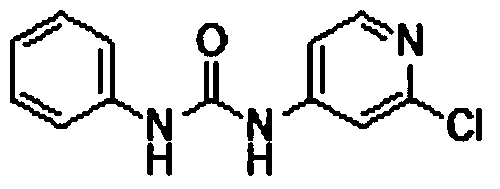
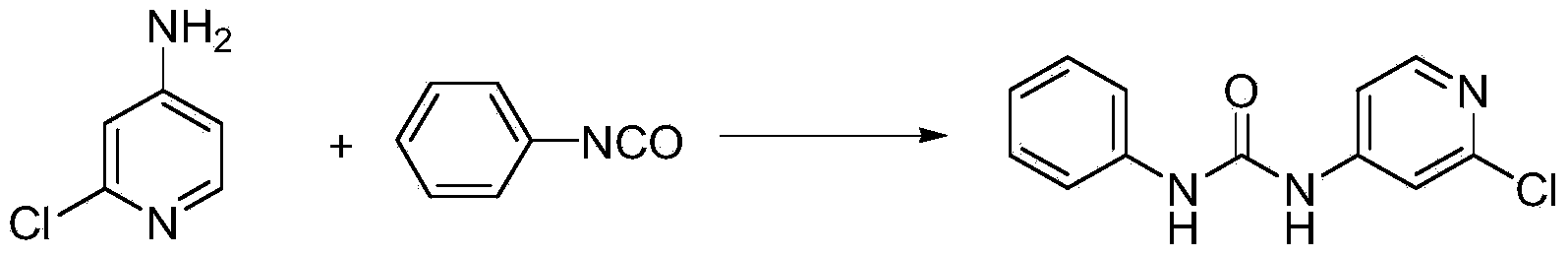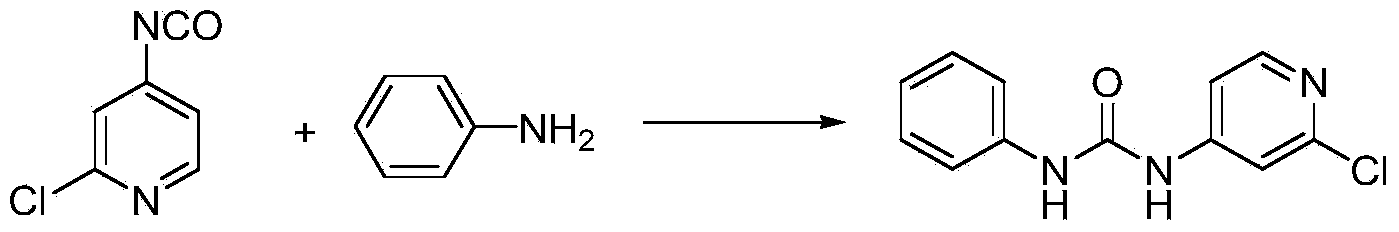Preparation method of 1-(2-chloro-4-pyridyl)-3-phenylurea
A pyridine phenyl urea and pyridyl technology are applied in the field of preparation of phenyl urea-type cytokinin efficient plant production regulators pyridine phenyl urea and 1--3-phenyl urea, and can solve the problem of difficult industrialized production and difficult raw materials. It is easy to remove, the preparation period is short, and the operation is simple and convenient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Its specific preparation method steps are as follows:
[0035] (1) Using 2-chloro-4-aminopyridine and phenyl chloroformate as raw materials, potassium carbonate, sodium carbonate, triethylamine, pyridine, N,N-dimethylaniline, 4-dimethylaminopyridine (DMAP ), N,N-diisopropylethylamine, 1,4-diazabicyclo[2.2.2]octane (DABCO), substituted pyridine and other inorganic bases or organic bases are used as acidic agents, Toluene, xylene, chlorobenzene, tetrahydrofuran (THF), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), dioxane, acetonitrile, ethylene glycol dimethyl ether, One of ethylene glycol diethyl ether, ethyl acetate, acetone, butanone, dichloromethane, and chloroform is used as solvent A; 2-chloro-4-aminopyridine and acid agent are dissolved in solvent A, and then added Phenyl chloroformate, stirred and reacted at -5-80°C, preferably 0-60°C, followed by liquid phase tracking to monitor the completion of the reaction, distilled to a part of the solvent under r...
Synthetic example 1
[0039] (1) Dissolve 38.8g of 2-chloro-4-aminopyridine and 33.5g of triethylamine in 400mL of methyl ethyl ketone, then add phenyl chloroformate, stir the reaction at 40°C, monitor the completion of the reaction by liquid phase tracking, and distill under reduced pressure Part of the solvent was removed, and a white solid was precipitated, which was filtered to obtain 73.2 g of phenyl 2-chloro-pyridine-4-carbamate, with a content of 99%.
[0040] (2) Add 50g of phenyl 2-chloro-pyridine-4-carbamate, 20.5g of aniline, and 2.1g of N,N-diisopropylethylamine into 300mL of tetrahydrofuran (THF) solution, reflux After the reaction was complete, tetrahydrofuran (THF) was distilled under reduced pressure, then an appropriate amount of water was added and stirred for 0.5 hours, and filtered under reduced pressure to obtain 48 g of white solid CPPU with a content of 98.8%.
Synthetic example 2
[0042] (1) Dissolve 38.8g of 2-chloro-4-aminopyridine and 26.3g of pyridine in 400mL of toluene solvent, then add phenyl chloroformate, stir the reaction at 30°C, monitor the completion of the reaction by liquid phase tracking, and distill under reduced pressure Part of the solvent was removed, and a white solid was precipitated, which was filtered to obtain 73.1 g of phenyl 2-chloro-pyridine-4-carbamate, with a content of 99.1%.
[0043] (2) Add 50g of phenyl 2-chloro-pyridine-4-carbamate, 20.5g of aniline, and 2.4g of 4-dimethylaminopyridine (DMAP) to 300ml of N,N-dimethylformaldehyde In the amide (DMF) solution, stir and react at 80°C. After the reaction is complete, add an appropriate amount of water to precipitate a solid, and filter under reduced pressure to obtain 47.88 g of white solid CPPU with a content of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com