Synthesis method of tianeptine sodium
A technology for tianeptine sodium and a synthesis method is applied in the field of synthesizing tianeptine sodium by spray-drying salt-forming, and can solve the problems of high cost, low product purity, low yield and the like of salt-forming sodium isonovate, Achieving the effect of high product yield and purity, good purity, and improving product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
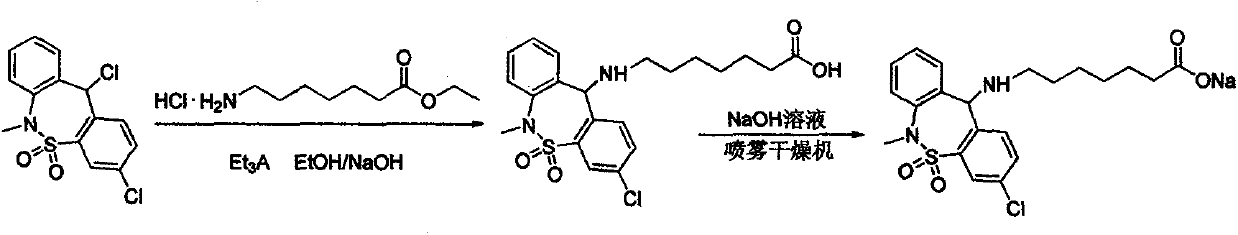
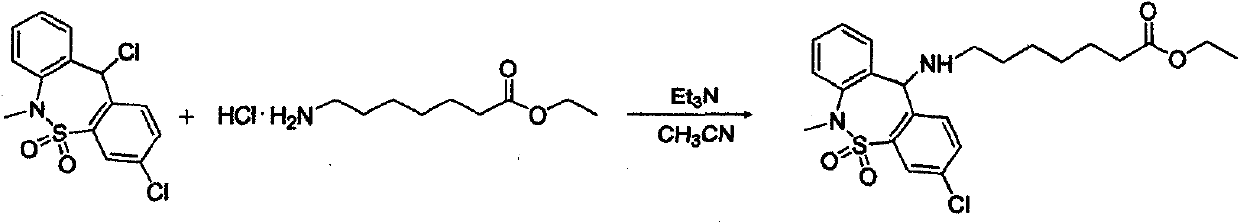
[0025] The first step: 7-[(3-chloro-6,11-dihydro-5,5-dioxo-6-methyldibenzo[c,f][1,2]thiazol-11yl)amino] Preparation of ethyl heptanoate:
[0026]
[0027] Into a 5L reaction flask, add 540g, 1.64mol (3,11-dichloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2]thiazepine in sequence Zhuo-5,5-dioxide); 430g, 2.05mol 7-aminoheptanoic acid ethyl ester hydrochloride, 2.6L acetonitrile, stir well, then add 368g, 3.64mol triethylamine, heat to 50°C-60°C After reacting for 2 hours, concentrate to dryness under reduced pressure; add 2.2 L of dichloromethane, 1.0 L of water and stir for 0.5 hours, let stand to separate layers, take the organic layer, wash with 1 L of water, combine the organic phases, and concentrate under reduced pressure to obtain a viscous substance 7 -[(3-Chloro-6,11-dihydro-5,5-dioxo-6-methyldibenzo[c,f][1,2]thiazol-11yl)amino]heptanoic acid ethyl ester 610g , 81% yield, 98.5% purity, reddish-brown, sticky substance.
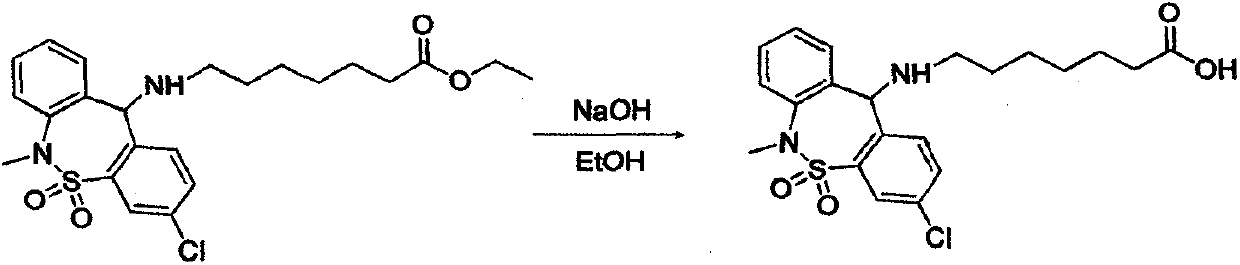
[0028] The second step: 7-[(3-chloro-6,11-dihyd...
Embodiment 2
[0035] The synthetic method of tianeptine sodium of the present invention is basically the same as that of Example 1, except that other inorganic alkali potassium hydroxide is used in the hydrolysis reaction of the second step ester, which is different from the alkali used in the first step hydrolysis reaction. The specific instructions are as follows:
[0036] The first step: 7-[(3-chloro-6,11-dihydro-5,5-dioxo-6-methyldibenzo[c,f][1,2]thiazol-11yl)amino] Preparation of ethyl heptanoate:
[0037]In a 5L reaction flask, add 540g (3,11-dichloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2]thiazepine-5 , 5-dioxide), 433g of ethyl 7-aminoheptanoate hydrochloride, 2500ml of acetonitrile, 370g of triethylamine, heated to 50°C-60°C for 2 hours, then concentrated to dryness under reduced pressure; cooled to room temperature by freezing, adding two Chloromethane 2L, water 1L, stir for 0.5 hours, let stand to separate layers, take the organic layer, wash with 1L of water, combine the organ...
Embodiment 3
[0043] The synthesis method of tianeptine sodium of the present invention is the same as that of Example 1, except that the molar ratio of the two starting reactants is adjusted.
[0044] The first step: 7-[(3-chloro-6,11-dihydro-5,5-dioxo-6-methyldibenzo[c,f][1,2]thiazol-11yl)amino] Preparation of ethyl heptanoate:
[0045] In a 5L reaction flask, add 540g (3,11-dichloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2]thiazepine-5 , 5-dioxide), 379g of ethyl 7-aminoheptanoate hydrochloride, 2500ml of acetonitrile, 375g of triethylamine, heated to 50°C-60°C for 2 hours and concentrated under reduced pressure to dryness. Freeze and cool down to room temperature, add 2 L of dichloromethane, 1 L of water and stir for 0.5 hours, let stand to separate layers, take the organic layer, wash with 1 L of water, combine the organic phases, and concentrate under reduced pressure to obtain a viscous 7-[(3-chloro- 6,11-dihydro-5,5-dioxo-6-methyldibenzo[c,f][1,2]thiazol-11yl)amino]heptanoic acid et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com