Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Methyl carbazate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Packaging 100, 500 g in poly bottle Application Methyl hydrazinocarboxylate (Methyl carbazate) was used in the preparation of methyl 3-(4-methyl-benzyl-idene) carbazate.It was also used in the synthesis of imidazo[1,5-d][1,2,4]tria zines.
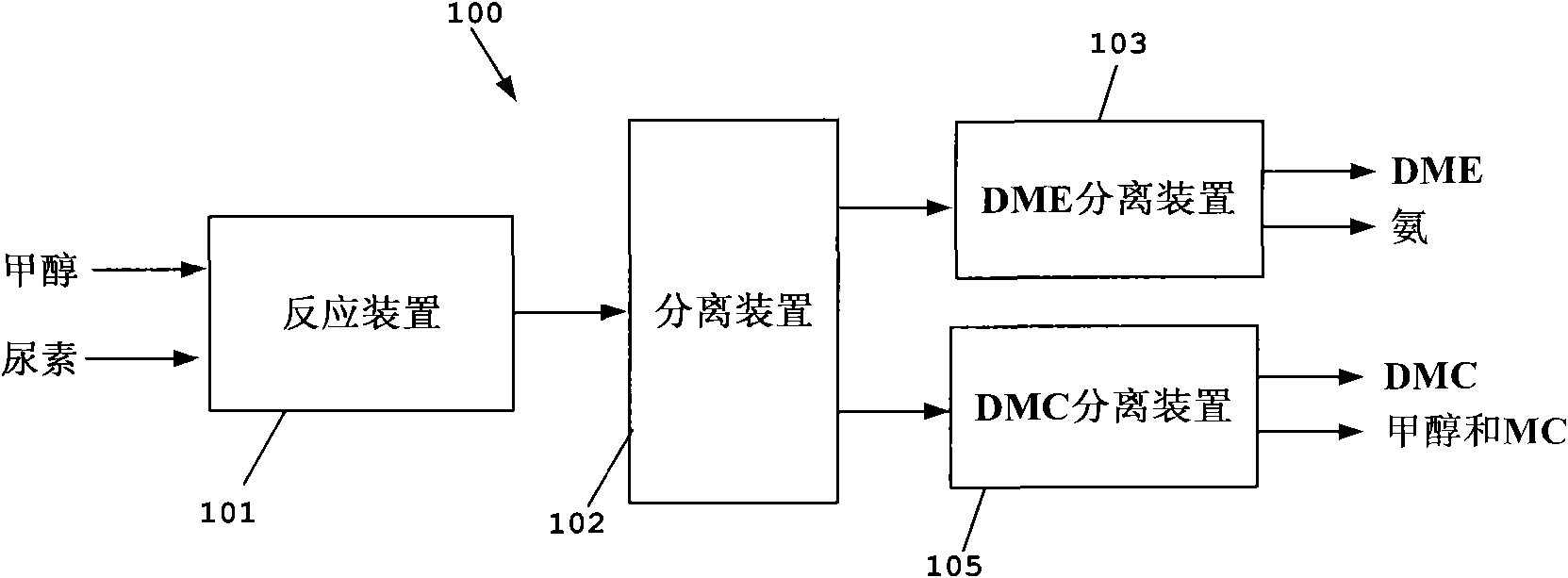
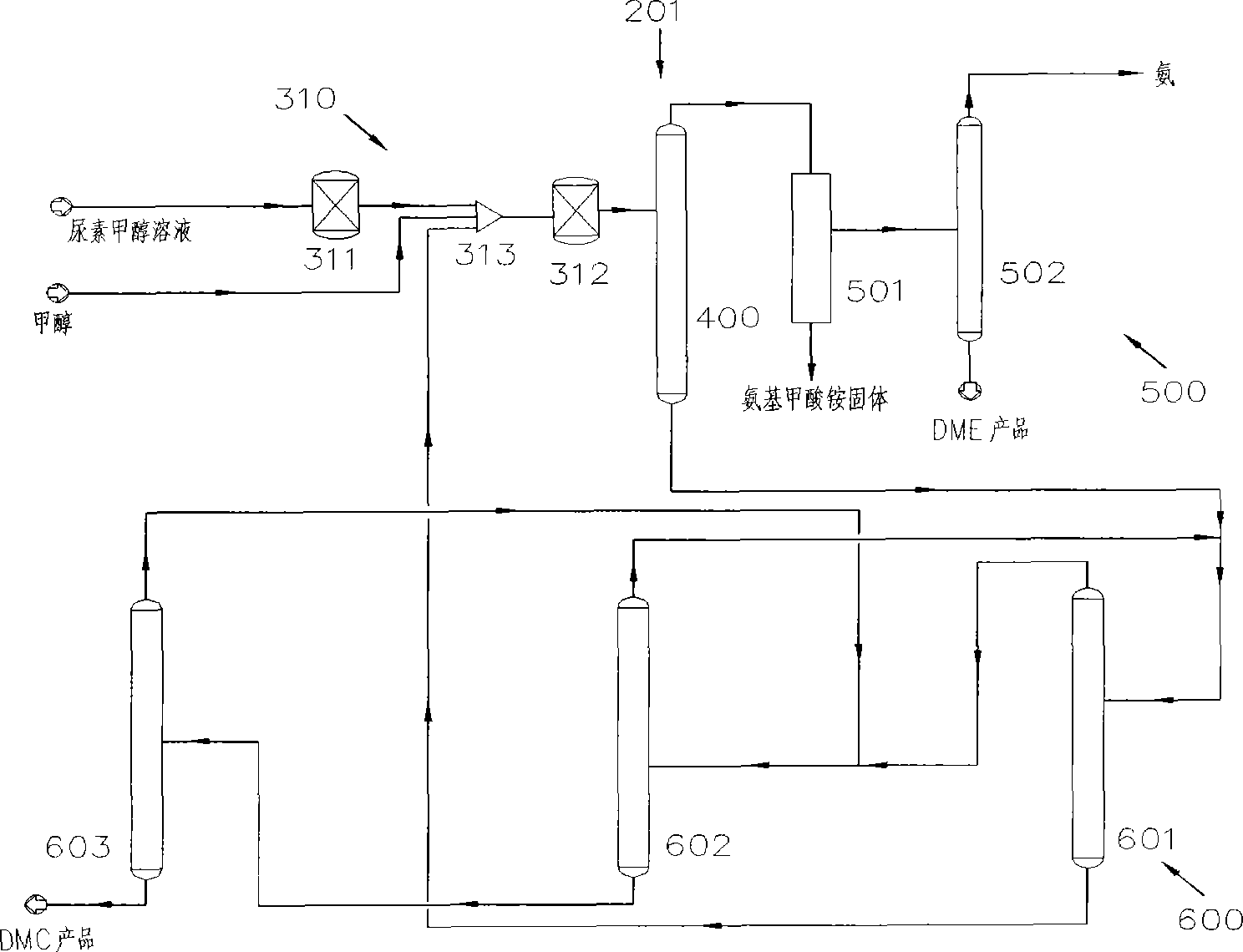
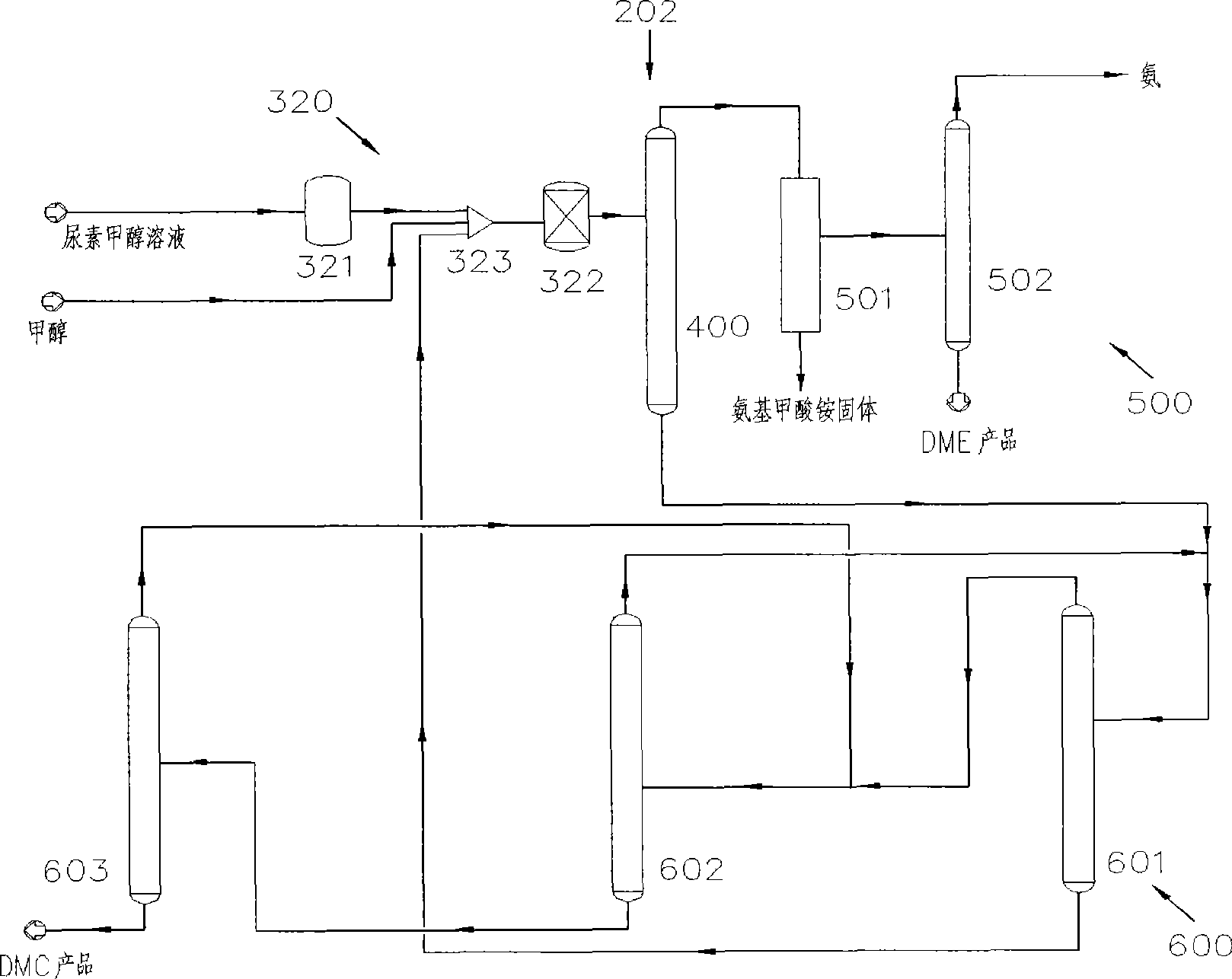
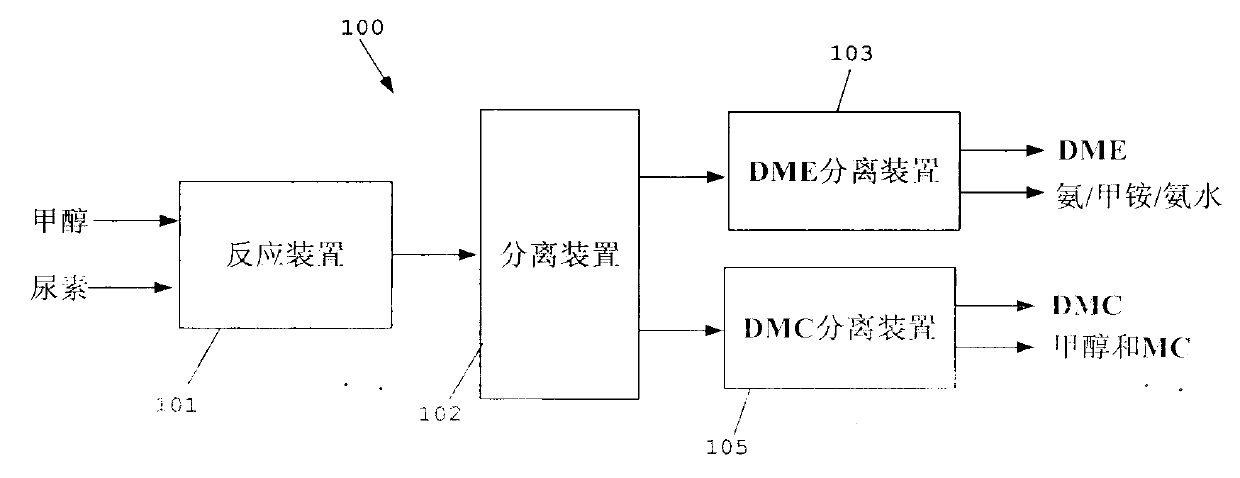
Process for co-producing dimethyl carbonate and dimethyl ether by urea alcoholysis method
InactiveCN102212009AIncrease productionQuality improvementOrganic compound preparationEther preparationMethyl carbamateMethyl carbazate
The invention provides a process for co-producing dimethyl carbonate and dimethyl ether by a urea alcoholysis method. The method comprises the following steps: adding methanol and urea in a reaction device for reaction so as to generate methyl carbamate and by-produce ammonia; further reacting generated methyl carbamate with methanol so as to generate dimethyl carbonate and by-produce ammonia, wherein a part of dimethyl carbonate is decomposed to generate dimethyl ether; separating out a material containing dimethyl ether and a material containing dimethyl carbonate from a material discharged from the reaction device; separating out dimethyl ether from the material containing dimethyl ether; and separating out dimethyl carbonate from the material containing dimethyl carbonate.
Owner:YASHENTECH CORP
Process for producing methyl carbamate
InactiveCN1683326AHigh yieldReduce consumptionCarbamic acid derivatives preparationOrganic compound preparationMethyl carbamateMethyl carbazate
The methyl carbamate producing process belongs to the field of fine chemical technology. The technological scheme of purifying methyl carbamate includes: dissolving urea in methanol to obtain methanol solution of urea of 0.01-10 mol / L concentration, reaction in reactor at 100-180 deg.c and 0.1-1.5 MPa for 0.5-10 hr, condensing in condensator to separate ammonia and making ammonia produce ammonia water, returning methanol with ammonia separated to the reaction system, and product purification. The present invention has high methyl carbamate yield, simplified operation, short reaction period, low power consumption and low cost.
Owner:山东康瑞集团有限公司 +1
Method for preparing 5-methylbenzimidazole-2-methyl carbamate
InactiveCN102351800ASimplify operating proceduresReduce manufacturing costOrganic chemistryFungicidesMethyl carbamateMethyl carbazate
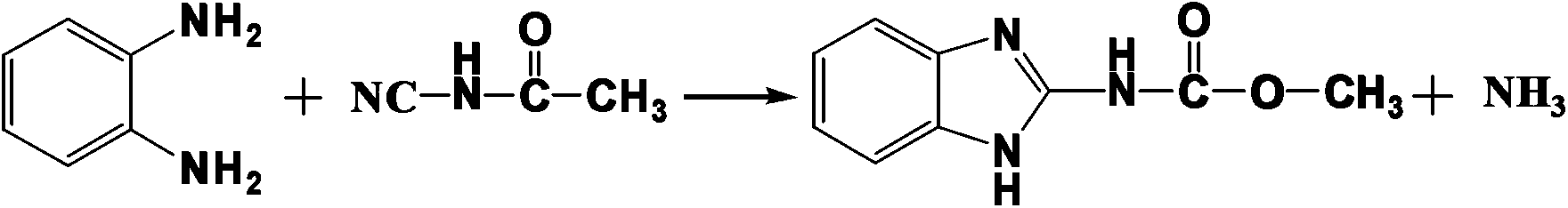
The invention discloses a method for preparing 5-methylbenzimidazole-2-methyl carbamate. Three reaction steps, namely cyanamide formation, acidification and ring closing are performed, operating procedures are simplified, strong base such as sodium hydroxide is adopted for cyanamide formation, hydrochloric acid is adopted for acidification, sodium thiosulfate is adopted for catalyzing ring closing, waste acid is reduced, and pollutants are reduced on the premise of ensuring product quality and yield. The method comprises the following step of: performing cyanamide formation reaction, acidification reaction and ring closing reaction on methylclhlorofonmate serving as a raw material to obtain the product, wherein in the cyanamide formation reaction, the methylclhlorofonmate is reacted with cyanamide under the alkaline condition to form methylcyanocarbamate salt; in the acidification reaction, the methylcyanocarbamate salt is reacted with the hydrochloric acid to form methylcyanocarbamate; and in the ring closing reaction, the methylcyanocarbamate and 4-methoxy-o-phenylenediamine are subjected to condensation reaction to form the 5-methylbenzimidazole-2-methyl carbamate.
Owner:NANJING FORESTRY UNIV
Synthetic method of N-(2-benzimidazolyl)-methyl carbamate
The invention discloses a synthetic method of N-(2-benzimidazolyl)-methyl carbamate. The method comprises the steps of by taking hydrogen cyanamide, methylclhlorofonmate and o-phenylenediamine as materials, adding strong alkali and weak acid salt in synthetic process; adding the strong alkali and weak acid salt to control the pH value of a reaction system in the reaction process, achieving the target of feeding once to control the pH of two-step reaction by the buffer effect, wherein the strong alkali and weak acid salt can be combined with and consume hydrogen chloride generated in the first step of reaction to counteract ammonia generated in the second step of reaction. The synthetic method is simple to operate, high in yield, and environment-friendly; the cost of acid-base is reduced; generation of impurities in reaction is reduced; and no waste water or residue is generated.
Owner:JINGBO AGROCHEM TECH CO LTD
Method and equipment for preparation of methyl carbamate
ActiveCN103254101AAvoid makingLower conversion rate per passCarbamic acid derivatives preparationOrganic compound preparationMethyl carbamateMethyl carbazate
Owner:ZHONGBEI UNIV
Nitrogen-containing sulfur substituent naphthalimide compound, preparation method and applications thereof
The present invention discloses a class of nitrogen-containing sulfur substituent naphthalimide compound, a preparation method and applications thereof, wherein the compound has a structure represented by a general formula Y, R is one selected from a six-membered ring containing hetero atom, the following structures defined in the specification, -NHCH2CH2OH, -NHCH2CH2N(CH3) and -NHCH2CH2CH2CH3, the hetero atom is at least one selected from N, O and S, the six-membered ring containing the hetero atom at least has a N atom, and R is bound with the mother nucleus in the general formula T through the N atom. According to the present invention, the methyldithiocarbazate is bounded into the active site of the naphthalimide to prepare the compound of the present invention, and the class of the compounds provide growth inhibition activities on breast cancer cells, cervical cancer cells and a variety of tumor cells with different tissue origins while provide low inhibition activities on human body normal cells, and have wide prospects in the tumor cell growth inhibition drug preparation. The general formula Y is defined in the specification.
Owner:DALIAN UNIV OF TECH
Synthesis of methyl carbamate and dimethyl carbonate (DMC) in presence of stripping with inert gas or superheated vapours and a reactor for the same
ActiveUS20150315134A1Safety arrangmentsCarbamic acid derivatives preparationMethyl carbamateMethyl carbonate
The invention relates to synthesis of methyl carbamate (MC) and dimethyl carabonate (DMC) in presence of stripping inert gas or superheated methanol vapors using packed column reactor and bubble column reactor.
Owner:COUNCIL OF SCI & IND RES
Synthetic method of methyl-4-(trifluoromethoxy) phenyl carbamate
InactiveCN102219712AImprove economyLow toxicityCarbamic acid derivatives preparationOrganic compound preparationCarbamateSide effect
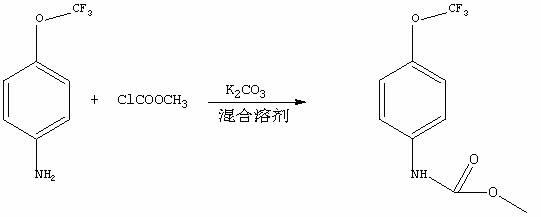
A synthetic method of methyl-4-(trifluoromethoxy) phenyl carbamate. Raw materials of 4-trifluoromethoxy-aniline, methyl chloroformate and alkalis and a mixed solvent of water and organic solvents are mixed and undergo directly a reaction to produce a product of methyl-4-(trifluoromethoxy) phenyl carbamate. The synthetic method comprises the following steps of 1, adding 4-trifluoromethoxy-aniline, sodium hydroxide, tetrahydrofuran and water into a container, mixing well and make the mixture undergo a reaction at a temperature of 0 to 5 DEG C for 2 hours, 2, heating the reaction products to room temperature and stirring for 30 minutes, adding slowly methyl chloroformate into the heated reaction products and stirring at room temperature for 6 to 7 hours, 3, cooling the reaction products obtained from the step 2 to 0 DEG C and stirring for 30 minutes, and 4, carrying out a suction filtration process for the reaction products obtained from the step 3 to collect solids, and washing and drying the solids to obtain desired products. The synthetic method has the advantages that the synthetic method is simple and practical, has a high efficiency and can realize an end product yield more than 95%; reagents adopted in synthesizing processes have the advantages of low toxicity, high economic efficiency, less side effects, less corrosion on equipment and less pollution on environment; and prices of raw materials are cheap thus a production cost is low and the synthetic method has a high economic efficiency and good application prospects.
Owner:NANKAI UNIV
Preparation method for novel insecticide indoxacarb
The invention discloses a preparation method for a novel insecticide indoxacarb. The preparation method comprises the following steps: taking (+)5-chlorine-1,3-dihydro-2-hydroxyl-1-oxo-2H-indene-methyl carboxylate and 4-(trifluoromethoxy)benzenaime as raw materials, protecting (+)5-chlorine-1,3-dihydro-2-hydroxyl-1-oxo-2H-indene-methyl carboxylate by virtue of fluorenylmethyl chloroformate, and carrying out cyclization to prepare 2-( fluorenylmethyl)-7-chlorinindene-[1,2-e][1,34]] oxadiazine-2,4a(3H, 5H)-dicarboxylic acid-4a-methyl ether; reacting trifluoromethoxyaniline and methylchloroformate with phosgene to prepare (chlorocarbonyl)[4-(trifluoromethoxy)phenyl] methyl carbamate; carrying out deprotection on 2-( fluorenylmethyl)-7-chlorinindene-[1,2-e][1,34]] oxadiazine-2,4a(3H, 5H)-dicarboxylic acid-4a-methyl ether in alkaline solution and then carrying out condensation reaction with (chlorocarbonyl)[4-(trifluoromethoxy)phenyl] methyl carbamate to prepare the indoxacarb. The process yield of the indoxacarb is high and the purity of the indoxacarb is good.
Owner:CHANGZHOU UNIV
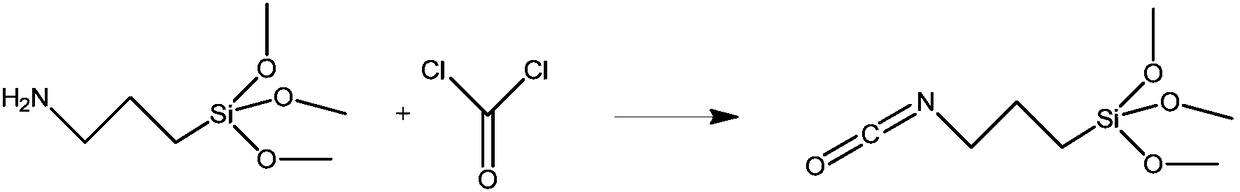
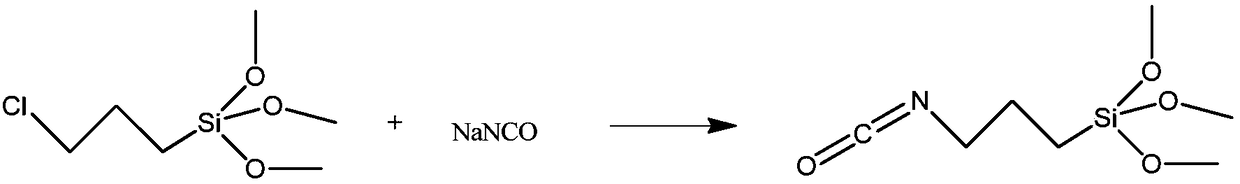
Method for preparing 3-isocyanatopropyltrimethoxysilane
InactiveCN109232638AImprove conversion rateRearrangement side reactions are less likely to occurGroup 4/14 element organic compoundsMethyl carbamateMethyltrichlorosilane
The invention relates to a method for preparing 3-isocyanatopropyltrimethoxysilane. The method is characterized in that [3-(trimethoxysilane)propyl]methyl carbamate is used as a raw material to reactwith methyl trichlorosilane, and thus 3-isocyanatopropyltrimethoxysilane is synthesized by one step. The preparation method provided by the invention is simple, the production process is safe, the reaction temperature is easy to control, production equipment is simple, and thus the method is suitable for industrial production.
Owner:大连鼎燕医药化工有限公司
Naphthalimide derivatives, preparation method and applications thereof
The invention discloses naphthalimide derivatives, a preparation method and applications thereof. The structure of the derivatives is represented by the formula (Y). In the formula (Y), the R represents a six-membered ring containing hetero-atoms, -NHCH2CH2OH, -NHCH2CH2N(CH3)2, or -NHCH2CH2CH2CH3, wherein the hetero-atoms are nitrogen atoms, oxygen atoms, or sulfur atoms, the six-membered ring containing hetero-atoms comprises at least one nitrogen atom, and the R is connected to the mother nucleus of a compound represented by the formula (T) through a nitrogen atom. The provided derivatives are prepared by grafting methyl carbazate to the active sites of naphthalimide. The derivatives can inhibit the growth of tumor cells from different tissues such as mammary glands, cervix, and the like, while normal human cells are barely influenced by the derivatives, and thus the derivatives have a wide prospect in preparation of drugs for inhibiting the growth of tumor cells.
Owner:DALIAN UNIV OF TECH
Method for preparing chlorocarbonyl[4-(trifluoromethoxy)phenyl] methyl carbamate
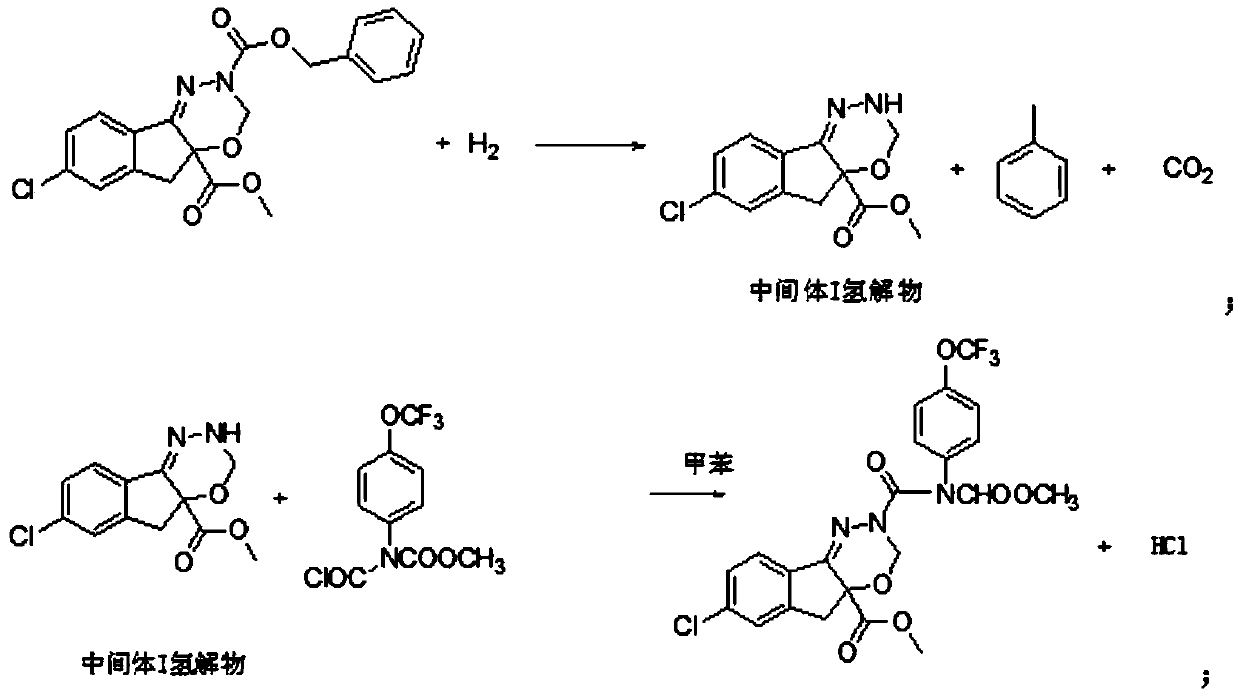
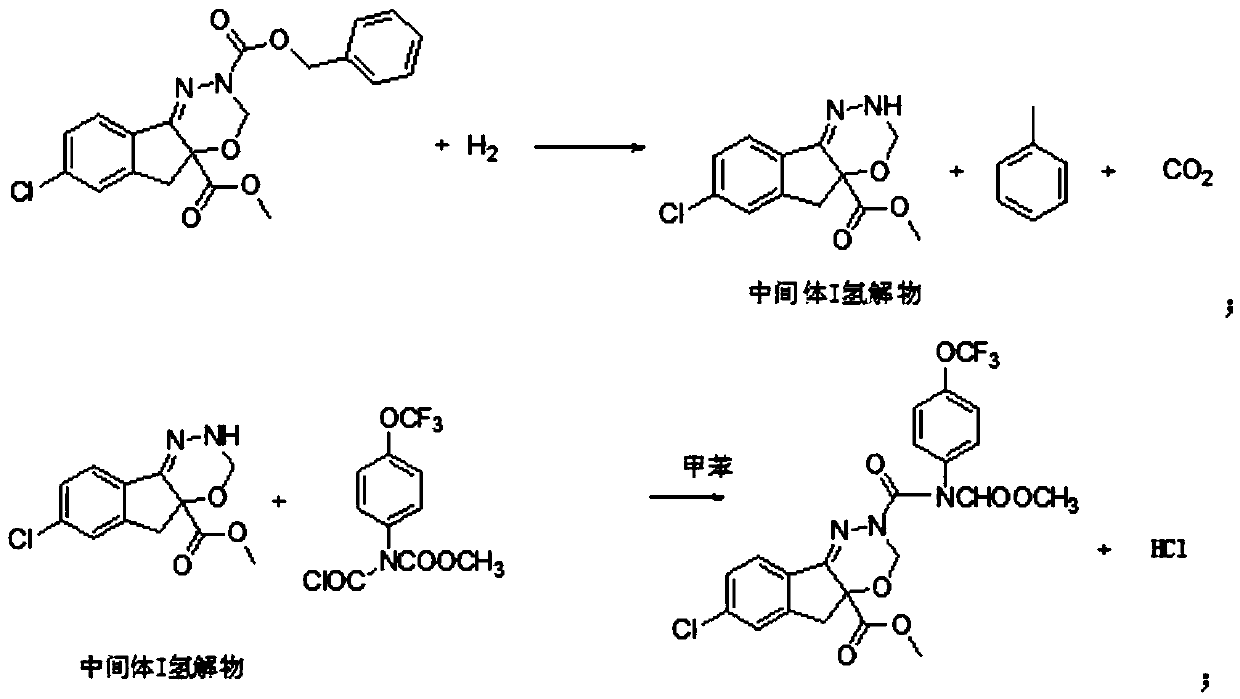
ActiveCN103936630AResidue reductionMild operating environmentCarbamic acid derivatives preparationOrganic compound preparationState of artMethyl carbamate
The invention relates to a method for preparing a drug intermediate and in particular relates to a method for preparing an intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl] methyl carbamate of an agricultural insecticide indoxacarb. Trifluoromethoxyaniline, methylchloroformate and triphosgene are mainly adopted as reactants, and the intermediate is prepared by using mixed alkali with higher safety. According to the method, the defect that a high-risk substance sodium hydride is needed to be adopted in the prior art is overcome, the operating environment is mild, the activity is improved through the mixed alkali process, and the hydrogen taking capacity is improved, so that the reaction speed is increased, the residues of raw materials are reduced, unnecessary side reactions are reduced, and the effects of shortening the reaction time and increasing the yield and product content are achieved; the method is greatly superior to an existing conventional preparation process, and a solid foundation is laid for preparing high-purity indoxacarb.
Owner:JINGBO AGROCHEM TECH CO LTD
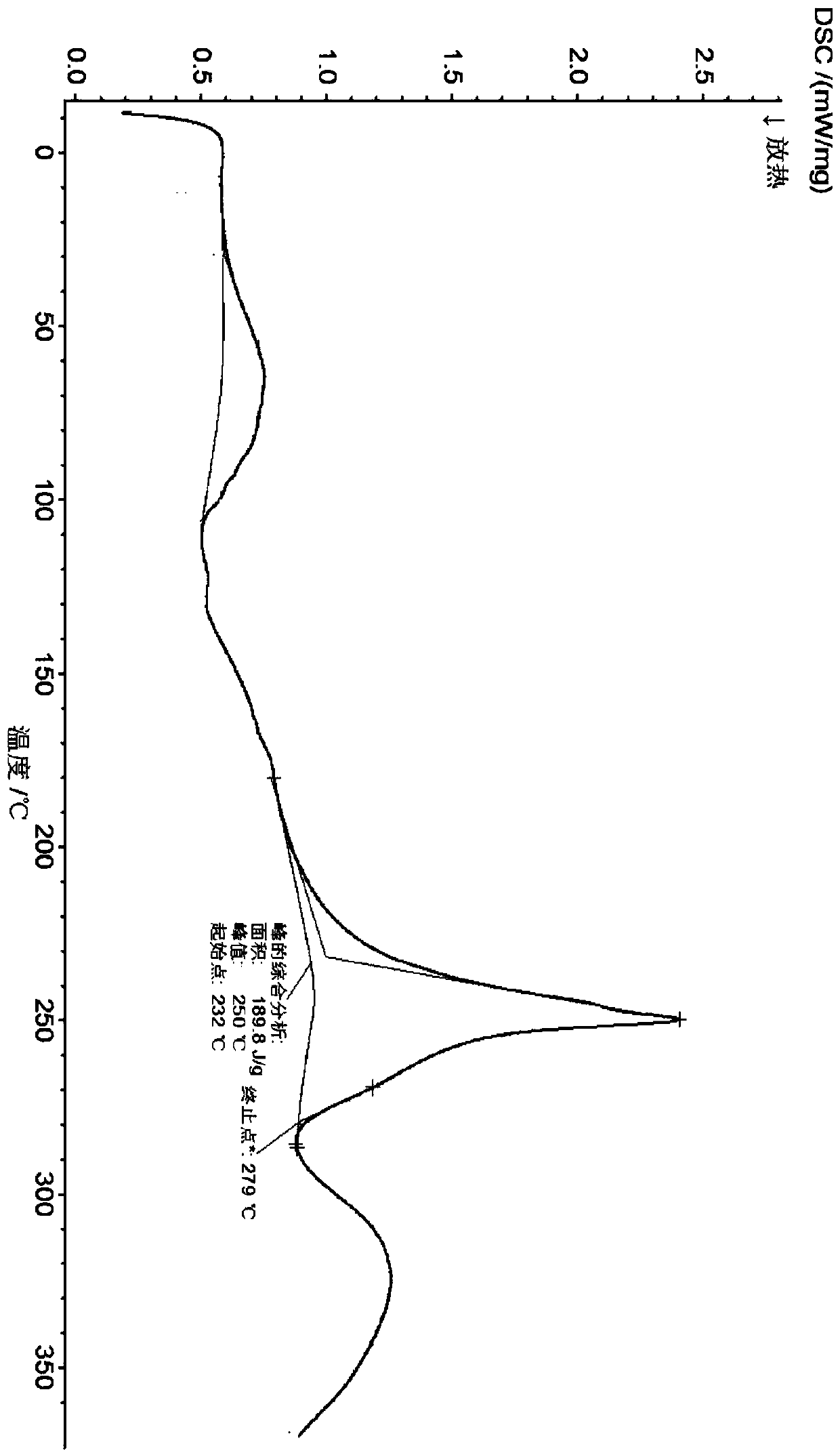
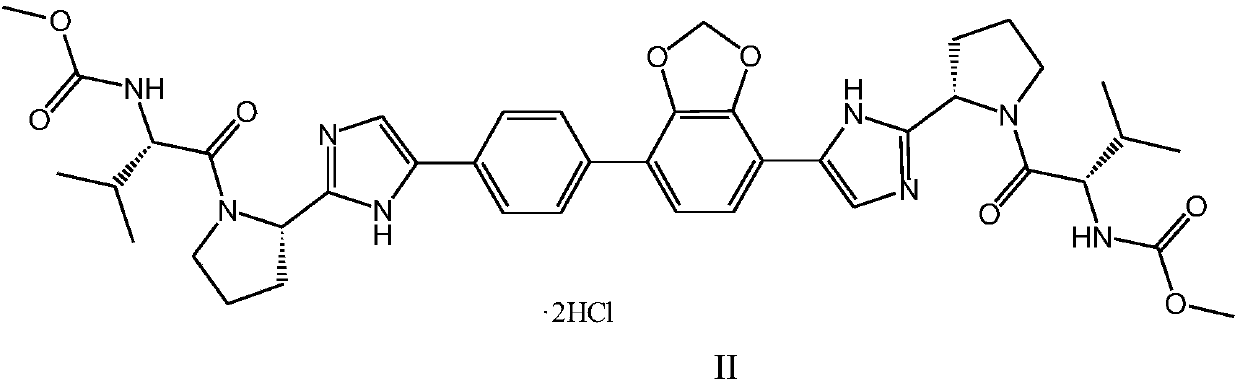
Crystalline methyl carbamate compound
ActiveCN108675998ALow hygroscopicityExcellent impurity controlOrganic active ingredientsOrganic chemistry methodsCrystallographyMethyl carbamate
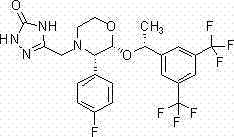
The invention relates to a crystalline methyl carbamate compound, namely a crystal form of N-[(2S)-1-[(2S)-2-{4-[7-(4-{2-[(2S)-1-[(2S)-2-[(methoxycarbonyl)amino]-3-methylbutyryl]pyrrolidin-2-yl]-1H-imidazol-4-yl}phenyl)-2H-1,3-benzodioxan-4-yl]-1H-imidazol-2-yl}pyrrolidin-1-yl]-3-methyl-1-oxybutan-2-yl]methyl carbamate dihydrochloride, a pharmaceutical composition containing the crystal form, anda use of the crystal form in preparation of drugs in treatment of hepatitis C virus.
Owner:BEIJING KAWINGREEN BIOTECH CO LTD
A preparing method of albendazole
InactiveCN105585533ASolve the problem of large impurities in 5-methylthiobenzimidazole-2-carbamate methyl esterLarge amount of solutionOrganic chemistryMethyl carbamateHydrazine compound
A preparing method of albendazole is disclosed. The method adopts 2-nitro-4-thiocyanatoaniline as a raw material, and includes salifying with an aqueous sodium hydroxide solution in an n-propanol solvent under nitrogen protection, reacting with bromopropane, and separating to obtain an n-propanol solution of 2-nitro-4-(propylthio)aniline, and therefore a problem that an impurity that is methyl 5-(methylthio)benzoimidazol-2-yl carbamate in products is high in content because steps of salifying in methanol with sodium sulfide and then reacting with bromopropane in processes at present is overcome. A technique of reducing the 2-nitro-4-(propylthio)aniline by utilizing hydrazine hydrate is adopted to replace a technique of reducing with sodium sulfide at present, thus overcoming a problem that sulfur-containing waste water is high in amount and difficult to treat in the sodium sulfide reduction technique. A methanol solution of methyl O-methyl isourea formate is adopted as a ring closing agent, thus overcoming a problem that waste water is high in amount in processes at present when cyanamide and an aqueous methyl formate solution are adopted as ring closing agents. The method is advantaged by a small waste water amount, capability of being environmental friendly, high product purity, and the like.
Owner:LIANYUNGANG YAHUI PHARMACHEM
Process for co-producing dimethyl carbonate and dimethyl ether by urea alcoholysis method
InactiveCN102212009BVast market potentialImprove qualityOrganic compound preparationEther preparationMethyl carbamateMethyl carbonate
Owner:YASHENTECH CORP
Method for preparing methyl carhamate
InactiveCN101570499AIncrease reaction rateHigh yieldCarbamic acid derivatives preparationOrganic compound preparationBoiling pointMethyl carbazate
Owner:KENLI SANHE NEW MATERIAL TECH
Preparation method of tert-butyl carbazate
ActiveCN102911084AReduce pollutionSimple processOrganic chemistryTert-butyl carbazateMethyl carbazate
The invention discloses a preparation method of tert-butyl carbazate. The method includes: using phenyl chloroformate and tert-butanol as raw materials, performing esterification in ionic liquid at the temperature of 30-40 DEG C under the action of solid base catalysts, adding hydrazine hydrate into esterification liquid after esterification is completed, performing substitution reaction at the temperature of 60-75 DEG C, and subjecting the reaction liquid to separation and purification after the substitution reaction is finished so that the tert-butyl carbazate is obtained. The preparation method is simple in process, high in yield, easy to operate, free of phosphine ligands and less in environmental pollution.
Owner:苏州卫优知识产权运营有限公司
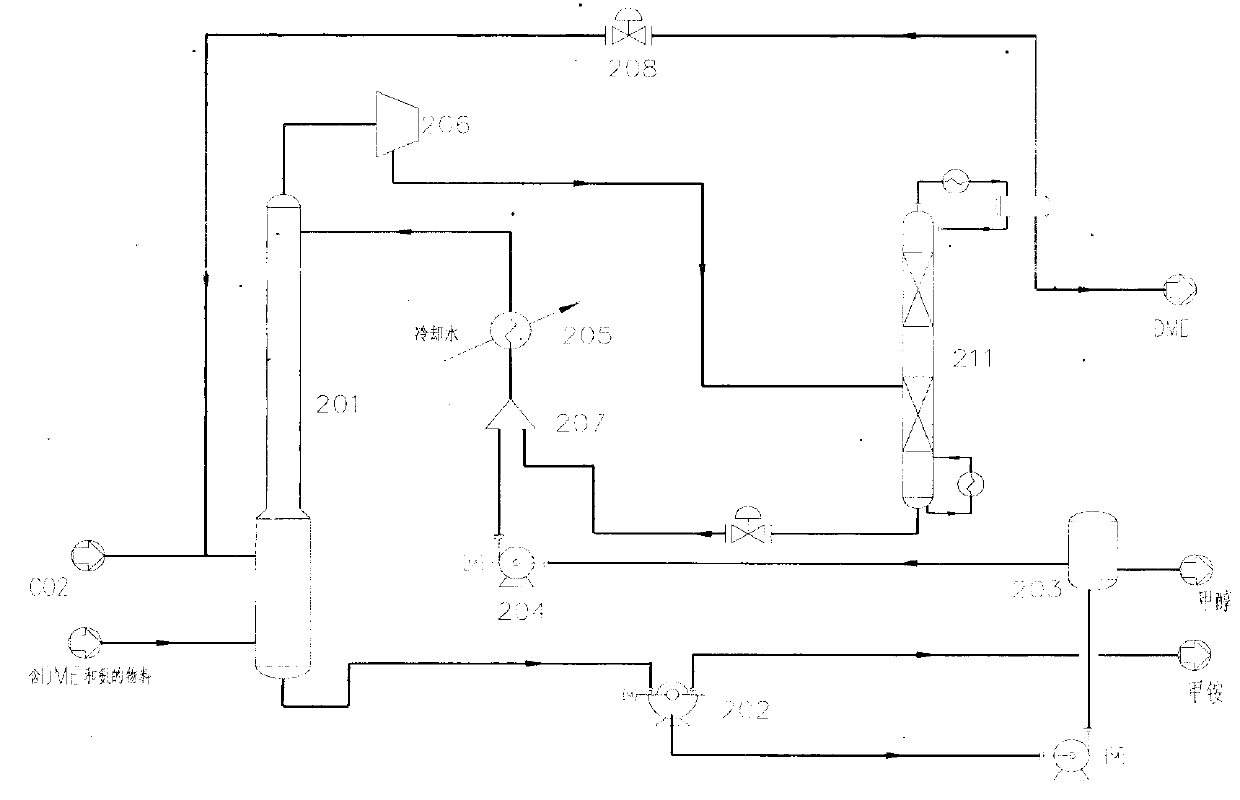
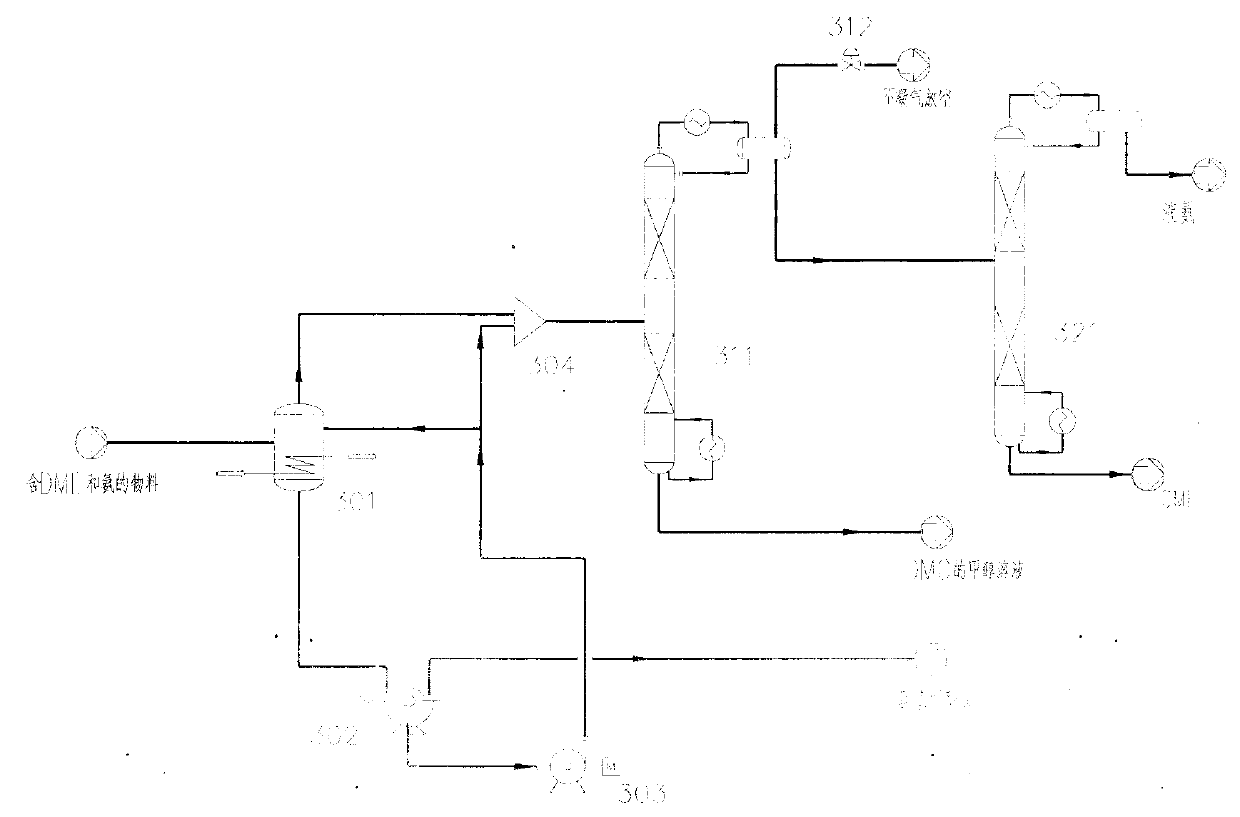
Method for separating dimethyl ether and recovering ammonia in co-production process for dimethyl carbonate and dimethyl ether via urea alcoholysis method
ActiveCN103288645AMeet supplyReduce manufacturing costEther separation/purificationOrganic compound preparationMethyl carbamateMethyl carbonate
The invention provides a method for separating dimethyl ether and recovering ammonia in a co-production process for dimethyl carbonate and dimethyl ether via a urea alcoholysis method. The method comprises the following steps of: adding methanol and urea in a reaction device, carrying out a reaction on methanol and urea to generate methyl carbamate and a by-product of ammonia, further carrying out a reaction on the generated methyl carbamate and methanol to generate dimethyl carbonate and the by-product of ammonia, and decomposing a part of dimethyl carbonate to generate dimethyl ether; separating out a material containing dimethyl ether and ammonia, and a material containing dimethyl carbonate from the output material of a reaction device; and separating out dimethyl ether from the material containing dimethyl ether and ammonia, converting ammonia to an ammonium carbamate solid, liquid ammonia or ammonia water, and recovering.
Owner:YASHENTECH CORP
Purification method for methyl N-chloroformyl-N-[(4-trifluoromethoxy)phenyl]carbamate
InactiveCN108586292AHigh quality contentEfficient separationCarbamic acid derivatives preparationOrganic compound preparationSolubilityPurification methods
The invention provides a purification method for methyl N-chloroformyl-N-[(4-trifluoromethoxy)phenyl]carbamate. The purification method comprises the steps of: 1) providing a to-be-purified methyl N-chloroformyl-N-[(4-trifluoromethoxy)phenyl]carbamate product; 2) mixing the to-be-purified methyl N-chloroformyl-N-[(4-trifluoromethoxy)phenyl]carbamate product with n-hexane and methyl acetate, heating the mixture to reflux, dissolving the components for more than one hour to obtain a mixed liquid; 3) cooling the mixed liquid to -2 DEG C to 2 DEG C, performing freeze-crystallization, and filteringand drying the mixture to prepare the purified product. In the method, by means of difference of the solubility of the methyl N-[(4-trifluoromethoxy)phenyl]carbamate and the methyl N-chloroformyl-N-[(4-trifluoromethoxy)phenyl]carbamate in the n-hexane and methyl acetate, impurities can be effectively separated, and mass percentage of the methyl N-chloroformyl-N-[(4-trifluoromethoxy)phenyl]carbamate in the product is increased to more than 96%.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
Method for producing methyl carbamate
ActiveCN110590605ATroubleshoot blocked technical issuesPerfect conditions for industrializationCarbamic acid derivatives preparationOrganic compound preparationTemperature controlMethyl carbamate
The invention provides a method for producing methyl carbamate. The method comprises the following steps: performing sectional temperature control on an exhaust channel of a reaction kettle, controlling the temperature of a pipeline section of the exhaust channel close to the reaction kettle to 4-20 DEG C, and increasing the temperatures of later pipeline sections gradually till the temperature ofa pipeline section of the exhaust channel far away from the reaction kettle is 75-85 DEG C; and performing pressurization on a reaction process for preparing methyl carbamate by using a urea alcoholysis method to release a gas, and maintaining the pressure of the reaction system in the reaction kettle to 1.8-2.8MPa. By adopting the method, the technical problem that the exhaust channel of the reaction kettle is blocked can be solved, industrial conditions of production of methyl carbamate can be completed, production reactions can be performed smoothly, and in addition, the yield of the methyl carbamate can be increased.
Owner:重庆化工职业学院
Process for purifying methyl carbamate
InactiveCN1683325AShorten separation timeReduce energy consumptionCarbamic acid derivatives preparationOrganic compound preparationTemperature controlMethyl carbamate
The methyl carbamate purifying process belongs to the field of fine chemical technology. The technological scheme of purifying methyl carbamate includes: spraying methyl carbamate solution into decompression distiller, spraying dewatered and heated gas into the decompression distiller in the direction opposite to that of the methyl carbamate solution to increase the gas-liquid contact area, maintaining the decompression distiller at 60-150 deg.c temperature and 0.001-0.1 MPa pressure, cooling the distilled gaseous methyl carbamate and separating. The methyl carbamate solution into the decompression distiller has temperature controlled in 70-100 deg.c, and the gas into the decompression distiller has temperature controlled in 80-100 deg.c and lagged entering time, and the distilled gaseous methyl carbamate has cooling temperature controlled in 56-60 deg.c. The process has short separation time, low power consumption, high product yield, and product purity over 95 %.
Owner:山东康瑞集团有限公司 +1
A kind of preparation method of indoxacarb insecticide
Owner:JINGBO AGROCHEM TECH CO LTD
Method for preparing 2-(2-chloro-1-ethidene)hydrazide methyl formate
The invention provides a method for preparing a method for preparing an aprepitant intermediate 2-(2-chloro-1-ethidene)hydrazide methyl formate. The method comprises the following steps: carrying out a condensation reaction between chloroacetonitrile and methyl hydrazinocarboxylate in a methanol / sodium methylate reaction solution by virtue of catalysis of glacial acetic acid, and performing after-treatment purification on the reaction solution by adopting acetone. The operation is simple, the purity of the obtained product is over 99%, and the method is high in yield and suitable for industrial production.
Owner:深圳万乐药业有限公司
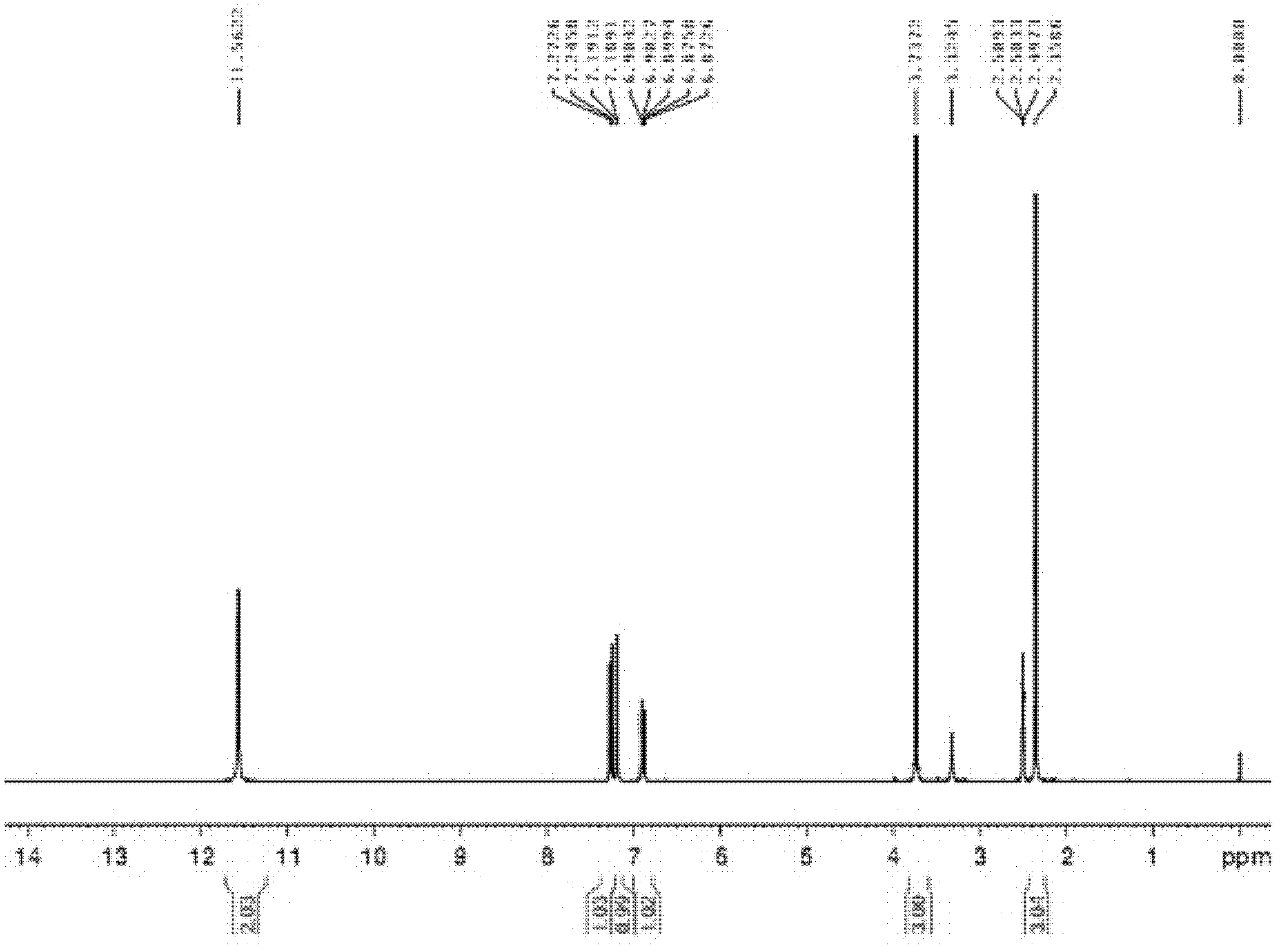
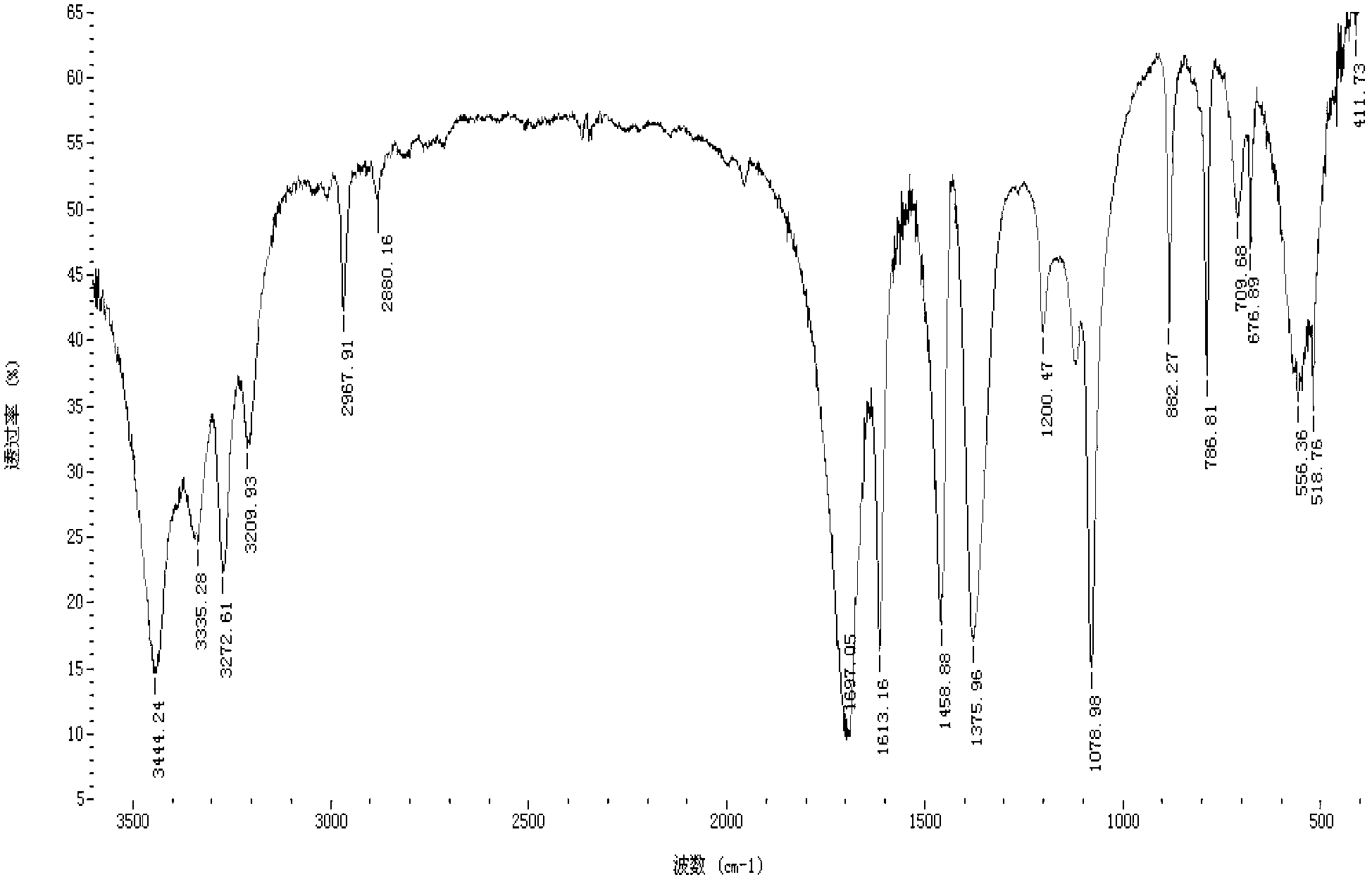
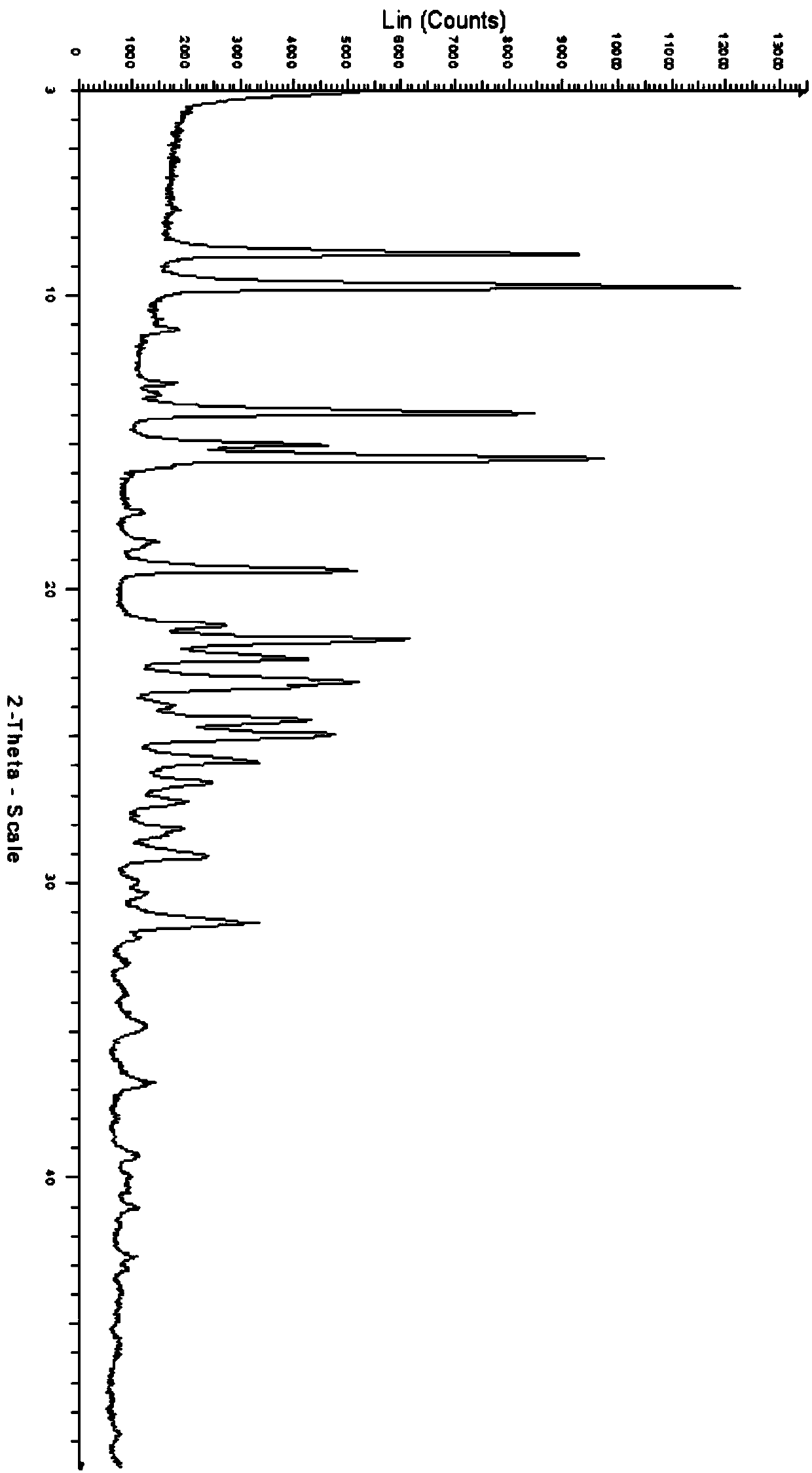
N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content analysis method
ActiveCN103033573AHigh column efficiencyNot easy to loseComponent separationMethyl carbamateMethyl carbazate
The invention relates to an N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content analysis method which comprises the following steps: separating and determining N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate in a sample by utilizing a gas chromatograph with a hydrogen flame ionization detector and a quartz capillary gas chromatographic column and taking diethyl phthalate as an internal standard substance; and through the comparison with the standard substance, calculating the N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content in the measured sample. The invention can effectively avoid the interference of inorganic salt and water, has the advantages of favorable precision, high recovery rate, high result accuracy and favorable reproducibility, and is especially applicable to the quality control on the pesticidal chemical intermediate product N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate, thereby achieving important functions and practical meanings for ensuring the quality of the final product.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
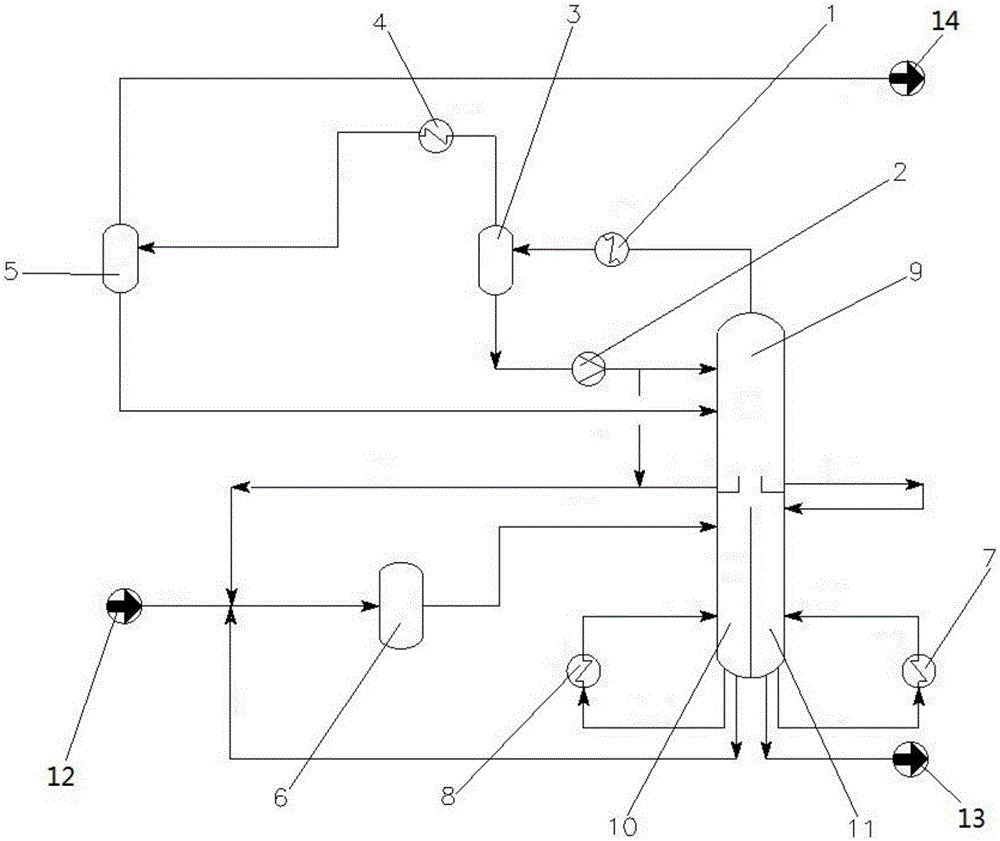
Method for preparing methyl carbamate by urea alcoholysis method and device thereof
ActiveCN106631901AEasy to makeSafe preparationCarbamic acid derivatives preparationOrganic compound preparationAlcoholMethyl carbamate
The invention discloses a method for preparing methyl carbamate by a urea alcoholysis method and a device thereof. The method comprises the steps that urea and methanol are mixed; then, a catalyst is added; the temperature is raised to 120 to 200 DEG C; reaction is performed for 0.8 to 8h; the temperature is lowered to room temperature; after filtering and separation, the methyl carbamate is obtained; the catalyst comprises 0.1 to 12 mass percent of active ingredients, 0.1 to 10 mass percent of auxiliary agent metal and the balance silicon oxide; the active ingredients are at least one of hydrochloride, nitrate or acetate of Mg, Ca, Zn, Fe, Cu or Pb; the auxiliary agent metal is at least one of La, K, Sr or Ce. The catalyst can be used for preparing the methyl carbamate by the urea alcoholysis method; the environment pollution cannot be caused; the process is simple; the large-scale production is easy; meanwhile, the preparation device can realize the industrialization of preparation of the methyl carbamate by the urea alcoholysis method.
Owner:中榆化工科技有限公司
SYP-7017 synthesis method
The present invention discloses a SYP-7017 synthesis method. The method is as follows: mixing 3, 5, 6-trichloropyridin-2-ol sodium, an alkane solvent, water and additives, then adding N-methoxy-N-[2-(bromomethyl)phenyl] methyl carbamate for reaction; after the reaction is completed, separating an oil phase, and removing the alkane solvent to obtain a crude SYP-7017 product. By the above method, the technological process of the synthesis of the SYP-7017 can be simplified, the safety of the process is improved, and pollution to environment is reduced.
Owner:JIANGSU BAOLING CHEM
Synthesis method for indoxacarb intermediate chloroformyl [4-(trifluoromethoxy) phenyl] methyl carbamate
ActiveCN109535036AReduce usageEasy to operateCarbamic acid derivatives preparationOrganic compound preparationMethyl carbamateSynthesis methods
The invention discloses a synthesis method for an indoxacarb intermediate chloroformyl [4-(trifluoromethoxy) phenyl] methyl carbamate and relates to the field of pesticide preparation. A process routeof acylating p-trifluoromethoxy aniline with phosgene and then reacting with methyl chloroformate is adopted: triggering reaction of p-trifluoromethoxy aniline and phosgene under low temperature, thereby acquiring p-trifluoromethoxy phenylcarbamyl chloride; triggering reaction of p-trifluoromethoxy phenylcarbamyl chloride and methyl chloroformate, thereby acquiring chloroformyl [4-(trifluoromethoxy) phenyl] methyl carbamate. In the invention, phosgene can directly react with p-trifluoromethoxy aniline; no catalyst is required; the steps of water shearing before action, water washing after reaction, and the like, in the prior art are avoided; the flow of phosgene introduced into the reaction system can be controlled; reaction process can be controlled; high-corrosivity sodium cyanide or low-stability mixed alkali used as an initiator in the prior art can be avoided; operation steps are simplified; reaction period is shortened; production cost is lowered; the synthesis method is easy for industrialization.
Owner:山东华阳农药化工集团有限公司
Improved method for synthesizing indoxacarb intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl]carbamic acid methyl ester
InactiveCN109704994ALow quality scoreHigh yieldCarbamic acid derivatives preparationOrganic compound preparationMethyl carbamateMethyl carbazate
The invention discloses an improved method for synthesizing indoxacarb intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl]carbamic acid methyl ester, and relates to the field of pesticide preparation. The method provided by the invention uses phosgene instead of solid phosgene and diphosgene without considering risk that the solid phosgene and the diphosgene are easily decomposed at high temperature and a phosgene toluene solution overflows easily and instantaneously at high temperature; at the same time, a manner that slurry is added dropwise reversely is adopted under the catalyst catalysis, a reaction is directly performed at room temperature, and strong-corrosivity sodium cyanide or a poorer-stability mixed alkali is avoided from being used as an initiator; the low-temperature reaction environment does not need to be strictly controlled, the operation steps are simplified, the reaction cycle is shortened, the production costs are reduced, and the reaction process can be controlled through control of an introduction speed of the phosgene; and the method reduces a mass fraction of sodium carbonate in the reaction system and improves a product yield.
Owner:山东华阳农药化工集团有限公司
Synthesis method of carbohydrazide
PendingCN114031524AMild reaction conditionsEasy to controlOrganic chemistryChemical synthesisDistillation
The invention discloses a synthesis method of carbohydrazide, and relates to the technical field of chemical synthesis, the synthesis method comprises the following steps: 1, synthesizing a methyl carbazate crude product by using dimethyl carbonate and hydrazine hydrate as raw materials; and performing reduced pressure distillation on the synthesized methyl carbazate crude product, and drying to obtain methyl carbazate; 2, synthesizing a carbohydrazide crude product by taking hydrazine hydrate and methyl carbazate obtained in the step 1 as raw materials; and carrying out reduced pressure distillation on the synthesized carbohydrazide crude product, and drying to obtain the carbohydrazide. According to the synthesis method of carbohydrazide, dimethyl carbonate and hydrazine hydrate serve as raw materials, carbohydrazide is synthesized at low temperature through a two-step method, the adopted raw materials are non-toxic and environmentally friendly, the whole reaction process is mild in condition, easy to control and high in reaction speed, and the obtained product is high in yield, few in side reaction and easy to purify.
Owner:DONGGUAN UPC IND & TRADE +1
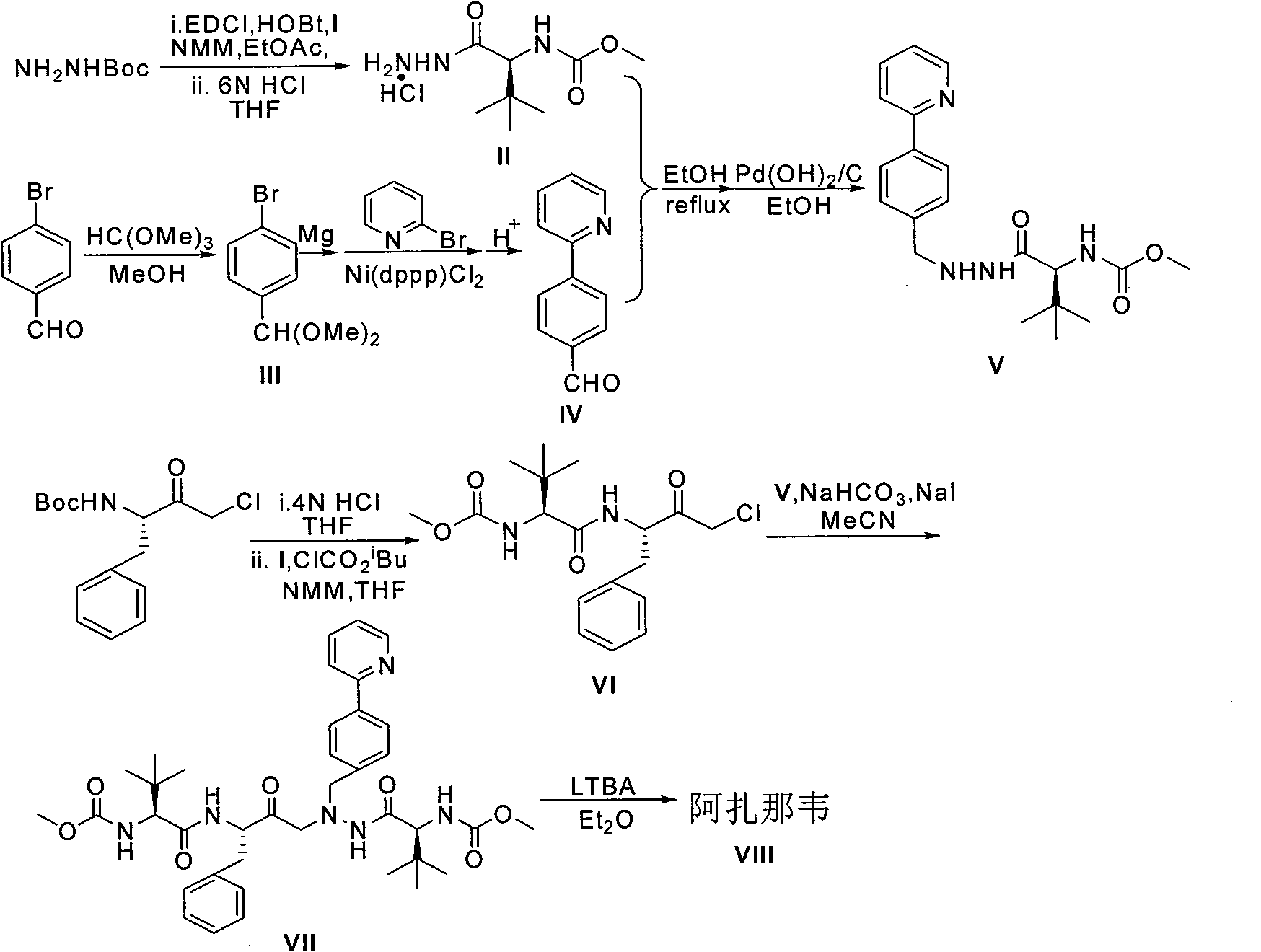
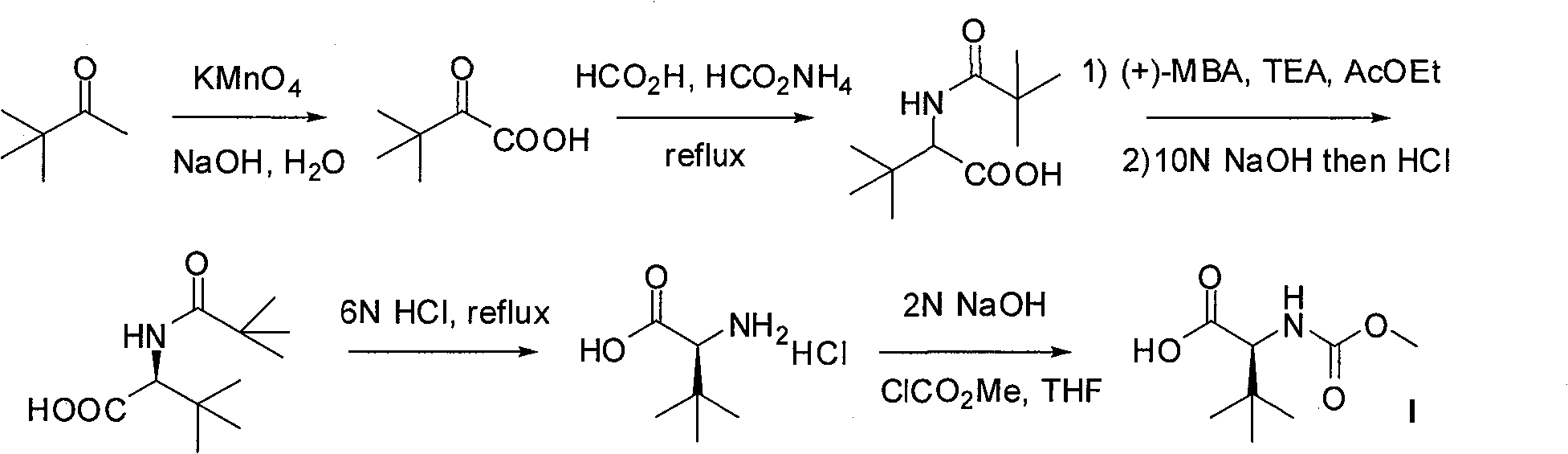
Novel synthetic method of HIV-1 protease inhibitor atazanavir
InactiveCN101391978BLow priceEasy recrystallization purificationOrganic chemistryBulk chemical productionMethyl carbamateMethyl carbazate
The invention relates to a new synthetic method of an HIV-1 protease inhibitor, Atazanavir, which adopts a convergent-typed synthetic strategy, introduces a construction unit, methoxycarbonyl-tert-lencyl, as an N atom protecting group in the whole early synthetic stage, and takes the diastereomeric selective reduction of aminoketone as the key and final reaction step of the new process. The method comprises the steps that: the compound of formula V, namely, N-1-[N-(methoxycarbonyl)-L-tert-leucine]-N-2-[4-(2-pyridyl)-phenmethyl]hydrazine, and the compound of formula VI, namely, (S)-1-((S)-4-chlorine-3-carbonyl-1-phenyl butane-2-yl-2-amino)-3, 3-dimethyl-1-carbonyl butane-2-yl-methyl carbamate, are treated with nucleophilic substitution reaction to generate the compound of formula VII, namely, 1-[4-(2-pyridyl)phenyl]-5(S)-2, 5-bis{[N-(methoxycarbonyl)-L-tert-leucineyl] amino}-4-carbonyl-6-phenyl-2-azahexane; and the compound of formula VII is treated with reduction reaction to generate the Atazanavir. The invention has the advantages of less process route steps, easily-controlled reaction conditions, simple and convenient operation, low-price and easily-obtained raw material, high product yield, low cost and being suitable for large-scale production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

















![Method for preparing chlorocarbonyl[4-(trifluoromethoxy)phenyl] methyl carbamate Method for preparing chlorocarbonyl[4-(trifluoromethoxy)phenyl] methyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cedac61b-2d60-47d2-bb50-11869bb5f486/BDA0000487225210000021.PNG)





![N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content analysis method N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content analysis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaba2b55-a057-4e54-9260-59a52e145089/BDA00002581603700021.PNG)
![N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content analysis method N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content analysis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaba2b55-a057-4e54-9260-59a52e145089/BDA00002581603700041.PNG)
![N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content analysis method N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate content analysis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaba2b55-a057-4e54-9260-59a52e145089/BDA00002581603700051.PNG)



![Synthesis method for indoxacarb intermediate chloroformyl [4-(trifluoromethoxy) phenyl] methyl carbamate Synthesis method for indoxacarb intermediate chloroformyl [4-(trifluoromethoxy) phenyl] methyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1bf5155f-fdf1-4968-a54b-7dd408ceeea3/HDA0001922027770000011.png)
![Synthesis method for indoxacarb intermediate chloroformyl [4-(trifluoromethoxy) phenyl] methyl carbamate Synthesis method for indoxacarb intermediate chloroformyl [4-(trifluoromethoxy) phenyl] methyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1bf5155f-fdf1-4968-a54b-7dd408ceeea3/HDA0001922027770000012.png)
![Improved method for synthesizing indoxacarb intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl]carbamic acid methyl ester Improved method for synthesizing indoxacarb intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl]carbamic acid methyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/477116b1-f748-4664-ad42-31e21c5eba93/HDA0001922031350000011.png)
![Improved method for synthesizing indoxacarb intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl]carbamic acid methyl ester Improved method for synthesizing indoxacarb intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl]carbamic acid methyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/477116b1-f748-4664-ad42-31e21c5eba93/HDA0001922031350000012.png)
![Improved method for synthesizing indoxacarb intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl]carbamic acid methyl ester Improved method for synthesizing indoxacarb intermediate chlorocarbonyl[4-(trifluoromethoxy)phenyl]carbamic acid methyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/477116b1-f748-4664-ad42-31e21c5eba93/201811599165.png)