Compound with antibacterial ability as well as preparation method and application thereof
A technology of compounds and capabilities, applied in the fields of organic chemistry, antibacterial drugs, resistance to vector-borne diseases, etc., can solve problems such as the crisis of effective clinical application of antibacterial drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
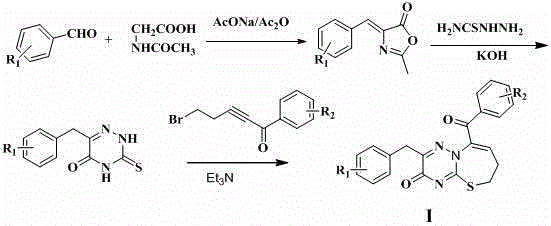
[0023] Preparation of 2-methyl-4-(4-chlorobenzylidene)oxazolone
[0024] Add 0.1 mol of p-chlorobenzaldehyde, 0.13 mol of acetylglycine, 0.12 mol of anhydrous sodium acetate and 50 g of acetic anhydride into a 100 mL three-neck flask in sequence, control the temperature at 90°C, stir for 5 h, and cool to room temperature. The solution became solid, suction filtered, the filter cake was washed with cold water, and the filter cake was dried to obtain 22.1 g of yellow powder, the yield was 100%, ESI-MS (m / z): 222.2 (M+H) + .
Embodiment 2
[0026] 3,4-dihydro-6-(4-chlorobenzyl)-3-thio-1,2,4-triazine-5(2 H ) - Preparation of ketones
[0027] Mix 2-methyl-4-(4-chlorobenzylidene)oxazolone (0.1mol, 22.1g) and KOH (11.2g) in 500ml of water, heat in a water bath, and let it reflux for 6 hours, and the reaction solution It became clear, then added thiosemicarbazide (0.12mol, 9g), and the mixture was reacted for 4.5h, then the pH of the reaction solution was adjusted with acetic acid to make it pH 4, and a solid precipitated, which was filtered and dried to obtain 13.5g of yellow powder, with a yield of 53.42 %, ESI-MS (m / z): 254.1 (M+H) + .
Embodiment 3
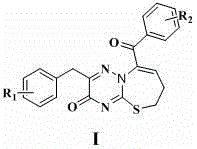
[0029] 2-(4-Chlorobenzyl)-9-(4-methoxybenzoyl)-6,7-dihydro-3H-[1,2,4]triazino[1,3]thiazepine Preparation of Zol-3-one (L1)
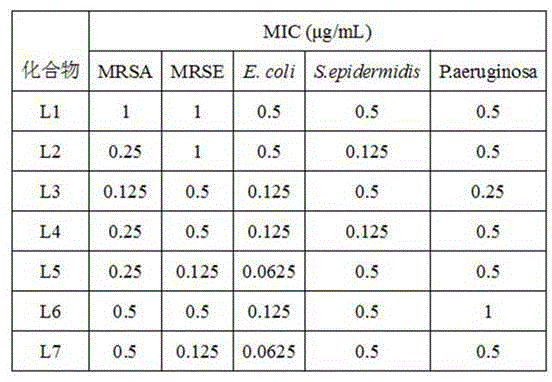
[0030] 3,4-dihydro-6-(4-chlorobenzyl)-3-thio-1,2,4-triazine-5(2 H )-ketone 0.01 mol, add 50 mL ethanol to dissolve, add dropwise 1-(4-methoxybenzoyl)-4-bromo-1-butyne 0.01 mol and triethylamine under stirring, reflux reaction for 3h, TLC monitoring After the reaction was completed, it was cooled to room temperature, and the reaction solution gradually precipitated solids. After suction filtration, washing with water, and ethanol recrystallization, 2.32 g of white crystals were obtained, with a yield of 52.74%. 1 H-NMR (600 MHz, DMSO- d 6 ): δ7.80 (2H, d, J = 8.4Hz), 7.48 (2H, d, J = 8.4Hz), 7.33 (1H, s), 7.21 (2H, d, J = 8.4Hz), 7.17 (2H, d, J = 8.4Hz), 3.91 (2H, s), 3.80 (3H, s), 2.63 (2H, m), 2.60 (3H, m); ESI-MS (m / z): 439.9 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com