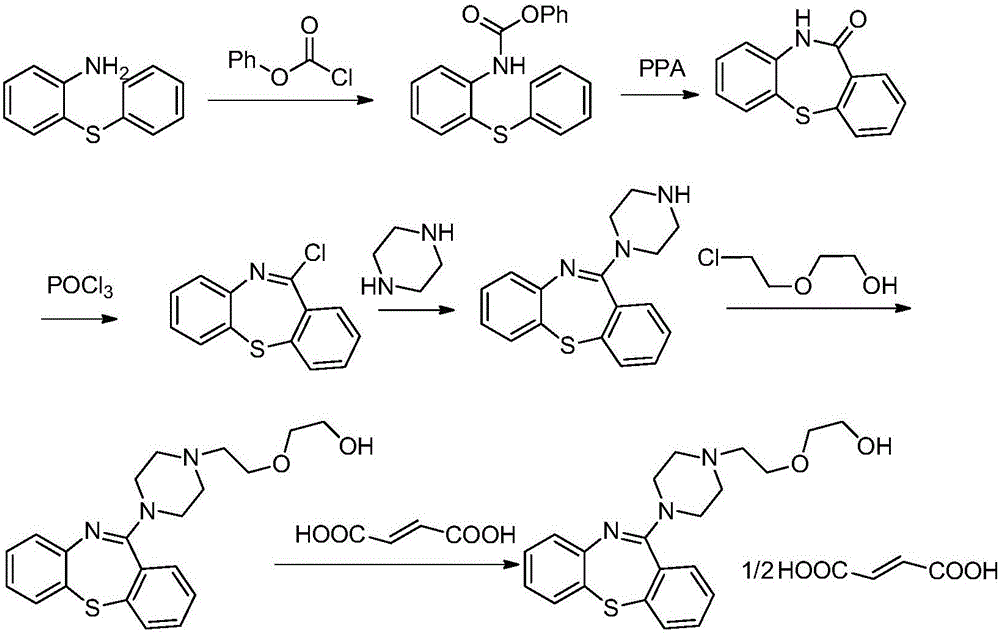
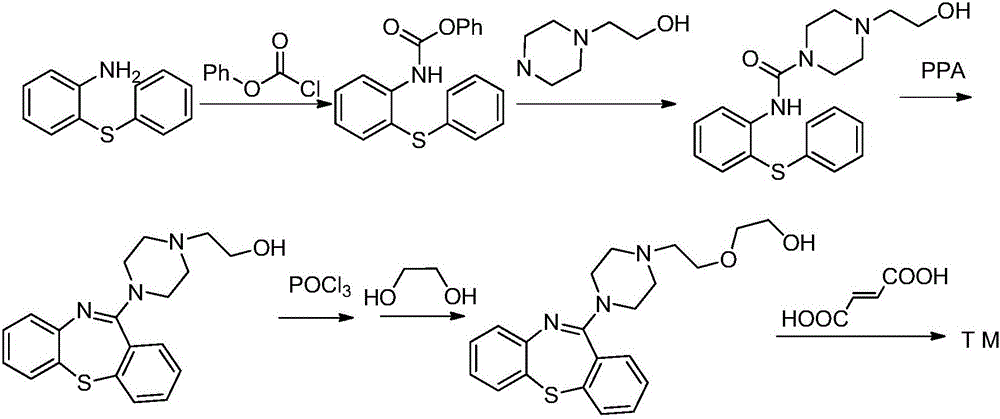
Quetiapine synthesizing method
A synthetic method, the technology of quetiapine, applied in the field of drug synthesis, can solve the problems of a large amount of iron-containing waste liquid, increase the production cost, and be difficult to handle, and achieve the effect of simple reaction conditions, high purity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation of embodiment 1 2-carboxy diphenyl sulfide (compound II)
[0062] Add 150mL tetrahydronaphthalene, o-chlorobenzoic acid (15.6g, 0.1mol) and copper powder (0.32g, 0.005mol) into the reaction flask, and when the system drops to about 0°C, add sodium hydroxide ( 6.0g, 0.15mol), thiophenol (11.1g, 0.1mol), heated up to 130 ° C for 6 hours after the addition (monitored by TLC during the reaction), cooled after the reaction, added 100mL of water, suction filtered to remove the copper powder, and the filtrate The layers were separated, and the pH of the aqueous phase was adjusted to 2-3 with hydrochloric acid. A large amount of white solid was precipitated, and 20.5 g of the crude product was obtained by suction filtration. The yield was 89%, and the purity (HPLC): 97.8%.
[0063] Molecular formula: C 13 h 10 o 2 S; molecular weight: 230.0; MS (m / z): 231.0 (M + +H).
Embodiment 2
[0064] Embodiment 2 Preparation of thioxanthone (III)
[0065] Add 2-carboxydiphenylsulfide (23g, 0.1mol, compound II) into the reaction flask, add 100mL of toluene to dissolve, cool down to about 0°C in an ice bath, add 20mL of concentrated sulfuric acid dropwise, and heat up to 80°C after the addition Continue to stir the reaction for 1h, and TLC monitors the degree of the reaction. After the reaction is completed, the reactant is poured into water, separated, the organic phase is washed to neutrality, dried, and toluene is removed, and recrystallized with ethanol to obtain 16g of a light yellow crude product. Rate: 75.5%. Purity (GC): 98.2%.
[0066] Molecular formula: C 13 h 8 OS; molecular weight: 212.0; MS (m / z): 213.0 (M + ).
[0067] 1 H NMR (400MHz, CDCl 3 )δ8.52(d, J=8.0Hz, 2H), 7.52-7.43(m, 4H), 7.37(t, J=7.6Hz, 2H); 13 C NMR (100MHz, CDCl 3 )δ 179.2, 137.1, 132.1, 129.6, 129.0, 126.1, 125.7; melting point: 207.5°C-209.1°C.
Embodiment 3
[0068] Embodiment 3 Preparation of thioxanthone oxime (compound VI)
[0069] Add thioxanthone (21.2g, 0.1mol, compound III) into the reaction flask, add 100mL of ethanol to dissolve, then add hydroxylamine hydrochloride (7.65g, 0.11mol, CAS: 5470-11-1), and heat up after the addition Stir the reaction under reflux for 3 hours, and monitor the progress of the reaction by TLC. After the reaction is completed, most of the solvent is removed by spinning, and crystals are precipitated by cooling, and 21.1 g of a white solid are obtained by suction filtration, yield: 93%. Purity (HPLC): 99.1%.
[0070] Molecular formula: C 13 h 9 NOS; molecular weight: 227.0; MS (m / z): 228.0 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com