a basic aqueous solution, a nitro group reduction reaction is conducted, cyclization and chlorination reactions are simultaneously carried out in the presence of a equivalent amount of halogenating agent, a reaction with piperazine is continuously conducted without separation, and a reaction with 2-haloethoxyethanol is conducted, thereby it is possible to economically producing Quetiapine, that is, 11-(4-[2-(2-hydroxyethoxy)ethyl]-1-piperazinyl)-dibenzo[b,f][1,4]thiazepine, in an environmentally friendly manner. Particularly, the process is advantageous in that economic efficiency is assured because of use of the low-priced starting material, use of an organic solvent is minimized because a reaction is conducted in an aqueous solution, and it is possible to achieve the environmentally friendly and economical process having high commercial usefulness because the number of reaction steps of the process is reduced and because generation of acidic waste is minimized.
Owner:SK BIOTEK
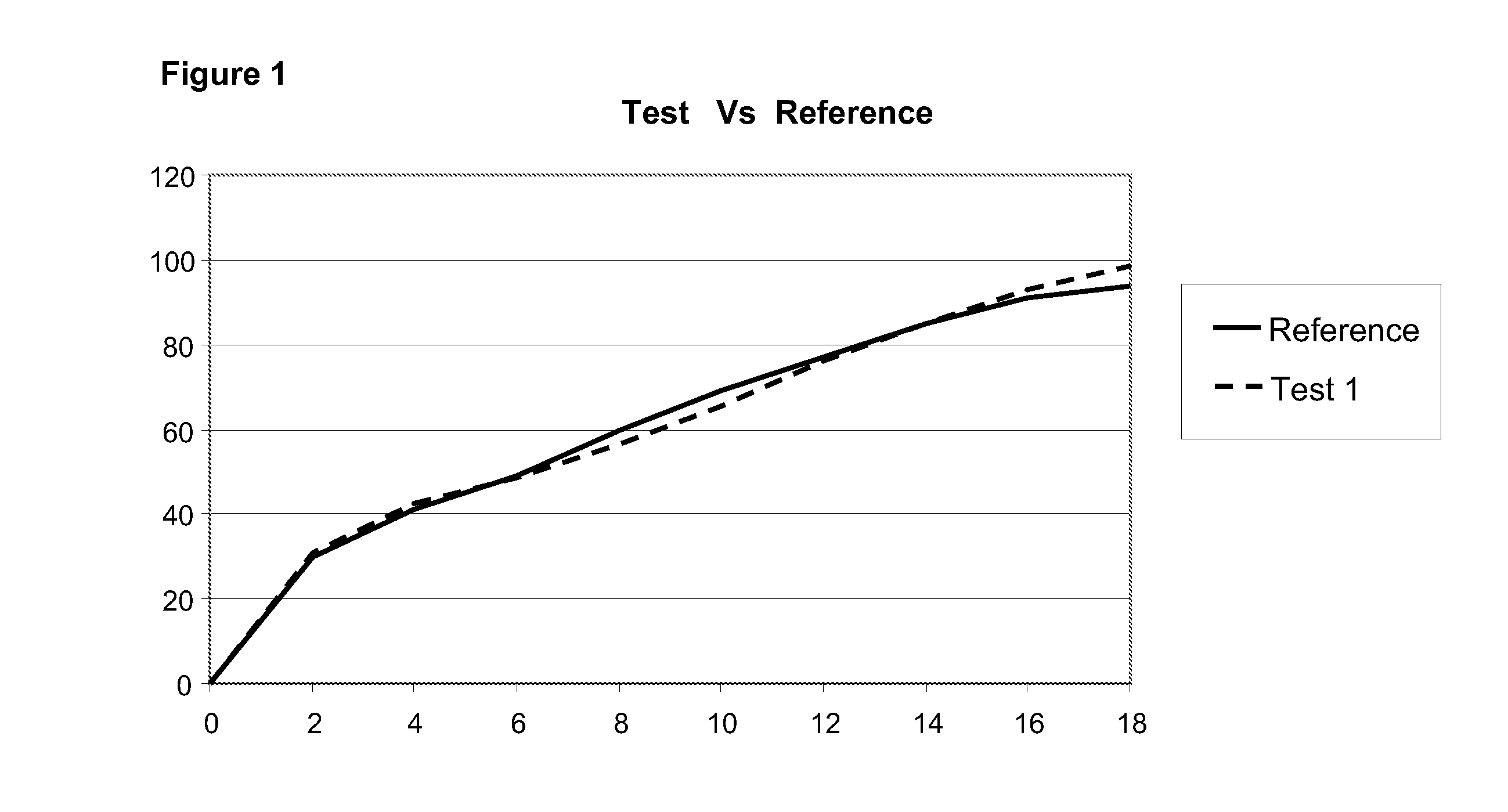
Pharmaceutical Compositions
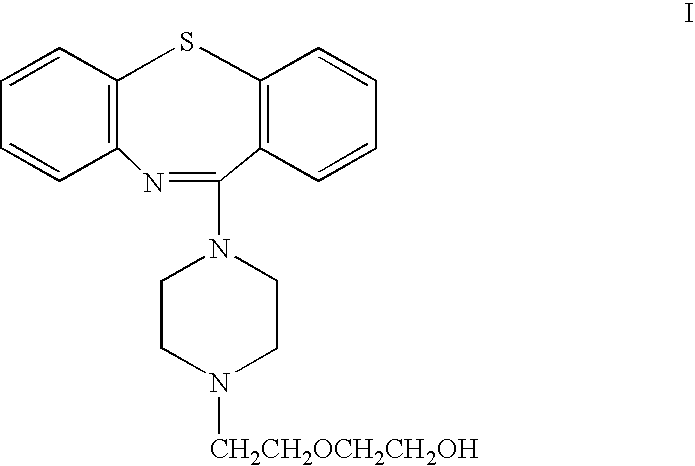
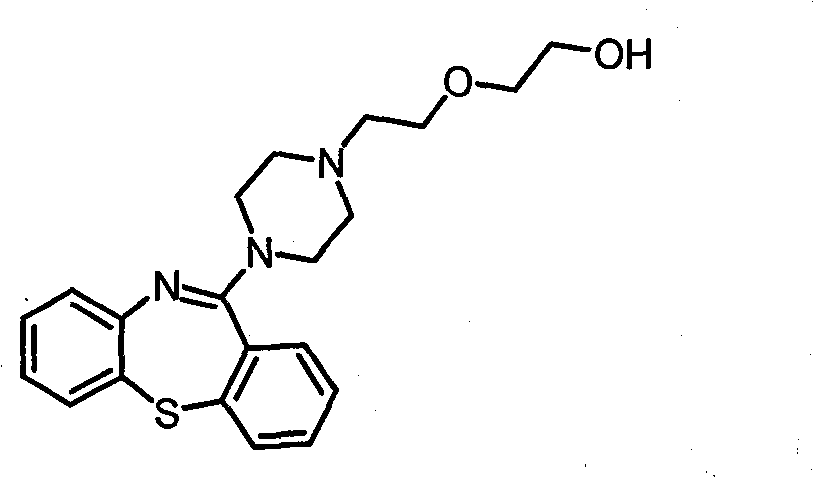
The present invention provides methods of treatment with a pharmaceutical composition, more particularly a sustained release pharmaceutical composition, comprising 11-[4-[2-(2-hydroxyethoxy)ethyl]-1-piperazinyl]dibenzo-[b,f][1,4]thiazepine or a pharmaceutically acceptable salt thereof, as well as new and improved methods for treating a variety of psychological disorders and conditions including, but not limited to, Mood Disorders and Anxiety Disorders and for treating one or more of the symptoms of these disorders.
Owner:ASTRAZENECA AB
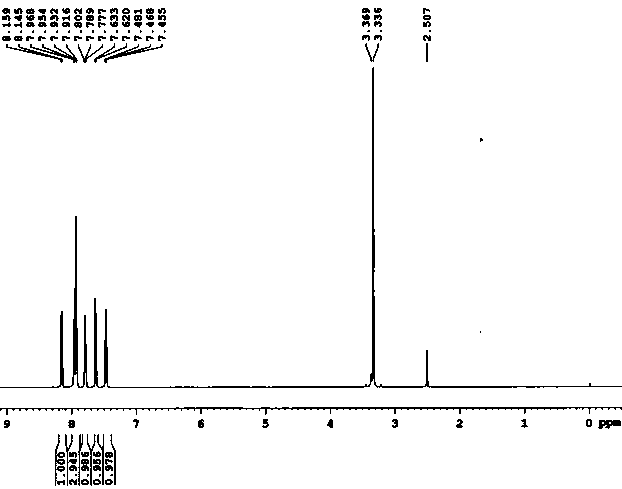
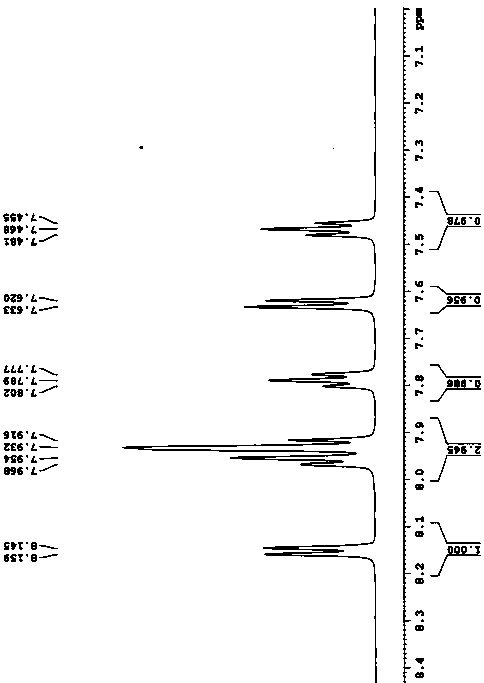
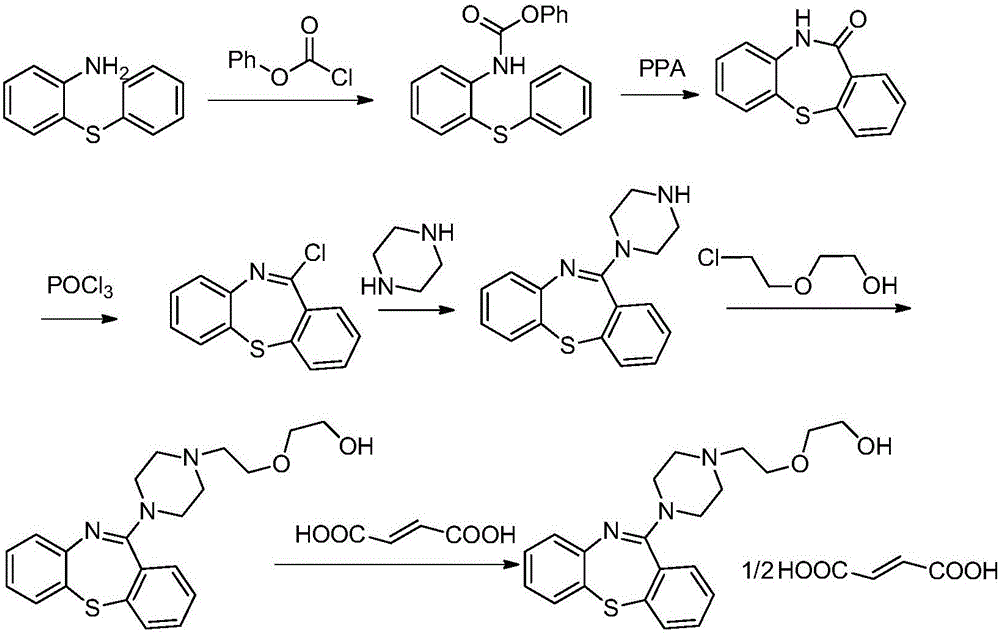
Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine
InactiveUS20070293471A1Short timeHigh yieldBiocideOrganic active ingredientsDibenzothiazepineThiazepine
Owner:IPCA LAB LTD
Substituted piperazines of azepines, oxazepines and thiazepines
InactiveUS20060270656A1Improved adverse event profileGood dopamine D bindingBiocideNervous disorderThiazepineHalogen
Described herein are antipyschotic compounds of formula (I) wherein: is an optionally benzo-fused five or six member aromatic ring having zero to three hetero atoms independently selected from N, O, and S; R1 is hydrogen, (C1-6) fluoroalkyl, (C3-6) cycloalkyl, or (C1-4) alkyl, wherein the (C1-4) alkyl is unsubstituted or substituted with hydroxy, methoxy, ethoxy, OCH2CH2OH, —CN, imidazolidin-2-one, phenyl, or tetrazole wherein tetrazole is unsubstituted or substituted with (C1-4) alkyl; R2 is H, halogen, (C1-6) fluoroalkyl, (C3-6) cycloalkyl, OR6, SR6, NO2, CN, COR6, C(O)OR6, C(OH)R6, CONR7R8, phenyl or (C1-6) alkyl, wherein the (C1-6) alkyl is unsubstituted or substituted with a hydroxy; R3 is hydrogen, (C1-6)fluoroalkyl , (C3-6) cycloalkyl, (C2-6) alkenyl, phenyl, monocyclic heteroaromatic, bicyclic heteroaromatic, or (C1-4)alkyl wherein (C1-4) alkyl is unsubstituted or substituted with a phenyl; R4 and R5 are independently selected from hydrogen, halogen, (C1-6) alkyl, (C1-6) fluoroalkyl, OR9, SR9, NO2, CN, or COR9; R6 is hydrogen, (C1-6) fluoroalkyl, or (C1-6) alkyl; R7 and R8 are independently hydrogen, or (C1-6) alkyl; R9 is hydrogen, (C1-6) fluoroalkyl, (C1-6) alkyl; Alk is (C1-4) alkylene unsubstituted or substituted with a hydroxy; Y is oxygen, sulfur, SO2, or a bond; X is CH2, C═O, S, O, or SO2; Z is hydrogen, halogen, (C1-6) alkyl, (C1-6)fluoroalkyl, —OH, (C1-6) alkoxy, (C1-6) fluoroalkoxy, (C1-6) alkylthio, (C1-6) acyl, (C1-4)alkylsulfonyl, —OCF3, —NO2, —CN, carboxamido which may be substituted on the nitrogen by one or two (C1-4) alkyl groups, and —NH2 in which one of the hydrogens may be replaced by a (C1-4) alkyl group and the other hydrogen may be replaced by either a (C1-4) alkyl group, a (C1-6) acyl group, or a (C1-4) alkylsulfonyl group; the phenyl of R1, R2 or R3 is independently unsubstituted or substituted with one to three substituents independently selected from Z; the monocyclic heteroaromatic of R3 is unsubstituted or substituted with one to three substituents independently selected from Z; the bicyclic heteroaromatic of R3 is unsubstituted or substituted with one to three substituents independently selected from Z; and salts, solvates, and crystal forms thereof. Also described are the use of the compounds of formula (I) as antagonists of the dopamine D2 receptor and as agents for the treatment of psychosis and bipolar disorders, and pharmaceutical formulations of the compounds of formula (I).
Owner:ELI LILLY & CO
Preparation of Heteroatom Ligand of Sulfur and Nitrogen and Its Application
ActiveCN103787935BReduce usageThe synthesis steps are simpleOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsThiazepineSulfur
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing tianeptine sodium intermediate
The invention discloses a method for preparing a tianeptine sodium intermediate 3-chloro-6-methyl dibenzo[c, f][1, 2] thiazepine-11-(6H)-ketone 5,5-dioxide. 4-chloro-2-(N-methyl-N-phenyl-sulfamoyl)-methyl benzoate is taken as a raw material for synthesizing the tianeptine sodium intermediate in one step. Compared with the prior art, the synthesis method is simple and convenient and has relatively good economic benefit.
Owner:山东诚汇双达药业有限公司
Novel preparation method of quetiapine
InactiveCN103724294AHigh yieldReduce consumptionCarboxylic acid salt preparationThiazepineQuetiapine
The invention discloses a high-efficient and simple method used for preparing high purity 1-[2-(2-hydroxyethoxy)ethyl]piperazine hydrochloride. The preparation method is used for replacing a method used for preparing quetiapine by reacting a free alkali derivative with 11-chloro-dibenzo[b, f](1, 4)thiazepine. According to the preparation method, low-temperature recrystallization is adopted for purifying 1-[2-(2-hydroxyethoxy)ethyl]piperazine hydrochloride so as to avoid residue of unknown piperazidine impurities in high-temperature purification processes, and high purity quetiapine can be obtained in subsequent reactions.
Owner:WUXI QIANHAO BIOPHARMA
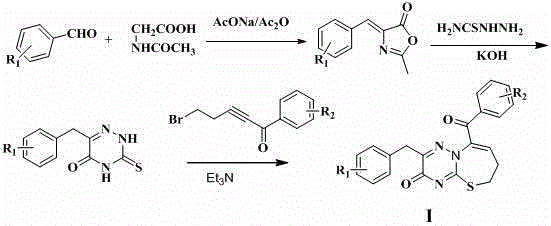
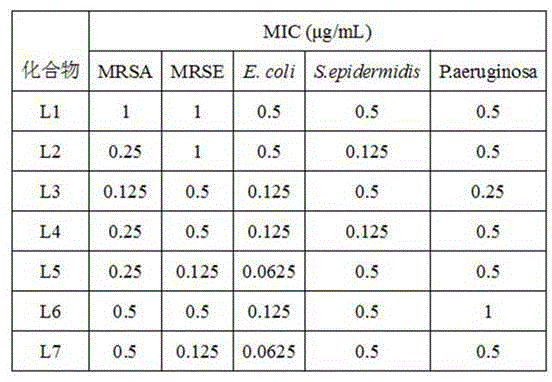
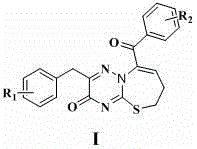
Compound with antibacterial ability as well as preparation method and application thereof
The invention discloses an application of a 2-aryl-9-aryl-6,7-dihydro-3H-[1,2,4]triazine[1,3]thiazepine-3-ketone derivative represented by the general formula I shown in the specification to antibacterial drugs. R1 and R2 are hydrogen, a methoxy group, a nitro group, a methyl group, halogen, a hydroxyl group, an acetyl group, a propionyl group, a benzoyl group, an alkoxy group, an amino alcoxy group and a carbamyl alkoxy group independently. The compound has an obvious inhibition effect on various bacteria such as MRSA (methicillin-resistant staphylococcus aureus), Escherichia coli, pseudomonas aeruginosa and the like and can be applied to preparation of the antibacterial drugs.
Owner:SHIJIAZHUANG UNIVERSITY
Method for increasing proportion of paradichlorobenzene in benzene chlorination product
InactiveCN103739441ARaise the ratioOrganic-compounds/hydrides/coordination-complexes catalystsHalogenated hydrocarbon preparationBenzeneChlorobenzene
The invention discloses a method for increasing a proportion of paradichlorobenzene in a benzene chlorination product. The method comprises the steps: adding a combined catalyst in benzene, introducing chlorine under a stirring condition, to obtain the chlorination product with high paradichlorobenzene content, wherein the temperature of the whole process is controlled to be 50-80 DEG C, and the amount of the introduced chlorine is controlled to ensure that the conversion rate of the benzene is more than 99 percent and the conversion rate of chlorobenzene is more than 93 percent, and in addition, the combined catalyst comprises a main catalyst and a co-catalyst, the main catalyst is one of iron powder, ferric trichloride, aluminum trichloride, antimony trichloride, antimony pentachloride or tin tetrachloride, the co-catalyst is a dibenzo thiazepine organic matter, and the weight ratio of the main catalyst to the co-catalyst is 1:1. According to the method, the proportion of the paradichlorobenzene in the product is effectively increased, the ratio of the paradichlorobenzene to orthodichlorobenzene can reach 4.8, the content of the orthodichlorobenzene is less than 0.4 percent, and the content of phenyl polychloride is less than 1 percent.
Owner:SOUTHEAST UNIV
Thiazepine inhibitors of HIV-1 integrase
The present invention discloses non-catechol compounds, such as thiazolothiazepines, and analogs and derivatives thereof, which are anti-integrase inhibitors. The compounds, which are useful as treatments for HIV disease, include compounds (I), (II), (III), or pharmaceutically acceptable salts thereof wherein A is thiazole, benzene, naphthalene, pyridine, pyrimidine, pyrazine, or quinoline; R is one or more of H, halogen, lower alkyl, lower alkoxy, NO2, lower ester or carboxylic acid; X—Y is CH2—S, S—CH2, CH2—O, CH2—S(O). S(O)—CH2, CH2—CH2, CH2—CH2—CH2, or CH2—CH2—CH2—CH2; R4 is H or hydroxy; R5 is H, phenyl, or alkylamine; W is S or O; and R6 is H, substituted or unsubstituted alkyl or amine; and Z is S, O, CH2, CH2CH2, or C═O.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine
Owner:IPCA LAB LTD
Preparation method of tianeptine sodium intermediate
ActiveCN105254587ALow reaction conditionsReaction conditions require simpleOrganic chemistryChemical synthesisThiazepine
The invention belongs to the technical field of chemical synthesis and particularly relates to a preparation method of a tianeptine sodium intermediate 3,11-dichloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2] thiazepine-5,5-dioxide. The method comprises steps as follows: 3-chloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2] thiazepine-11-alcohol-5,5-dioxide is added to an organic solvent, a chloro-substituted reagent is added, the mixture is heated to have a reflux reaction, cooled to the room temperature after the reaction is finished, filtered and dried, and then the tianeptine sodium intermediate 3,11-dichloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2] thiazepine-5,5-dioxide is prepared. According to the method, industrial hydrochloric acid commonly used on the market is used as the chloro-substituted reagent, requirements for a reaction condition are not high, requirements for equipment are low, the production process is safe, controllable and environment-friendly, the industrial hydrochloric acid raw material is low in price and easy to obtain, the cost is reduced, and the method is more suitable for industrial production.
Owner:山东诚汇双达药业有限公司
Quetiapine synthesizing method
ActiveCN105859653ASuitable for mass productionPreparation cost Raw materials are cheap and easy to getOrganic chemistryBeckmann rearrangementThiazepine
The invention discloses a quetiapine synthesizing method. O-chlorobenzoic acid with the low price is adopted as a starting material to react with thiophenol, and then ring closure is performed to obtain thioxanthone. Hydroxyl amination and Beckmann rearrangement are performed to obtain a key intermediate dibenzo[b,f][1,4]thiazepines-11-(10H)one, chlorination is performed, then, a reaction is performed on 1-(2-hydroxyethoxy)ethylpiperazine with the existence of acid-binding agent to obtain quetiapine, and the quetiapine and fumaric acid form a salt in an absolute ethyl alcohol system to obtain a product. According to the quetiapine synthesizing method, raw materials are low in price and easy to obtain, the steps are simple, operation is easy, and the cost can be effectively lowered. According to the method, the high-purity quetiapine can be obtained, the liquid phase purity of the obtained semi-fumaric acid quetiapine obtained through salt forming is 99% or above, and the quetiapine synthesizing method can be applied to the field of medicine.
Owner:JIAXING UNIV
Preparation method of tianeptine sodium impurity D
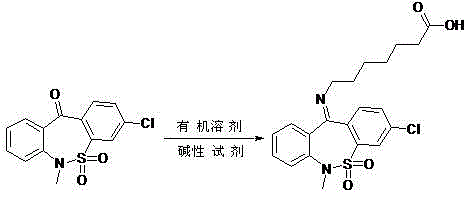
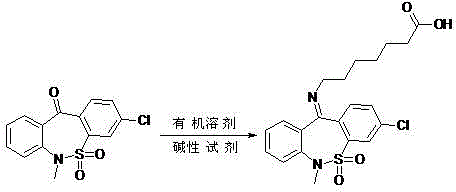
ActiveCN102977053ASolving quality control challengesHigh purityOrganic chemistryReference sampleThiazepine
The invention relates to a preparation method of tianeptine sodium impurity D. The 3-chlorine-6-methyl dibenzo [c, f][1, 2] thiazepine-11 (6H)- ketone S, S-dioxide and 7-amino heptylic acid hydrochloride are used as starting materials, a crude product is prepared by dehydration under alkaline condition, and the crude product is refined to prepare the 7-[(3-chlorine-6,11- dihydro-6- methyl dibenzo [c, f][1,2] thiazepine-11-yl)- imino group]- heptylic acid S, S-dioxide, namely the tianeptine sodium impurity D. The preparation method provided by the invention is short in synthetic route, simple to operate and high in product purity, and can provide qualified reference sample for the quality control of tianeptine sodium.
Owner:SHANDONG CHENGCHUANG BLUE OCEAN PHARM TECH CO LTD
Preparation method of quetiapine fumarate
The invention discloses a preparation method of quetiapine fumarate with high purity, which is suitable for industrialization and includes: taking 11-piperazine-dibenzo[B, F][1, 4]thiazepine dihydrochloride as the initial raw material, N-substituting and salt-forming to generate quetiapine fumarate. The highly finished product obtained by the method has high purity, the operating method is simple, the production cost is low, the yield is high, the preparation method is more suitable for industrialization, and the reaction time is shortened by adding a phase-transfer catalyst.
Owner:HAINAN SHENGKE LIFE SCI RES INST
1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound and preparation method thereof
The invention mainly aims to provide a 1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound and a preparation method thereof. According to the method, the 1, 10a-dihydro-2H-pyridine [1, 2-d][1, 4] thiazepine compound can be efficiently synthesized through reaction of sulfur-containing onium salt and allene at 65 DEG C only under the condition that dichloromethane is used as a solvent without participation of other reagents. The invention also provides a preparation method of the 1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound. Other reaction reagents do not need to beadded, an aza-six-membered ring and seven-membered ring skeleton can be simply constructed and is expected to be applied to synthesis work of drug molecules, reaction raw materials are easy to obtain, reaction operation is simple, other noble metal catalysts do not need to be added, aftertreatment is convenient, substrate applicability is wide, the yield is generally very high, and inert gas protection is not needed in the preparation process. The reaction conditions are mild, the reaction can be quickly and smoothly carried out at 65 DEG C and large-scale preparation is easy.
Owner:SHENZHEN POLYTECHNIC
1,4-thiazepine medicinal compound for nursing skin ulcer as well as preparation method and application thereof
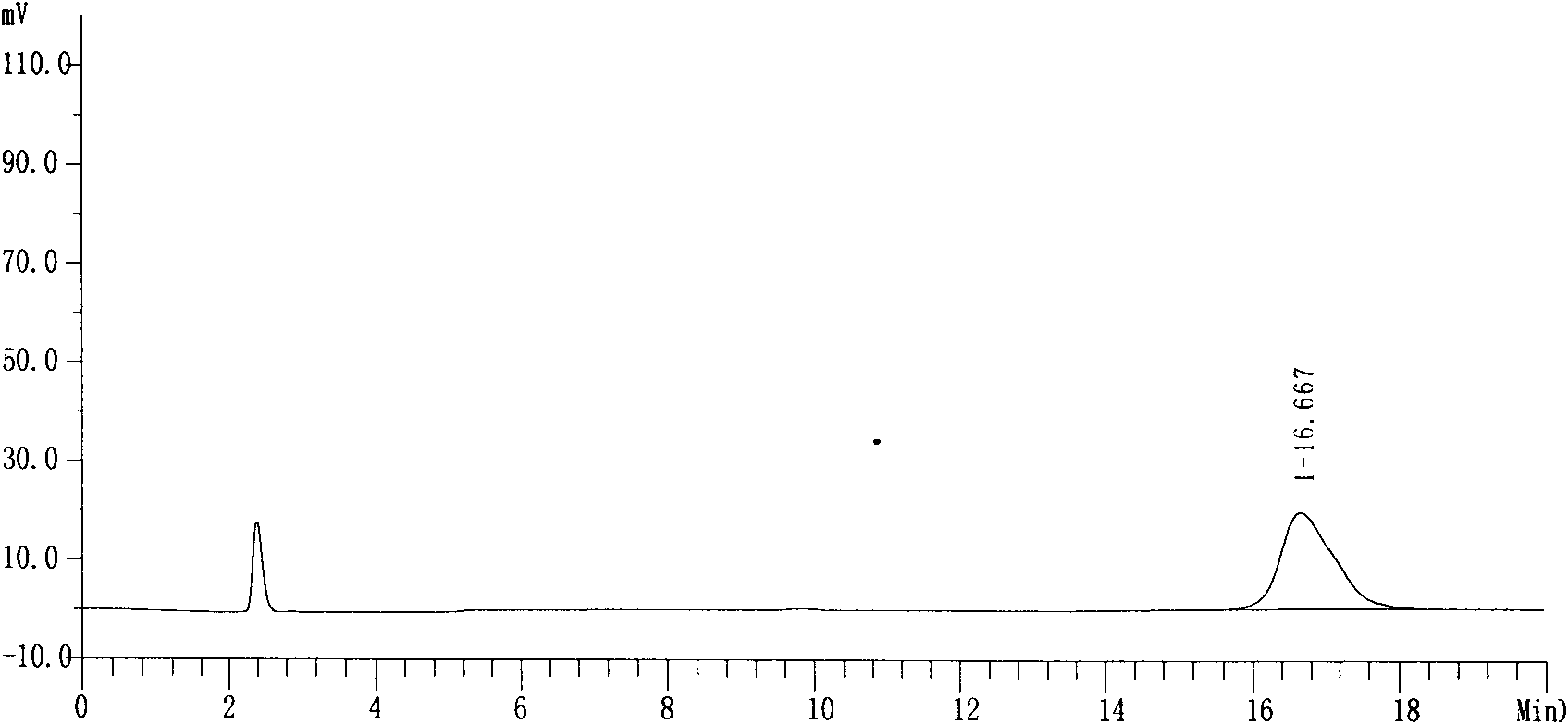
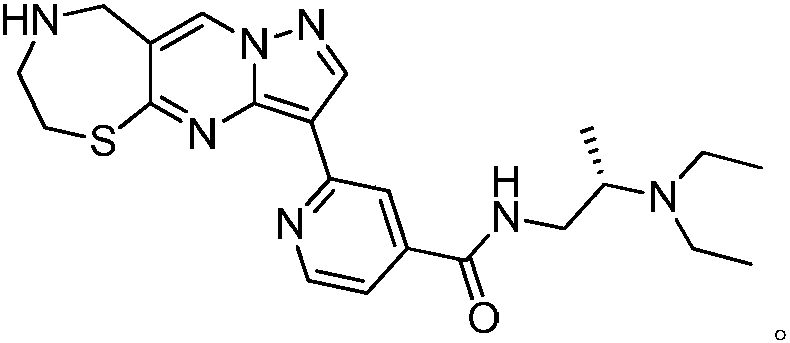
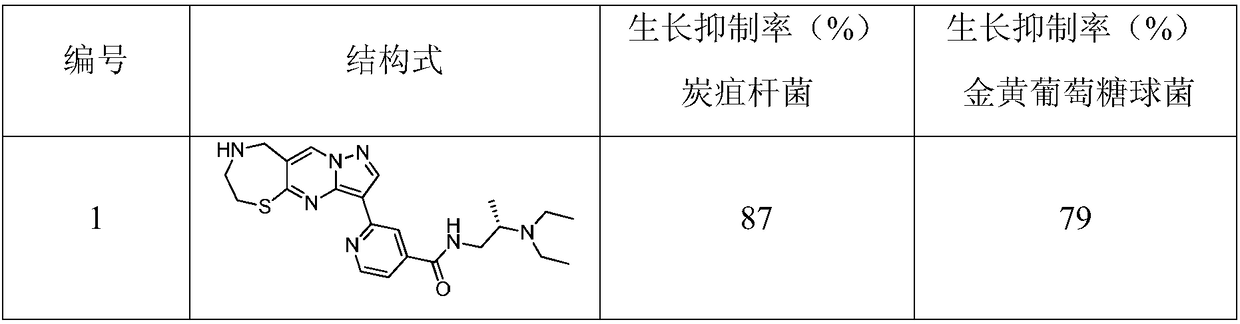
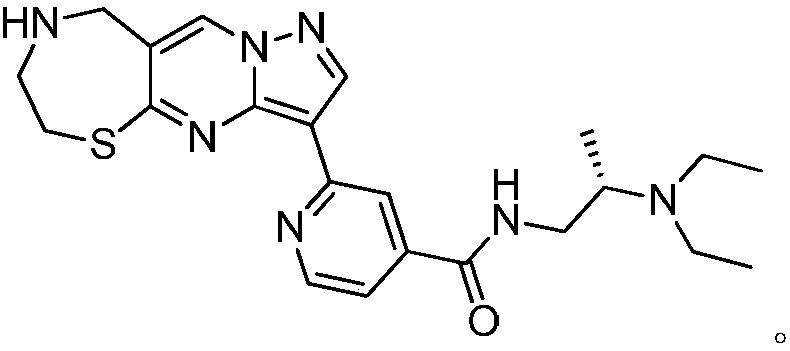
InactiveCN108191888APromote secretionStrong inhibitory activityAntibacterial agentsOrganic active ingredientsThiazepineMedicine
The invention discloses a 1,4-thiazepine medicinal compound for nursing a skin ulcer as well as a preparation method and application thereof, and belongs to the technical field of pharmaceutical synthesis. The key point of the technical scheme is characterized in that the structural formula of the 1,4-thiazepine medicinal compound for nursing the skin ulcer is as shown in the following descriptions. The invention also discloses the preparation method of the 1,4-thiazepine medicinal compound for nursing the skin ulcer and the application of the 1,4-thiazepine medicinal compound for nursing theskin ulcer to the preparation of the 1,4-thiazepine medicinal composition for nursing the skin ulcer. The 1,4-thiazepine medicinal compound prepared by the preparation method has better inhibitory activity for both bacillus anthracis and staphyloccocus aureus which easily cause the skin ulcer, is further made into an ointment to carry out a clinical nursing experiment, and is discovered that the nursing effect is more obvious.
Owner:THE FIRST AFFILIATED HOSPITAL OF HENAN UNIV OF SCI & TECH
Preparation method of 11-chlorodibenzo[b,f][1,4]thiazepine in presence of Vilsmeier reagent
The invention discloses a preparation method of 11-chlorodibenzo[b,f][1,4]thiazepine in presence of a Vilsmeier reagent. The preparation method comprises the following steps: by using 10H-dibenzo[b,f][1,4]thiazepine-11-one as shown in a structural formula (I) and the Vilsmeier reagent as shown in a structural formula (III) as raw materials, preparing the 11-chlorodibenzo[b,f][1,4]thiazepine as shown in the structural formula (II) through a heating reaction in an organic solvent, wherein the Vilsmeier reagent is prepared from di-(trichloromethyl)carbonic ester as shown in a structural formula (a) and DMF (Di-Methyl Formamide) as shown in a structural formula (b). The preparation method provided by the invention has the advantages of short reaction time, simplicity and convenience in operation, simple post-treatment, less pollution and low cost and the like and is a chemical synthesis method with a better popularization and application prospect.
Owner:ZHEJIANG UNIV OF TECH +1
Internediate for preparing quetiapin and preparation method of the intermediate
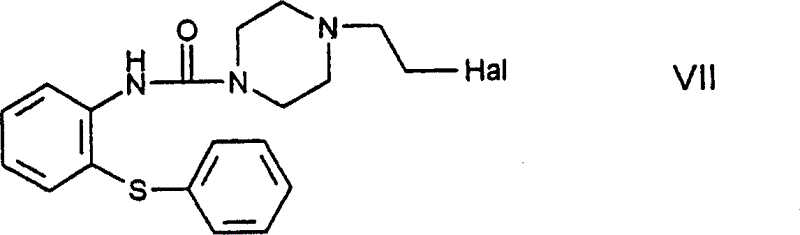
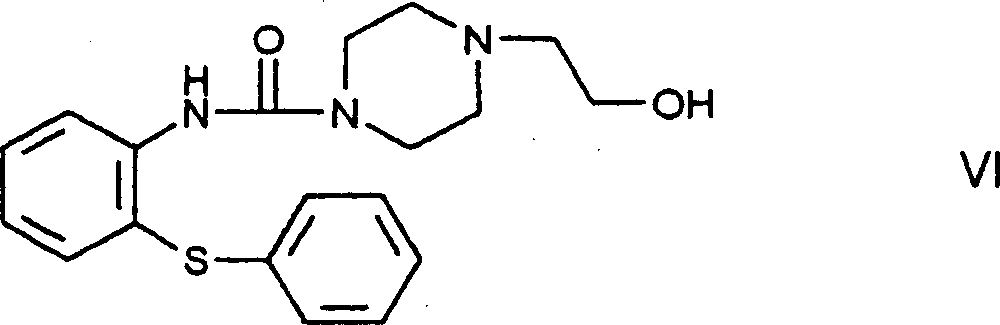
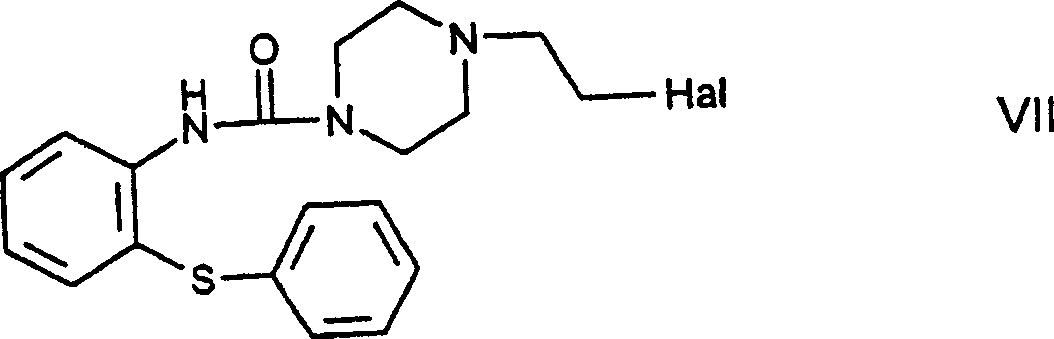
The invention refers to a novel process for the preparation of 11-[4- / 2-(2-hydroxyethoxy)ethyl / -1-piperazinyl]dibenzo[b,f]-1,4-thiazepine of the formula (I) known as quetiapine. According to the invention, a haloethylpiperazinylthiazepine derivative of the formula (VIII), wherein Hal stands for a halo atom, is reacted with ethylene glycol.
Owner:EGIS GYOGYSZERGYAR NYILVANOSAN MUKODO RESZVENY TARSASAG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for the preparation of 11-amino-3-chloro-6, 11-dihydro-5, 5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine Process for the preparation of 11-amino-3-chloro-6, 11-dihydro-5, 5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/35b3e04f-9806-4475-8970-adecfcaafb63/US06441165-20020827-C00001.png)
![Process for the preparation of 11-amino-3-chloro-6, 11-dihydro-5, 5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine Process for the preparation of 11-amino-3-chloro-6, 11-dihydro-5, 5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/35b3e04f-9806-4475-8970-adecfcaafb63/US06441165-20020827-C00002.png)
![Process for the preparation of 11-amino-3-chloro-6, 11-dihydro-5, 5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine Process for the preparation of 11-amino-3-chloro-6, 11-dihydro-5, 5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/35b3e04f-9806-4475-8970-adecfcaafb63/US06441165-20020827-C00003.png)


![3,4-dihydrobenzo[f][1,4]thiazepine compound or salts of same and medical use of same 3,4-dihydrobenzo[f][1,4]thiazepine compound or salts of same and medical use of same](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/64c8d796-b7ae-4f07-8728-a8af11fc99c4/HSA00000081542300011.PNG)
![3,4-dihydrobenzo[f][1,4]thiazepine compound or salts of same and medical use of same 3,4-dihydrobenzo[f][1,4]thiazepine compound or salts of same and medical use of same](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/64c8d796-b7ae-4f07-8728-a8af11fc99c4/HSA00000081542300012.PNG)
![3,4-dihydrobenzo[f][1,4]thiazepine compound or salts of same and medical use of same 3,4-dihydrobenzo[f][1,4]thiazepine compound or salts of same and medical use of same](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/64c8d796-b7ae-4f07-8728-a8af11fc99c4/DSA00000081542000011.PNG)




![Process for the preparation of 11-amino-3-chloro-6,11-dihydro-5,5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine Process for the preparation of 11-amino-3-chloro-6,11-dihydro-5,5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13c3c05d-06ed-4450-b693-26e5def053e6/US20010037021A1-20011101-C00001.png)
![Process for the preparation of 11-amino-3-chloro-6,11-dihydro-5,5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine Process for the preparation of 11-amino-3-chloro-6,11-dihydro-5,5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13c3c05d-06ed-4450-b693-26e5def053e6/US20010037021A1-20011101-C00002.png)
![Process for the preparation of 11-amino-3-chloro-6,11-dihydro-5,5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine Process for the preparation of 11-amino-3-chloro-6,11-dihydro-5,5-dioxo-6-methyl-dibenzo[c,f][1,2]thiazepine and application to the synthesis of tianeptine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13c3c05d-06ed-4450-b693-26e5def053e6/US20010037021A1-20011101-C00003.png)
![Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d36962c-963c-4422-b2f6-648f84065f1f/US20070225494A1-20070927-C00001.png)
![Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d36962c-963c-4422-b2f6-648f84065f1f/US20070225494A1-20070927-C00002.png)
![Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d36962c-963c-4422-b2f6-648f84065f1f/US20070225494A1-20070927-C00003.png)
![Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8497bcd4-e526-472a-949a-1870d73df918/US20070293471A1-20071220-C00001.png)
![Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8497bcd4-e526-472a-949a-1870d73df918/US20070293471A1-20071220-C00002.png)
![Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8497bcd4-e526-472a-949a-1870d73df918/US20070293471A1-20071220-C00003.png)






![Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a83ef501-b7a3-450f-95ad-bdc3a71a911c/US07847094-20101207-C00001.png)
![Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a83ef501-b7a3-450f-95ad-bdc3a71a911c/US07847094-20101207-C00002.png)
![Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine Industrial preparation of 11-[4-{2-(2-hydroxyethoxy) ethyl}-1-piperazinyl] dibenzo [b,f]-[1,4]thiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a83ef501-b7a3-450f-95ad-bdc3a71a911c/US07847094-20101207-C00003.png)


![1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound and preparation method thereof 1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/45210ae7-f080-4354-a96a-94268c05cbf8/HDA0002448347730000011.png)
![1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound and preparation method thereof 1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/45210ae7-f080-4354-a96a-94268c05cbf8/HDA0002448347730000012.png)
![1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound and preparation method thereof 1, 10a-dihydro-2H-pyridine [1, 2-d] [1, 4] thiazepine compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/45210ae7-f080-4354-a96a-94268c05cbf8/HDA0002448347730000021.png)
![Preparation method of 11-chlorodibenzo[b,f][1,4]thiazepine in presence of Vilsmeier reagent Preparation method of 11-chlorodibenzo[b,f][1,4]thiazepine in presence of Vilsmeier reagent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0f6f443-f176-432d-9bbb-9298af67715e/BDA00003289782200011.PNG)
![Preparation method of 11-chlorodibenzo[b,f][1,4]thiazepine in presence of Vilsmeier reagent Preparation method of 11-chlorodibenzo[b,f][1,4]thiazepine in presence of Vilsmeier reagent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0f6f443-f176-432d-9bbb-9298af67715e/BDA00003289782200031.PNG)
![Preparation method of 11-chlorodibenzo[b,f][1,4]thiazepine in presence of Vilsmeier reagent Preparation method of 11-chlorodibenzo[b,f][1,4]thiazepine in presence of Vilsmeier reagent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0f6f443-f176-432d-9bbb-9298af67715e/FDA00003289782100011.PNG)