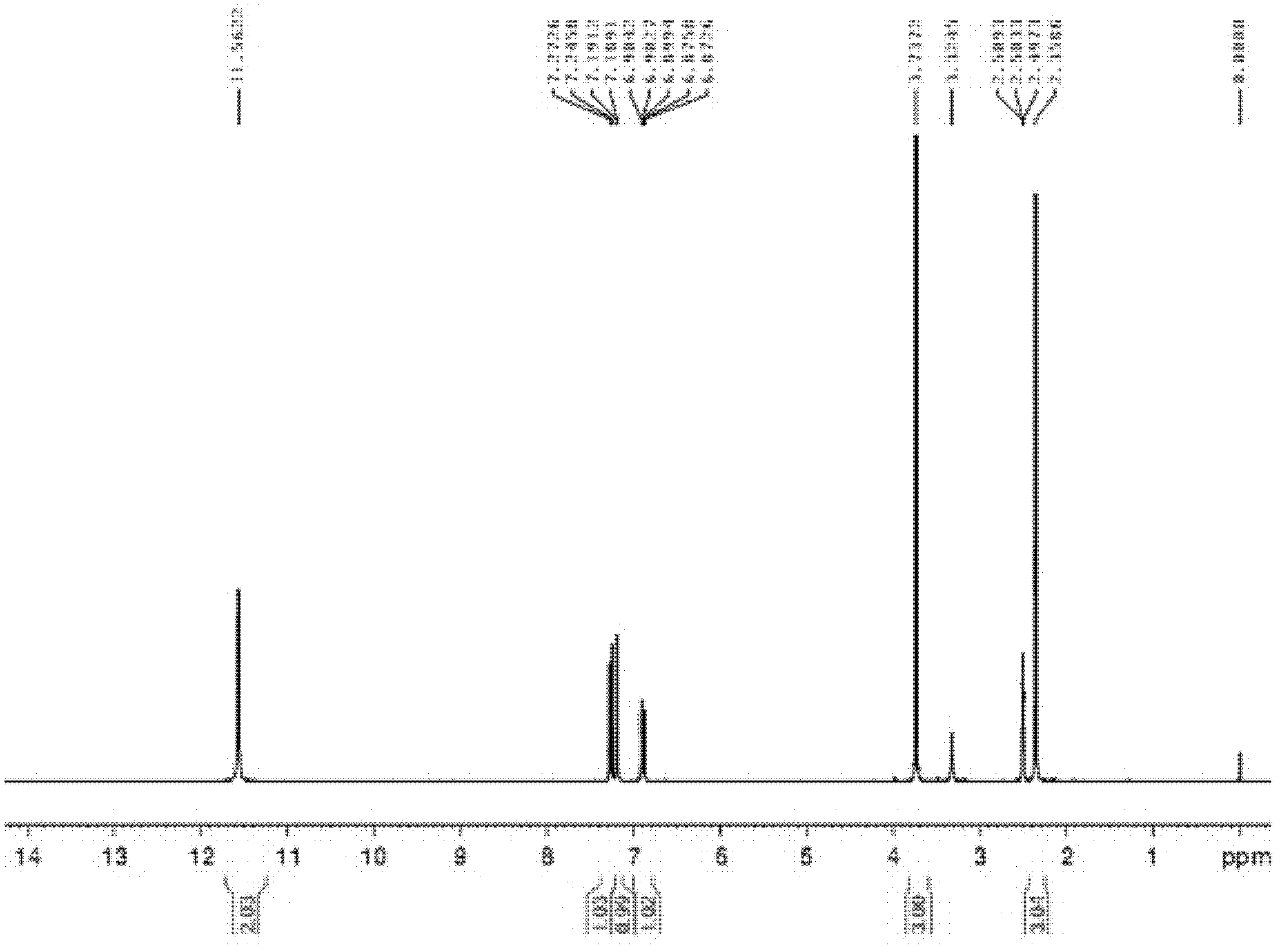
Method for preparing 5-methylbenzimidazole-2-methyl carbamate
A technology of methyl carbamate and methyl cyanocarbamate, which is applied in the field of preparation of fungicides, can solve the problems of long reaction steps, difficulty in industrial production, and high toxicity, and achieve the effects of mild reaction conditions, reduced dosage, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In a 250mL four-necked flask with a thermometer and a stirring device, add 16.8g of 50% cyanamide solution (containing 0.2mol of cyanamide), 60ml of methanol solvent, 64g of 25% NaOH solution (containing 0.4mol of NaOH) and 0.5 g benzyltrimethylammonium chloride was used as a phase transfer catalyst, and 18.9 g (0.2 mol) of methyl chloroformate was uniformly added dropwise at a rate of 30 g / h, kept at 45 ° C for about 3 hours, and the reaction solution was cooled, and then poured into a large amount of ice-water mixture, white crystals are precipitated, suction filtration under 760mmHg vacuum condition, the filter cake is washed with methanol and washed to neutrality successively, and dried to obtain 18.7g of methyl cyanocarbamate sodium salt, the yield is 76.8 %.
[0033] In a 250mL four-necked flask equipped with a thermometer and a stirring device, mix and react 18.3g (0.15mol) of sodium methyl cyanocarbamate and 30ml of 5mol / L hydrochloric acid (containing 0.15mol o...
Embodiment 2
[0036] In a 250mL four-necked flask with a thermometer and a stirring device, add 33.6g of 50% cyanamide solution (containing 0.4mol of cyanamide), 60ml of methanol solvent, 64g of 25% NaOH solution (containing 0.4mol of NaOH) and 0.5 g benzyltrimethylammonium chloride was used as a phase transfer catalyst, and 18.9 g (0.2 mol) of methyl chloroformate was uniformly added dropwise at a rate of 30 g / h, kept at 45 ° C for about 3 hours, and the reaction solution was cooled, and then poured into a large amount of ice-water mixture, white crystals are precipitated, suction filtration under 760mmHg vacuum condition, the filter cake is washed with methanol and washed to neutrality successively, and dried to obtain 21.0g of methyl cyanocarbamate sodium salt, the yield is 86.0 %.
[0037] In a 250mL four-neck flask equipped with a thermometer and a stirring device, mix and react 18.8g (0.15mol) of sodium methyl cyanocarbamate and 60ml of 5mol / L hydrochloric acid (containing 0.3mol of h...
Embodiment 3
[0040] In a 250mL four-necked flask with a thermometer and a stirring device, add 33.6g of 50% cyanamide solution (containing 0.4mol of cyanamide), 60ml of methanol solvent, 96g of 25% NaOH solution (containing 0.6mol of NaOH) and 0.5 g benzyltrimethylammonium chloride was used as a phase transfer catalyst, and 18.9 g (0.2 mol) of methyl chloroformate was uniformly added dropwise at a rate of 30 g / h, kept at 45 ° C for about 3 hours, and the reaction solution was cooled, and then poured into a large amount of ice-water mixture, white crystals are precipitated, suction filtration under 760mmHg vacuum condition, the filter cake is washed with methanol and washed to neutrality successively, and dried to obtain 20.1g of methyl cyanocarbamate sodium salt, the yield is 82.2 %.
[0041] In a 250mL four-necked flask equipped with a thermometer and a stirring device, mix and react 18.3g (0.15mol) of sodium methyl cyanocarbamate and 50ml of 5mol / L hydrochloric acid (containing 0.25mol o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com