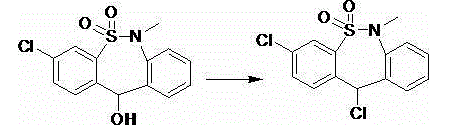
Preparation method of tianeptine sodium intermediate
A technology for tianeptine sodium and intermediates, which is applied in the field of chemical synthesis, can solve problems such as environmental pollution, complexity, hidden dangers, etc., and achieves the effects of low requirements for reaction conditions, simple equipment requirements, and safe production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 31.0 g of 3-chloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2]thiazepin-11-ol-5,5-dioxide to 124 g Add 18.2g of industrial hydrochloric acid to toluene, heat and reflux at 110°C-114°C to separate water for 2 hours, after the reaction is completed, cool to room temperature, filter, and dry to obtain the intermediate 3,11-dichloro-6,11 of tianuptine sodium -Dihydro-6-methyl-dibenzo[c,f][1,2]thiazepine-5,5-dioxide 31.0g, purity>99.0%, molar yield 94.5%.
Embodiment 2
[0025] Add 61.9 g of 3-chloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2]thiazepin-11-ol-5,5-dioxide to 310 g Add 73.0g of industrial hydrochloric acid to toluene, heat and reflux at 110°C-114°C to separate water for 5 hours, after the reaction is completed, cool to room temperature, filter, and dry to obtain the intermediate 3,11-dichloro-6,11 of tianuptine sodium -Dihydro-6-methyl-dibenzo[c,f][1,2]thiazepine-5,5-dioxide 62.6g, purity>99.0%, molar yield 95.5%.
Embodiment 3
[0027] Add 31.0 g of 3-chloro-6,11-dihydro-6-methyl-dibenzo[c,f][1,2]thiazepin-11-ol-5,5-dioxide to 186 g Add 18.2g of industrial hydrochloric acid to dichloromethane, heat and reflux at 40°C-44°C for 7 hours, after the reaction is completed, cool to room temperature, filter, and dry to obtain the intermediate 3,11-dichloro-6,11 -Dihydro-6-methyl-dibenzo[c,f][1,2]thiazepine-5,5-dioxide 30.8g, purity>99.0%, molar yield 94.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com